Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acid-base disorders can be quantified by using the physiologic, base-excess, and physiochemical approaches (Stewart method). The bicarbonate [HCO 3 − ] buffer system plays a central role in the physiologic and base-excess methods. They share nearly common explanations while describing the types and mechanisms behind acid-base disturbances. However, Stewart’s more recent (1980s) acid-base approach differs significantly from the other two approaches by not attributing a central role to [HCO 3 − ], as HCO 3 − alone does not determine metabolic acid-base disorders. The Stewart approach adapts a method based on charge differences between strong cations and anions. According to Stewart, acid-base balance in the body is determined by “strong” and “weak” electrolytes that are present in body fluid compartments as cations and anions. Strong electrolytes are fully dissociated in aqueous solution, and weak electrolytes are partially dissociated. He proposed that changes in plasma [H + ] are related to water dissociation and not addition or removal of H + from the plasma. Stewart derived six equations that, when solved simultaneously, yield the [H + ] of a solution, and these equations operate strictly under the physiochemical principles of electroneutrality (sum of cations is equal to sum of anions), conservation of mass (amount of substance in a solution is constant unless added or removed), and law of mass action (dissociation equilibriums of all incompletely dissociated substances must be met at all times). Stewart proposed that [H + ] and HCO 3 − are dependent variables and that changes in hydrogen ion [H + ] and subsequently the pH are influenced by three independent variables: namely, partial pressure of carbon dioxide (pCO 2 ). When pCO 2 increases, [H + ] raises, and when it decreases, [H + ] decreases. Increases in pCO 2 define respiratory acidosis, and decreases in pCO 2 respiratory alkalosis.
Total concentration of weak acids [A TOT ]: weak acids are not completely dissociated in aqueous solution and are mainly represented by albumin and phosphate. Increase and decrease in [A TOT ] increases and decreases [H + ], respectively (e.g., hypoalbuminemia causes metabolic alkalosis and hyperphosphatemia results in metabolic acidosis).
Strong ion difference (SID): difference between the total concentration of strong cations (Na + , K + , Ca 2+ , and Mg 2+ ) and strong anions (Cl − , SO 4 − , and anions of organic acids). Concentrations of Na + and Cl − are the main determinants of SID because of their magnitude, and normal values range from 39 to 42 mEq/L based on concentrations of Na + and Cl − (SID = Na + − Cl − = 140 − 100 = 40 mEq/L). This simplest estimation is called apparent SID (SID a ). When other anions like plasma [HCO 3 − ] and the anionic equivalence of albumin and phosphate are included in the calculation of SID, the estimation is called effective SID (SID e ). SID e is difficult to calculate at the bedside and needs to be estimated from blood pH, pCO 2 , and the plasma concentrations of albumin and phosphate using a formula or a nomogram. SID is always positive, as Na + concentration is greater than Cl − . Because of the electroneutrality principle, SID is zero in healthy individuals, as the positivity is balanced by the negative charge present on HCO 3 − , albumin, and phosphate. Changes in SID result in metabolic acid-base disturbances. Metabolic acidosis develops because of a decrease in cations or increase in anions resulting in SID <39 mEq/L. Similarly, metabolic alkalosis develops when SID >42 mEq/L.
The difference between SID a and SID e is called strong anion gap (SIG) and represents unmeasured anions like ketones, SO 4 2 − , and urate. SIG is similar to anion gap (AG) and ranges from 0 to 2 mEq/L. A SIG value >2 mEq/L reflects metabolic acidosis resulting from the presence of more anions than cations (e.g., ketones in diabetic ketoacidosis).
The Stewart method describes six acid-base disorders based on variations in the three independent variables, as shown in Table 15.1 . ,
| Independent Variable | Acidosis | Alkalosis |
|---|---|---|
|
↑pCO 2 | ↓pCO 2 |
|
||
| Water excess | ↓SID, [↓Na + ] | |
| Water deficit | ↑SID [↑Na + ] | |
| Hyperchloremia | ↓SID | |
| Hypochloremia | ↑SID | |
|
|
|
| 3. Nonvolatile weak acids: [A TOT ] | ||
| Serum albumin | ↑[Alb] | ↓[Alb] |
| Inorganic phosphate | ↑[Pi] | ↓[Pi] |
Stewart’s methodology is mechanistic and useful in analyzing complex acid-base disorders, especially with SIG being superior to AG in detecting anions such as ketones. Compared with the traditional physiologic approach that is far more descriptive and widely adapted, the Stewart approach requires additional measurements of multiple ions and the use of complex calculations that hinder swift bedside clinical deployment. Interpreting SID with its two formulations, SID a and SID e , is cumbersome, and classification of metabolic acid-base disorders is unduly complex. No quantitative assessment of the secondary compensatory responses to primary changes in SID e , [A TOT ], and pCO 2 is offered by the Stewart approach. This is a major drawback and can risk misdiagnosis of the secondary responses as independent, simple acid-base disorders. Hence this chapter focuses on the physiologic approach with the bicarbonate-carbonic acid buffer system that remains central in assessing acid-base disorders.
On a daily basis, the body’s metabolic processes generate 10,000–15,000 mEq of volatile acids and 1–2 mEq/kg of fixed acids that must be buffered and excreted to maintain the pH within a narrow range of 7.35–7.45. Chemical buffers and the pulmonary and renal systems operate interdependently to regulate and maintain acid-base balance. The most important buffer is the bicarbonate-carbonic acid (HCO 3 – and H 2 CO 3 ) system, which acts immediately to buffer the extracellular fluid. The relationship between pH, HCO 3 − , and carbon dioxide (CO 2 ) is described by the Henderson-Hasselbalch equation:
The number 6.10 represents the dissociation constant for the reaction; 0.03 represents the solubility coefficient of CO 2 in blood. PCO 2 is the partial pressure of CO 2 in the blood.
Normal acid-base status is maintained by pulmonary excretion of volatile acids and by renal excretion of fixed acids and the formation of bicarbonate. To maintain acid-base balance, the kidney must reabsorb all of the filtered bicarbonate (about 4000 mEq/day) and excrete the fixed daily acid load. Reabsorption occurs mostly in the proximal tubule (>90%) and, to a lesser degree, in the collecting tubule. Renal excretion of acid is achieved by combining hydrogen ions (H + ) with urinary buffers to be excreted as titratable acids, such as phosphate, urate, and creatinine, or with ammonia to form ammonium. The ammonia buffering system is especially important because other buffers are filtered in fixed concentrations and can be depleted by high acid loads; by contrast, tubular cells actively regulate ammonia production in response to changes in acid load.
When acid-base derangements occur, the blood pH is returned toward normal, initially by chemical buffering, followed by pulmonary ventilation, and finally by renal regulation of acid-base excretion. The PaCO 2 is finely regulated by changes in tidal volume and minute ventilation. A decrease in pH is sensed by arterial chemoreceptors and leads to increases in tidal volume or respiratory rate. Pulmonary regulation occurs over minutes to hours. The kidney controls pH through the regulation of H + excretion, bicarbonate reabsorption, and the production of new bicarbonate. Reabsorption of bicarbonate is equivalent to removing free H + . Changes in renal acid-base handling occur hours to days after changes in acid-base status.
Acid-base disorders occur when a change in the normal value of the blood pH results from abnormal renal or pulmonary function or when an acid or base load overwhelms excretory capacity. Acidemia refers to a decrease in the blood pH below the normal range, whereas alkalemia refers to an increase in the blood pH above the normal range. Acidosis is a process that tends to decrease the blood pH and occurs by a fall in the plasma bicarbonate concentration and/or an elevation in PaCO 2 . In contrast, alkalosis is a process that tends to raise the blood pH through an elevation in the plasma bicarbonate concentration and/or a fall in PaCO 2 . Although acidemia cannot be present without acidosis and alkalemia cannot be present without alkalosis, acidosis or alkalosis can exist at any blood pH.
The four primary acid-base disorders are classified as respiratory or metabolic. A respiratory disturbance occurs when acidosis or alkalosis results from a primary change in the PaCO 2 . Respiratory acidosis is a disorder that elevates the PaCO 2 and reduces the pH; respiratory alkalosis is a disorder that reduces the PaCO 2 and elevates the pH. A metabolic disturbance occurs when acidosis or alkalosis results from a primary change in the plasma bicarbonate concentration. Metabolic acidosis is a disorder that reduces the plasma bicarbonate concentration and pH; metabolic alkalosis is a disorder that elevates the plasma bicarbonate concentration and pH. Compensation refers to physiologic respiratory and renal changes by which the body attempts to return the pH toward normal in response to primary acidosis or alkalosis. , Compensation does not return the pH back to a completely normal value.
A simple acid-base disorder is a single primary acid-base disorder (respiratory acidosis, respiratory alkalosis, metabolic acidosis, or metabolic acidosis) with appropriate respiratory or renal compensation for that disorder. A mixed acid-base disorder is characterized as the simultaneous presence of two or more primary acid-base disorders and is frequently encountered in intensive care unit (ICU) patients. The arterial blood pH will depend on the direction and magnitude of disturbances. Mixed acid-base disorders can be suspected from the patient’s history and whenever the measured compensatory values of either bicarbonate or PaCO 2 differ significantly from what is expected.
Disturbances in acid-base balance lead to predictable responses that serve to limit the magnitude of change of the blood pH. The expected compensatory responses to primary acid-base disturbances are listed in Table 15.2 . , The magnitude of the compensatory response is proportional to the severity of the primary acid-base disturbance. The Henderson-Hasselbalch equation shows that pH is determined by the ratio of the plasma HCO 3 − concentration and PaCO 2 , not by either value in isolation. In each acid-base disorder, compensatory renal or respiratory responses act to minimize the change in pH by minimizing alterations in the ratio. Metabolic disorders result in respiratory compensation (change in PaCO 2 ); respiratory acid-base disorders result in metabolic compensation (change in HCO 3 − concentration).
| Primary Acid-Base Disorders | Primary Defect | Effect on pH | Compensatory Response | Expected Range of Compensation | Limits of Compensation |
|---|---|---|---|---|---|
| Respiratory acidosis | Alveolar hypoventilation (↑P co 2 ) | ↓ | ↑Renal HCO 3 − reabsorption (HCO 3 − ↑) | Acute: Δ[HCO 3 − ] = +1 mEq/L for each ↑ ΔP co 2 of 10 mm Hg | [HCO 3 − ] = 38 mEq/L |
| Chronic: Δ [HCO 3 − ] = +4 mEq/L for each ↑ ΔP co 2 of 10 mm Hg | [HCO 3 − ] = 45 mEq/L | ||||
| Respiratory alkalosis | Alveolar hyperventilation (↓ P co 2 ) | ↑ | ↓Renal HCO 3 − reabsorption (HCO 3 − ↓) | Acute: Δ [HCO 3 − ] = −2 mEq/L for each ↓ ΔP co 2 of 10 mm Hg | [HCO 3 − ] = 18 mEq/L |
| Chronic: Δ [HCO 3 − ] = −5 mEq/L for each ↓ ΔP co 2 of 10 mm Hg | [HCO 3 − ] = 15 mEq/L | ||||
| Metabolic acidosis | Loss of HCO 3 − or gain of H + (↓ HCO 3 − ) | ↓ | Alveolar hyperventilation to ↑ pulmonary CO 2 excretion (↓P co 2 ) |
|
|
| Metabolic alkalosis | Gain of HCO 3 − or loss of H + (↑ HCO 3 − ) | ↑ | Alveolar hypoventilation to ↓ pulmonary CO 2 excretion (↑ P co 2 ) | P co 2 = +0.6 mm Hg for Δ [HCO 3 − ] of 1 mEq/L P co 2 = 15 + [HCO 3 − ] | P co 2 = 55 mm Hg |
In metabolic acidosis, a low plasma HCO 3 − concentration decreases the pH, stimulating medullary chemoreceptors to increase ventilation and thereby decrease PaCO 2 and restore the pH toward normal. In general, for metabolic acidosis, respiratory compensation results in a 1.25 mm Hg decrease in PaCO 2 for every 1.0 mEq/L reduction in the plasma HCO 3 − concentration down to a minimum PaCO 2 of 10–15 mm Hg. The expected PaCO 2 in a simple metabolic acidosis can be calculated by the Winters formula :
This formula may be used in patients with metabolic acidosis to evaluate whether the observed PaCO 2 is an appropriate compensatory response or whether there is additional respiratory acidosis (PaCO 2 greater than predicted) or respiratory alkalosis (PaCO 2 less than predicted).
Respiratory compensation to metabolic alkalosis should raise the PCO 2 by about 0.6–0.75 mm Hg for every 1 mEq/L increase in the plasma bicarbonate concentration. The expected PaCO 2 may be estimated by the following formula:
In metabolic alkalosis, a high pH induces hypoventilation with a resultant rise in PaCO 2 and decrease in pH. However, hypoxemia induced by progressive hypoventilation eventually activates oxygen-sensitive chemoreceptors to stimulate ventilation and generally limits the compensatory pulmonary response to a PaCO 2 of <55 mm Hg.
Metabolic acidosis occurs via increased bicarbonate loss, decreased excretion of acid, an imbalance between production and consumption of endogenous acids, or administration of exogenous acid. The clinical significance of metabolic acidosis depends on the severity of the disorder. Without appropriate intervention, metabolic acidosis can progress to life-threatening changes in cardiac, neurologic, and metabolic function, as listed in Box 15.1 . Metabolic acidosis can be classified as high AG metabolic acidosis or non-AG (hyperchloremic) metabolic acidosis.
Obtundation and coma
Hyperactivity of sympathetic nervous system
Decreased cerebral metabolism
Decreased response to catecholamines
Increased minute ventilation
Subjective dyspnea
Respiratory muscle fatigue
Decreased contractility of myocardium
Core vasculature blood pooling (venoconstriction and arterial dilatation)
Decreased cardiac response to catecholamines
Tachyarrhythmias
Hyperkalemia (inorganic acidemia)
Hyperphosphatemia
Increased protein catabolism
Calculation of the serum AG is a useful tool in the evaluation of metabolic acidosis. The serum AG represents the difference in the measured cations (mainly sodium) and the measured anions (chloride and bicarbonate). Mathematically, this is represented as follows:
Based on the law of electroneutrality, the concentration of cations should be equal to the concentration of anions in the human body. Certain cations and anions are not measured on routine laboratory chemistry panels, and the serum AG quantifies these unmeasured anions. This can be represented as follows:
Therefore:
Calcium, magnesium, gamma globulins, and potassium are the major “unmeasured” cations and account for approximately 11 mEq/L under normal conditions ( Fig. 15.1 ). Although potassium is routinely measured in chemistry panels, the concentration of potassium in the blood is negligible compared with that of sodium, chloride, and bicarbonate, so potassium is not typically included as a “measured” cation in the AG equation. Negatively charged plasma proteins (albumin), sulfates, phosphates, and other organic anions are the major “unmeasured” anions and account for 20–24 mEq/L. Thus the normal AG is about 12 mEq/L (23 − 11). Under normal circumstances, the serum AG is typically 12 ± 4 mEq/L but can vary depending on the laboratory method used. Therefore the established normal range provided by the particular laboratory that performs the testing should be used.
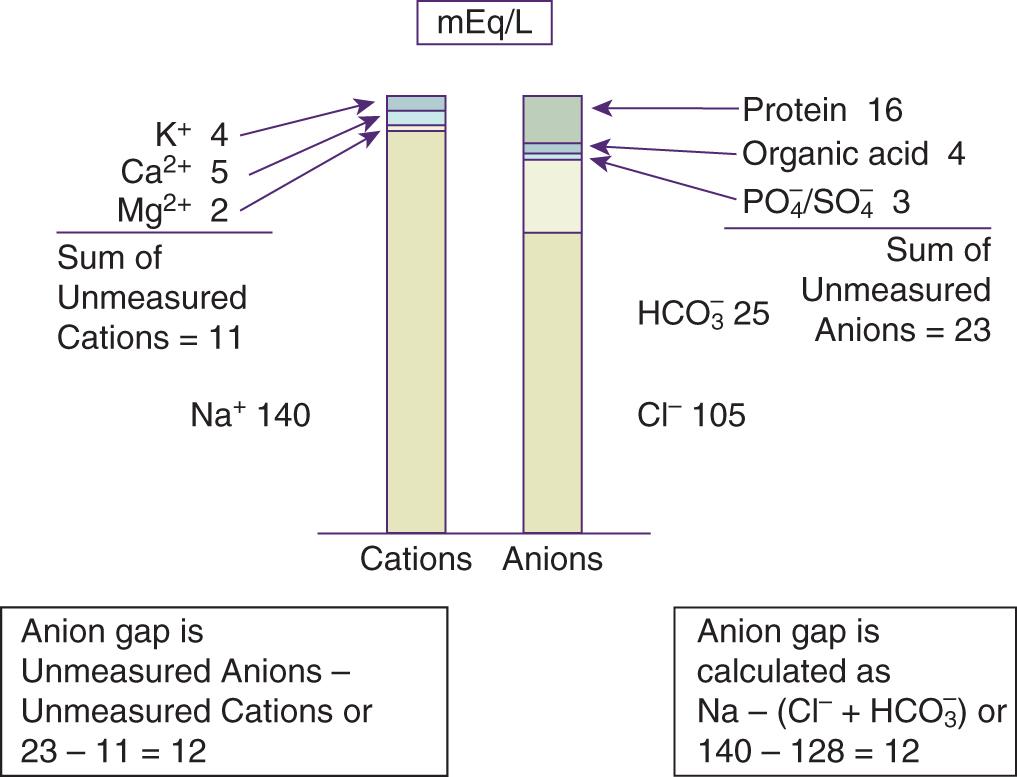
The AG can be affected by increases or decreases in the UC or UA. The most important contributor to a normal serum AG is albumin, which has a negative charge at a physiologic pH. In patients with hypoalbuminemia, as commonly observed in critical illness or malnutrition, the AG must be corrected for low albumin. For each 1 g/dL fall in the plasma albumin concentration from a normal albumin concentration, the AG falls about 2.5 mEq/L. This can be represented as follows:
Other causes of a low or negative AG that may be important to consider for diagnostic and treatment purposes in ICU patients are listed in Table 15.3 and include hypercalcemia, hypermagnesemia, lithium intoxication, paraproteinemias such as multiple myeloma, and halide (bromide or iodide) intoxication.
| Decreased Anion Gap | Increased Anion Gap |
|---|---|
| Increased cations (not Na + ) | Increased anions (not Cl − or HCO 3 − ) |
| ↑Ca 2+ , Mg 2+ | ↑Albumin concentration |
| ↑Li + | Alkalosis |
| ↑IgG | ↑Inorganic anions |
| Decreased anions: |
|
|
|
|
|
|
↑Organic anions |
| Laboratory error |
|
|
|
|
|
* Albumin is the major unmeasured anion. A decline in serum albumin of 1.0 g/dL from the normal value of 4.5 g/dL decreases the anion gap by 2.3–2.5 mEq/L. Correction is very important to diagnose anion gap acidosis in the setting of hypoalbuminemia.
The contributors to serum AG in the normal physiologic state and in high AG and non-AG metabolic acidosis are depicted in Fig. 15.2 .
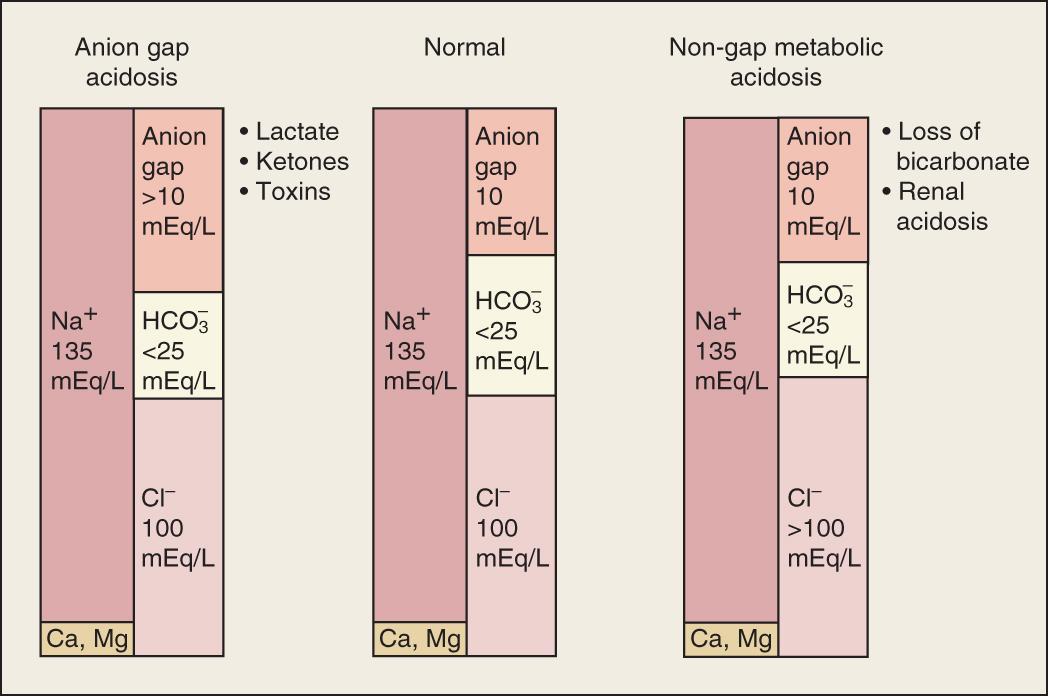
High AG metabolic acidosis develops from excessive production, ingestion, or retention of a strong acid or a compound metabolized to a strong acid. These include negatively charged acids such as ketones, lactate, and sulfates, in addition to metabolites of methanol, ethylene glycol, or salicylate, which accumulate in place of the consumed HCO 3 − and cause a high AG. Other causes of an increased AG include hyperalbuminemia or uremia (increased anions) and hypocalcemia or hypomagnesemia (decreased cations) (see Table 15.3 ).
The presence of a significantly elevated AG (AG >20 mEq/L) always represents metabolic acidosis, regardless of the pH or plasma bicarbonate concentration. In the event of a mixed acid-base disorder with a normal blood pH, a high AG points toward underlying metabolic acidosis, which otherwise may be missed. Therefore the serum AG should always be calculated when assessing acid-base disorders, especially in the ICU setting. The common causes of high AG acidosis in the ICU are lactic acidosis, ketoacidosis, toxin-induced acidosis, and renal failure ( Box 15.2 ).
Diabetic ketoacidosis (acetoacetate)
Alcoholic (beta-hydroxybutyrate)
Starvation
l -Lactic acid acidosis (types A and B)
d -Lactic acid acidosis
Renal failure: sulfate, phosphate, urate, hippurate
Ethylene glycol → glycolate, oxalate
Methyl alcohol → formate
Salicylate → ketones, lactate, salicylate
Paraldehyde → organic anions
Toluene → hippurate (commonly presents with normal anion gap)
Propylene glycol → lactate
Pyroglutamic acidosis (acetaminophen use) → 5-oxoproline
Diarrhea
Fistula, external
Proximal renal tubular acidosis (RTA type 2)
Acetazolamide
Classic distal renal tubular acidosis (low serum K + ) RTA type 1
Generalized distal renal tubular defect (high serum K + ) RTA type 4
NH 4 Cl ingestion
Sulfur ingestion
Dilutional acidosis
Late stages in treatment of diabetic ketoacidosis
L -lactic acidosis is among the most frequent causes of elevated AG metabolic acidosis in the ICU and is associated with a high mortality. , l -lactate refers to the L (levo) enantiomer of lactic acid and is directly measured by the standard serum lactate assay. Lactic acid concentration should be measured directly if lactic acidosis is expected because the serum AG has a sensitivity and specificity of <80% in identifying elevated lactate levels. Thus a normal AG does not rule out lactic acidosis. Jansen et al. have shown that lactate levels serve as a prognosis indicator that can signal underlying deterioration, prompt more aggressive management, and help avoid unnecessary treatment when the condition stabilizes. Lactic acidosis occurs whenever production of lactate exceeds its utilization. In most cases of clinically significant lactic acidosis, there is evidence of defective utilization and increased production, depending on the etiology of lactic acidosis.
Pyruvate is the precursor of lactate and is produced in the cytoplasm from glucose metabolism via glycolysis in the Embden-Meyerhof pathway. Pyruvate normally undergoes oxidative decarboxylation by mitochondrial pyruvate dehydrogenase (PDH) to acetylcoenzyme A and then ultimately to CO 2 and H 2 O. This process results in the synthesis of 36 moles of adenosine triphosphate (ATP) and requires oxidized nicotinamide adenine dinucleotide (NAD + ). Pyruvate can also enter the Cori cycle in the liver and renal cortex and be converted back to glucose. Oxidative phosphorylation, ATP synthesis, and reoxidation of NADH are inhibited during hypoxia. This anaerobic metabolism leads to an increased NADH/NAD + ratio, increased conversion of pyruvate to lactate, and synthesis of 2 molecules of ATP, rather than the 36 generated via the tricarboxylic acid cycle. The overall result of anaerobic metabolism is increased lactate levels, an elevated lactate/pyruvate ratio, greater glucose utilization, and lower energy production.
Traditionally, lactic acidosis has been categorized as type A or type B. Type A lactic acidosis is characterized by an impaired mitochondrial oxidative capacity in the setting of tissue hypoxia, whereas type B lactic acidosis is the result of dysregulation of cell metabolism rather than hypoxia ( Box 15.3 ). Most cases of type A lactic acidosis are the result of reduced oxygen delivery because of reduced tissue perfusion from shock or cardiopulmonary arrest. Other causes are carbon monoxide poisoning and severe anemia. Type B lactic acidosis is classified as type B1 (related to underlying diseases like malignancies or liver disease), type B2 (related to the effect of drugs and toxins), and type B3 (associated with inborn errors of metabolism). The most common drugs associated with type B2 lactic acidosis include biguanides (e.g., metformin), reverse transcriptase inhibitors, acetylsalicylic acid (ASA), propofol, and linezolid, among others (see Box 15.3 ). Sepsis is a common cause of lactic acidosis in the ICU. Lactate level predicts increased mortality in patients with and without sepsis, even at 1 year post-hospitalization. Sepsis-induced lactic acidosis has been conventionally classified as type A lactic acidosis because of inadequate oxygen supply and augmented anaerobic metabolism. However, the lack of response to increased oxygen delivery, the absence of tissue hypoxia, and normal tissue ATP levels suggest that lactate formation during sepsis may be the result of dysregulation of cellular metabolism. , Decreased clearance of lactate, rather than increased production, has been demonstrated in sepsis. In addition, increased pyruvate production, decreased PDH activity, regional differences in lactate production, and decreased clearance of lactate have been implicated as possible contributors to lactic acidosis. Decreased muscle PDH activity has been shown in sepsis, and it can be restored by dichloroacetate, suggesting that lactic acidosis during sepsis is the result of functional inhibition of PDH, leading to enhanced conversion of pyruvate to lactate. ,
Poor tissue perfusion
Shock
Cardiogenic
Hemorrhagic
Septic
Profound hypoxemia
Severe asthma
Severe anemia
Carbon monoxide poisoning
Liver disease
Diabetes mellitus
Catecholamine excess
Endogenous
Exogenous
Thiamine deficiency
Ketoacidosis
Seizure
Malignancy
Intracellular inorganic phosphate depletion
Intravenous (IV) fructose
IV xylose
IV sorbitol
Alcohols metabolized by alcohol dehydrogenase
Ethanol
Methanol
Ethylene glycol
Propylene glycol
Mitochondrial toxins
Salicylate intoxication
Cyanide poisoning
2,4-Dinitrophenol ingestion
Nonnucleoside anti–reverse transcriptase drugs
Metformin
Inborn errors of metabolism
Pyroglutamic acidosis
Kombucha tea
Short bowel syndrome
Ischemic bowel
Small bowel obstruction
Treatment of lactic acidosis requires identification and correction of the underlying cause. The therapeutic goal in type A lactic acidosis is restoration of tissue oxygen delivery through hemodynamic and/or respiratory support. The use of sodium bicarbonate in lactic acidosis is controversial and not supported by clinical studies. Intravenous (IV) administration of sodium bicarbonate may increase lactate production, decrease portal vein flow, decrease ionized calcium levels, lower intracellular pH, and worsen cardiac output. , , Bicarbonate increases extracellular pH only if ventilation removes the excess CO 2 generated; otherwise, hypercapnia can lower intracellular pH and impair cellular function. , , Bicarbonate can worsen tissue oxygen delivery if the arterial pH increases more than the intracellular pH, with a leftward shift in the oxyhemoglobin dissociation curve. If tissue hypoxia is present, the use of bicarbonate can stimulate glycolysis mediated by the pH-sensitive rate-limiting enzyme phosphofructokinase and paradoxically increase lactate production. Sodium bicarbonate should be administered cautiously when the arterial pH is less than 7.15 because a pH below this value will promote the development of decreased responsiveness to catecholamines, arrhythmias, cardiac depression, and hemodynamic instability.
Alternative buffering agents, such as tris-hydroxymethyl aminomethane (THAM), carbicarb, and dichloroacetate (DCA) have not shown any clinical benefit in patients with lactic acidosis. Carbicarb and DCA are unavailable in the United States. THAM can bind to both CO 2 and metabolic acids. Protonated THAM is excreted by the kidney through glomerular filtration, together with bicarbonate or another anion. Thus THAM can increase the buffering capacity of blood without generating CO 2 but is less effective in patients with anuria. Reported toxicities of THAM include hyperkalemia, hypoglycemia, and respiratory depression. THAM has not been specifically evaluated as a therapeutic agent for lactic acidosis in clinical trials. Carbicarb, an equimolecular mixture of sodium bicarbonate and sodium carbonate, has a buffering capacity similar to sodium bicarbonate but generates less CO 2 . Animal studies have demonstrated inconsistent benefits of carbicarb in lactic acidosis, and one human study comparing the effects of sodium bicarbonate with carbicarb in metabolic acidosis found no benefit. , DCA stimulates the activity of the mitochondrial PDH enzyme complex indirectly through inhibition of the PDH kinase and hence decreases lactate production. Data from animal studies and one placebo-controlled double-blind clinical trial demonstrated that DCA improved acid-base status but not hemodynamics or survival. ,
Dialysis can theoretically be used to treat lactic acidosis because it supplements bicarbonate, removes lactate, prevents decreased ionized calcium, avoids volume overload, and removes drugs associated with lactic acidosis such as metformin. , However, the same potential risks of worsening lactic acidosis with bicarbonate administration through the dialysate exist. Furthermore, in severe lactic acidosis, the quantity of lactate cleared by dialysis is much less than the quantity of lactate generated. Continuous dialysis modalities are preferred over intermittent dialysis in hemodynamically unstable patients and can deliver bicarbonate at a lower rate. Evidence supporting intermittent or continuous dialysis for treatment of lactic acidosis is anecdotal at best, and prospective controlled trials are warranted.
D -lactate refers to the D (dextro) enantiomer of lactic acid. d -lactic acidosis is an uncommon form of lactic acidosis that occurs in patients with jejunoileal bypass, small bowel resection, or other causes of short bowel syndrome resulting from bacterial overgrowth. In these patients, abnormally large amounts of glucose and starch are metabolized to d -lactic acid by gram-positive intestinal anaerobes such as lactobacilli. , d -lactic acid is then absorbed into the systemic circulation and causes acidemia that tends to persist because d -lactate is not recognized by l -lactate dehydrogenase, the enzyme that converts l -lactate into pyruvate. Patients typically present with recurring episodes of metabolic acidosis after a carbohydrate meal in addition to neurologic abnormalities, including confusion, ataxia, slurred speech, and memory loss. The diagnosis can be easily missed because the d -isomer responsible for the acidosis is not detected by the standard assay for lactate and requires a special assay for detection. Therapy for d -lactic acidosis includes administration of sodium bicarbonate to correct the acidosis, oral antibiotics to decrease gram-positive anaerobic colonic bacteria, and a low-carbohydrate diet to reduce carbohydrate delivery to the colon. d -lactic acidosis has also been described in patients who have large amounts of propylene glycol and in those with diabetic ketoacidosis (DKA). ,
Ketoacidosis is a common complication in patients with insulin-dependent diabetes mellitus but can also be seen in chronic alcoholism and starvation (see Box 15.2 ). It results from the overproduction of ketone bodies, leading to accumulation of ketones in the plasma (ketonemia) and urine (ketonuria).
DKA occurs in patients with insulin-dependent diabetes mellitus and results from severe insulin deficiency in the setting of increased metabolic demand, such as from a concurrent infection or myocardial infarction. It can also occur from poor compliance with insulin or missed injections. Insulin deficiency results in decreased glucose uptake, glycogen store depletion, lipolysis, and fatty acid oxidation leading to increased ketoacid production (acetoacetate and beta-hydroxybutyrate). Symptoms can progress from polydipsia, polyuria, nausea, vomiting, dyspnea, and diffuse abdominal pain to confusion, lethargy, and somnolence. Laboratory findings include hyperglycemia, increased serum AG, ketonemia, ketonuria, and increased plasma osmolality. The diagnosis is established by measuring plasma and urine ketone levels. However, clinicians must be aware that the nitroprusside reaction used in standard plasma and urine tests only measures acetone and acetoacetate levels and not beta-hydroxybutyrate levels, which is the predominant ketone in severe untreated DKA. Therefore laboratory analysis for ketones may be falsely negative. High plasma glucose levels can cause dilutional hyponatremia because the osmotic effect of hyperglycemia causes the movement of water into the intravascular space. For each 100 mg/dL of glucose over 100 mg/dL, the plasma sodium level is lowered by approximately 1.6 mEq/L. In spite of severely depleted total body potassium from osmotic diuresis, plasma potassium levels are initially elevated or within the normal range from insulin deficiency. ,
The major goals of treatment are rapid volume expansion, correction of hyperglycemia, correction of acid-base and electrolyte disturbances, and identification and treatment of the precipitating cause. Adults should initially receive a rapid infusion of 1 L of isotonic saline with repeat boluses as necessary to prevent hemodynamic collapse. When the blood pressure and heart rate have stabilized and the patient is euvolemic, isotonic saline can be switched to 0.45% saline at a slower rate to replace the free water lost by osmotic diuresis. Insulin is typically administered as a 10- to 20-unit IV bolus (0.15 units/kg) followed by an infusion of 5–7 units/hour (0.1 unit/kg/hour). Insulin inhibits lipolysis and gluconeogenesis and allows for the conversion of ketones to bicarbonate. If the blood sugar falls below 250 mg/dL, the rate of insulin should be decreased to 0.03 u/kg/hour and 5%–10% dextrose should be added to the fluids. Once the AG normalizes, subcutaneous insulin should be administered while the insulin infusion is continued for another 1–2 hours. Potassium replacement should be administered at 10–20 mEq/hour if the plasma potassium level is less than 5.3 mEq/L and if renal failure is not present. Plasma potassium levels should be measured frequently, and the infusion should be stopped if hyperkalemia occurs. Small amounts of IV sodium bicarbonate should only be administered if the arterial pH is less than 6.9, with frequent monitoring of pH and serum AG. IV phosphate replacement can be given if the initial level is less than 1.0 mg/dL. Plasma phosphorus levels can be high initially because of the transcellular shift of phosphate out of the cell in the setting of acidosis and insulin deficiency. ,
Hemodialysis-dependent patients with DKA are managed differently. Insulin administration is frequently the only treatment needed for DKA management in these patients. Anuric dialysis patients usually present with signs of extracellular volume (ECV) expansion rather than volume depletion because osmotic diuresis cannot occur in the absence of kidney function. Therefore dialysis patients do not require IV fluids unless they have evidence of extracellular fluid loss such as vomiting, diarrhea, or excessive insensible losses. If volume depletion is present, small amounts of isotonic saline should be carefully administered with close monitoring of respiratory and hemodynamic parameters. When volume overload is apparent, immediate hemodialysis is the therapy of choice. Hemodialysis will also help correct hyperglycemia resulting from diffusive clearance of glucose but must be performed in conjunction with insulin administration, as insulin is the only therapy that will halt lipolysis and gluconeogenesis. Dialysis-dependent patients with DKA should not receive routine potassium supplementation because total body potassium stores may be high and patients are unable to excrete a potassium load. Urgent dialysis is indicated if hyperkalemia is present with electrocardiographic findings. Similarly, significant metabolic acidosis can only be corrected with hemodialysis. ,
Euglycemic DKA is an emerging entity that has been recognized in patients with both type 1 and type 2 diabetes mellitus who present with increased AG acidosis, ketosis, and relatively normal serum glucose <200 mg/dL. The pathophysiology is thought to be related to decreased hepatic gluconeogenesis during fasting or enhanced urinary excretion of glucose. Common causes of euglycemic DKA include SGLT2 inhibitors, pregnancy, glycogen storage diseases, and chronic liver disease. Less common causes include pancreatitis, alcohol use, cocaine intoxication, gastroparesis, and Duchenne muscular dystrophy.
Alcoholic ketoacidosis (AKA) occurs in the setting of chronic alcoholism, recent binge drinking, minimal oral intake, and persistent vomiting. It is characterized by elevated plasma ketone levels, high AG, and normal or only slightly elevated plasma glucose level. Prolonged starvation results in decreased insulin activity, glycogen depletion, increased counterregulatory hormone production, dehydration, and increased lipolysis and fatty acid oxidation with accumulation of ketoacids. The metabolism of ethanol itself promotes ketoacidosis by leading to an accumulation in reduced NADH. Reduced NADH then results in impaired conversion of lactate to pyruvate, preferential conversion of pyruvate to lactate, and a shift toward beta-hydroxybutyrate production. Beta-hydroxybutyrate is the predominant ketone in AKA. As mentioned, the standard nitroprusside test for detecting ketones only detects acetoacetate and may be falsely negative or only minimally positive in AKA, leading to an underestimation of the degree of ketoacidosis. ,
Patients with AKA frequently present with a mixed acid-base disturbance. They can have elevated AG metabolic acidosis from ketoacidosis and lactate, metabolic alkalosis from persistent vomiting, and chronic respiratory alkalosis from liver disease. Magnesium and phosphate levels may be low because of increased urinary excretion and poor nutrition.
The mainstay of treatment is hydration with 5% dextrose in isotonic saline. Before glucose administration, thiamine should be given to avoid precipitating Wernicke encephalopathy. Carbohydrate and fluid replacement reverse the pathophysiologic derangements that lead to AKA by increasing plasma insulin levels and suppressing the release of glucagon and other counterregulatory hormones. Dextrose stimulates the oxidation of NADH and aids in normalizing the NADH/NAD + ratio. Insulin should be avoided because it can lead to hypoglycemia, especially as the patient’s endogenous insulin levels rise with carbohydrate and fluid repletion. Bicarbonate is only recommended if the plasma pH is less than 7.1 and the acidosis is not responding to IV fluids. Hypophosphatemia, hypokalemia, and hypomagnesemia should be corrected. Glucose infusion can exacerbate hypophosphatemia, resulting in rhabdomyolysis if not repleted.
Starvation, as mentioned earlier, results in ketoacidosis because of the increase in counterregulatory hormones and a decrease in insulin level, promoting fatty acid oxidation, gluconeogenesis, and ketone production. However, in comparison to the potentially severe ketoacidosis that develops in uncontrolled diabetes and alcoholic states, ketoacid levels do not typically exceed 10 mEq/L with fasting. This is probably because of the insulin level, which, though lower, is still enough to limit the production of free fatty acids and thus ketoacidosis , ( see Boxes 15.2 and 15.3 ).
Accumulation of the metabolites of methanol—ethylene glycol, diethylene glycol, and propylene glycol—causes a high AG and increased plasma osmolal gap. Intoxication should be suspected in any patient who presents with high AG metabolic acidosis, renal failure, and neurologic findings, and treatment should be initiated early.
The normal range of plasma osmolality is 285–290 mOsm/kg. Plasma osmolality (Posm) can be estimated from the following formula:
The plasma osmolal gap represents the difference between the measured and calculated plasma osmolality. A difference of higher than 10 mOsm/kg is considered an osmolal gap. Accumulation of the noted alcohols will typically produce an osmolal gap of higher than 20 mOsm/kg. Methanol gives rise to the greatest increment in plasma osmolality, followed by ethylene glycol, propylene glycol, and finally diethylene glycol. The absence of an osmolal gap does not exclude an alcohol-related intoxication. Furthermore, although the plasma osmolal gap may support the diagnosis of ingestion of a toxic alcohol, plasma toxicology screens specifically looking for the toxins are considered the gold standard.
Other causes of high AG metabolic acidosis that can be associated with an elevated plasma osmolal gap include lactic acidosis, ketoacidosis, advanced chronic kidney disease (CKD), formaldehyde ingestion, and paraldehyde ingestion. The plasma osmolal gap is usually less pronounced in these disorders (≤15–20 mOsm/kg), and plasma toxicology is not typically performed. Substances that can cause an osmolal gap without metabolic acidosis are ethanol, isopropyl alcohol ingestion, infusion of nonconductive glycine, sorbitol or mannitol solutions, severe hyperproteinemia, and severe hyperlipidemia.
Methanol, used as a laboratory and industrial solvent, is commonly found in windshield wiper fluid, de-icing products, gas-line antifreeze, and various paint solvents and thinners. It is metabolized by alcohol dehydrogenase (ADH) to formaldehyde, which is further metabolized by aldehyde dehydrogenase (ALDH) to formic acid. The most common symptoms of methanol intoxication are abdominal pain and visual disturbances, including decreased visual acuity, photophobia, and blurred vision. Formic acid is the main toxic metabolite responsible for retinal, ophthalmic, and neural toxicity. Permanent blindness may occur because of optic nerve atrophy. High AG metabolic acidosis is the result of generation of formic acid and increased production of lactic acid. Lactic acidosis results from impaired cellular respiration by formate or increased generation of NADH during the metabolism of methanol. , Imaging findings in acute methanol intoxication include bilateral necrosis of the putamen, diffuse white matter necrosis, and subarachnoid hemorrhage on brain computed tomography (CT) and magnetic resonance imaging (MRI).
Ethylene glycol is typically found in radiator antifreeze in addition to various solvents and paint formulations. It is metabolized by ADH to glycolaldehyde, then to glycolic acid by ALDH, which is further metabolized to glyoxylic acid and finally oxalic acid. The metabolites are responsible for neurologic, cardiopulmonary, and renal toxicity. Typically, neurologic abnormalities occur initially, followed by cardiopulmonary dysfunction, and finally renal dysfunction. Neurologic findings include coma, seizures, meningeal signs, external ocular paralysis, and delayed onset of cranial nerve deficits. Cardiopulmonary findings include tachycardia, hyperventilation, and heart failure. Oxalic acid combines with plasma calcium to form calcium oxalate, which leads to hypocalcemia, QTc prolongation, and risk of ventricular arrhythmias. Calcium oxalate crystals precipitate in the renal tubules, causing flank pain, oliguria, and renal failure. Calcium oxalate crystals are present in the urine 4–8 hours after ingestion of ethylene glycol and can be visualized by direct urine microscopy. High AG metabolic acidosis is the result of generation of glycolic, glyoxylic, and oxalic acids and increased production of lactic acid. Measurement of plasma ethylene glycol levels can confirm poisoning. ,
Methanol and ethylene glycol poisoning are treated with fomepizole or ethanol, which inhibits ADH and prevents the formation of toxic metabolites. Fomepizole (15 mg/kg loading dose, then 10 mg/kg every 12 hours) is preferred over ethanol because of its easier dosing regimen and better side-effect profile. The indications for antidotal therapy are a plasma concentration of methanol or ethylene glycol higher than 20 mg/dL and two of the following: osmolal gap higher than 10 mOsm/kg, arterial pH lower than 7.3, plasma bicarbonate level lower than 20 mEq/L, and the presence of urinary oxalate crystals. Indications for hemodialysis are severe metabolic acidosis (pH <7.25), visual abnormalities, renal failure, electrolyte abnormalities not responsive to treatment, hemodynamic instability despite ICU treatment, and plasma concentration higher than 50 mg/dL. In ethylene glycol toxicity, pyridoxine and thiamine are administered to increase the metabolism of glycolic and glyoxylic acid to the less-toxic metabolites glycine and alpha-hydroxy-beta-ketoadipate. In methanol toxicity, folic acid or folinic acid increases the breakdown of formic acid to CO 2 and water. ,
Diethylene glycol is present in brake fluid and is used as an illegal adulterant in ethanol spirits or in medication. Diethylene glycol is oxidized by ADH to 2-hydroxyethoxyacetaldehyde and then via ALDH to 2-hydroxyethoxyacetic acid. Acute oliguric and nonoliguric renal failure are frequent. Treatment consists of hemodialysis and fomepizole.
Propylene glycol is a solvent for unstable drugs, including benzodiazepines, phenytoin, nitroglycerin, and some topical medications. The majority of intoxications have resulted from excessively large or rapidly infused IV injections of propylene glycol–containing medications such as benzodiazepines. Neurologic depression is the primary manifestation of acute propylene glycol poisoning. Metabolic acidosis is attributed to the generation of lactic acid during metabolism of propylene glycol. Discontinuation of propylene glycol–containing medication can lead to correction of the acidosis within 24 hours in most patients. In the face of extremely high blood concentrations, hemodialysis is extremely effective in rapidly reducing plasma propylene glycol levels.
Use of isopropyl alcohol is the most common cause of toxic alcohol exposure in the United States; it is found in rubbing alcohol, hand sanitizer gels, and other antiseptic preparations. It is metabolized by ADH to acetone, without production of AG acidosis. Toxicity is mainly limited to gastrointestinal (GI) effects, such as hemorrhagic gastritis and neurologic depression. Isopropyl alcohol is the only toxic alcohol that causes ketosis without acidosis. In comparison to other toxic alcohols, isopropyl alcohol intoxication is usually managed supportively. Hemodialysis may increase the rate of elimination of both isopropyl alcohol and acetone and should be considered for patients with deteriorating mental status or hemodynamic instability.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here