Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Primary tumors arising from the peritoneum are rare and are usually of mesenchymal origin.
These tumors are related to benign but locally aggressive fibroblast proliferation or fibromatosis. The morbidity associated with these tumors is related to their locally aggressive behavior with involvement of adjacent organs. They are uncommon, occurring in 2 to 4 per million people per year, and do not show the features of neoplasia. The cause is unknown, but there is an association with pregnancy and estrogen administration. They can occur either sporadically or in association with familial adenomatous polyposis. Familial adenomatous polyposis is an inherited syndrome characterized by innumerable polyps predominantly occurring in the colon. Gardner’s syndrome is now considered to be a part of familial adenomatous polyposis, and both these conditions have a mutation of the APC gene.
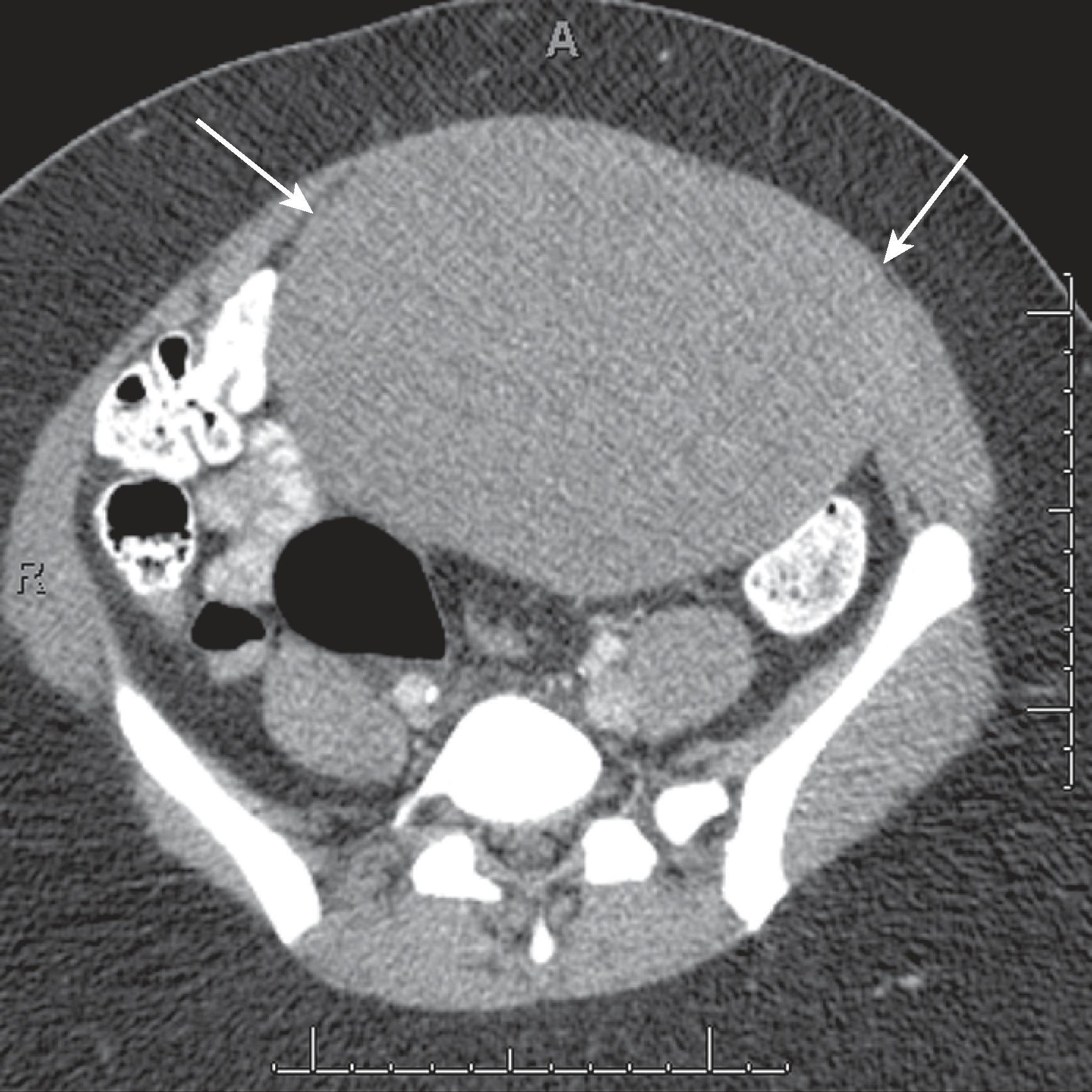
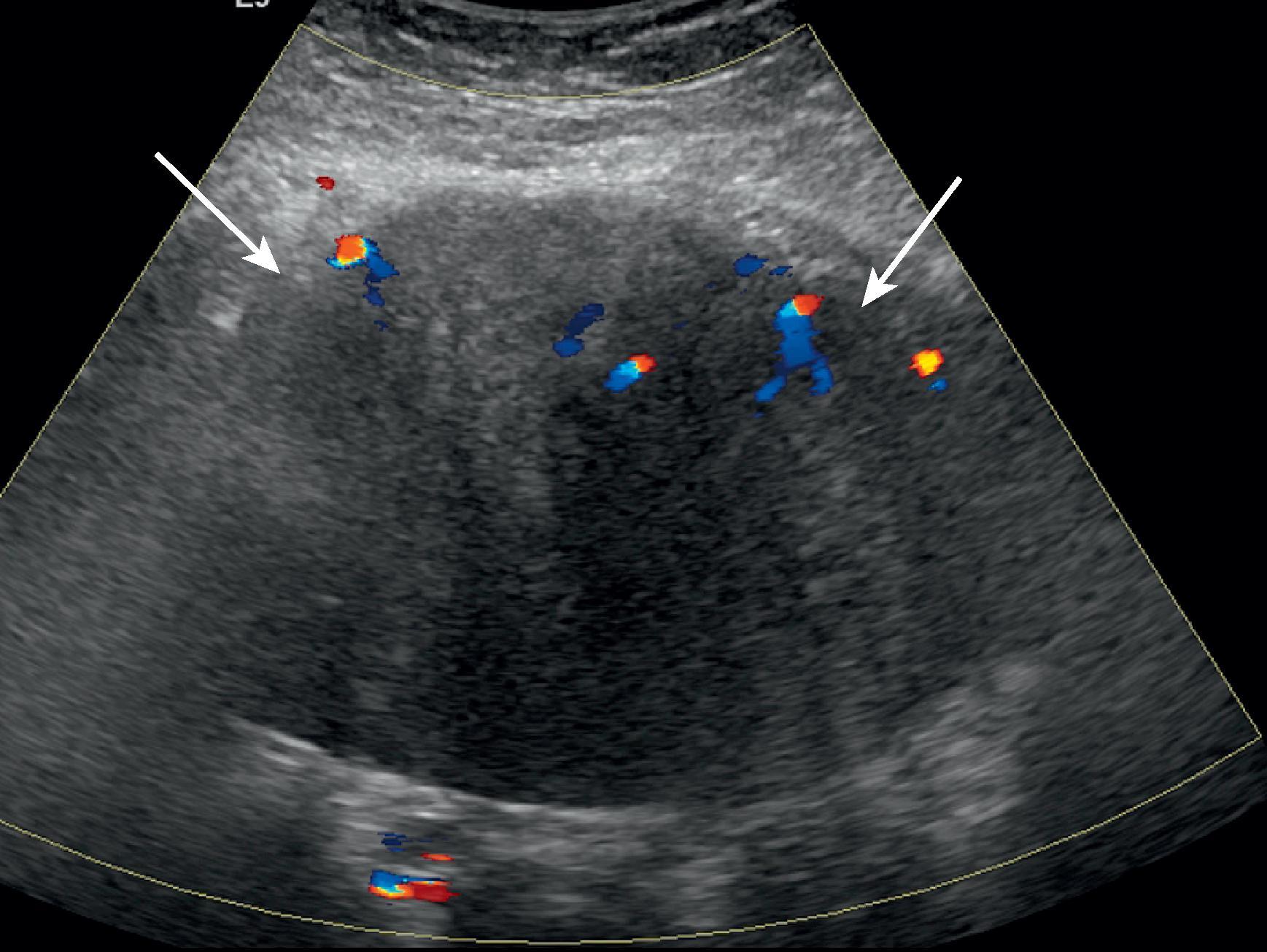
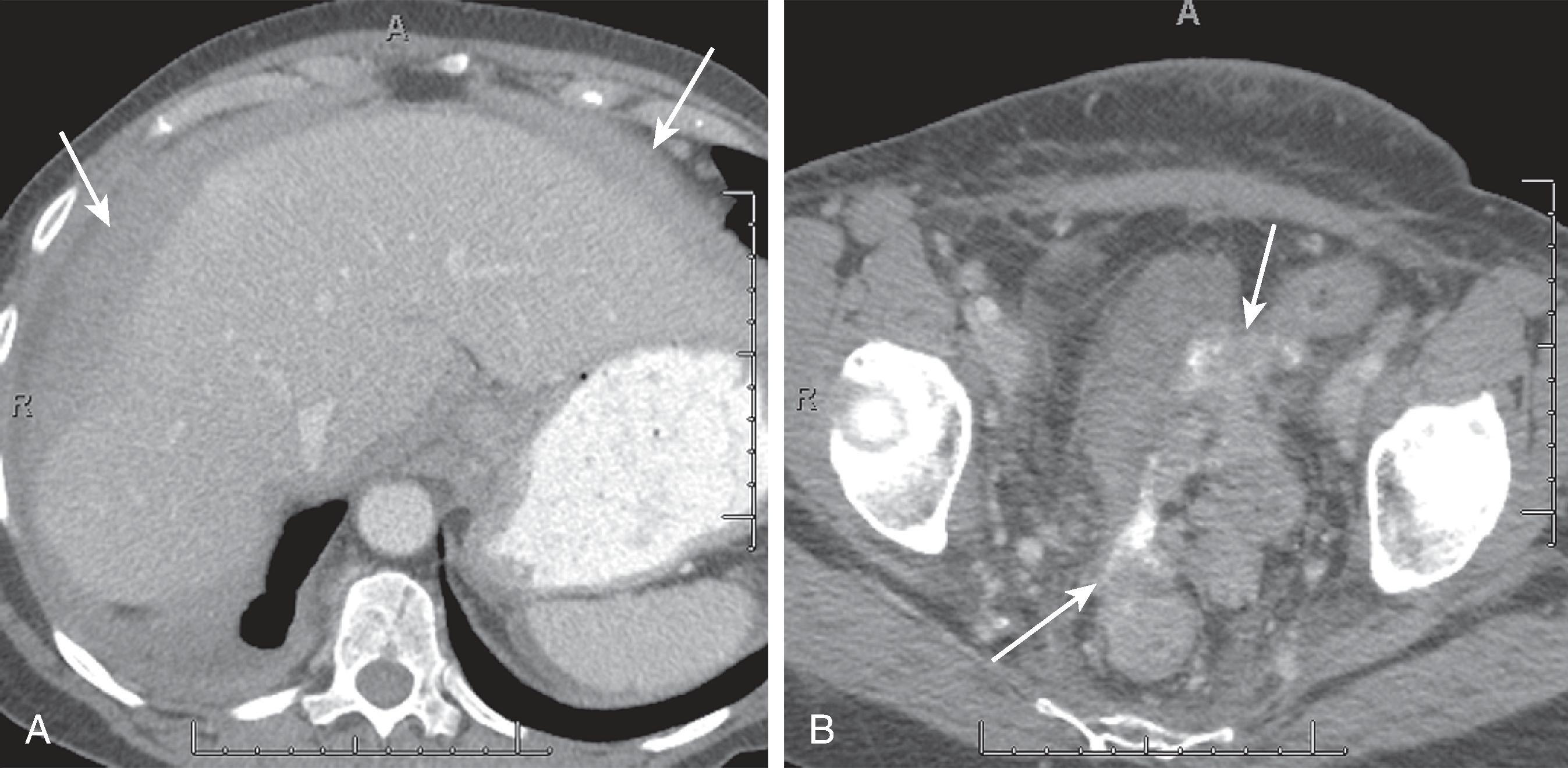
Desmoid tumors can occur in 5% to 25% of patients with familial adenomatous polyposis. About half of the abdominal desmoids occur intra-abdominally, and the other half are found in the abdominal wall. Nearly one-third of abdominal desmoids cause pain. Abdominal desmoids can involve the abdominal wall, mesentery, or retroperitoneum ( Figs. 66.1 and 66.2 ). Many of these tumors are often associated with prior surgery, such as colectomy, and can recur at the surgical site. Surgery is thought to stimulate desmoid tumor growth. The most common site for mesenteric desmoids is at the base of the small bowel mesentery. Tumors can vary in size and range from a few centimeters to extremely large lesions. Desmoids are pseudoencapsulated lesions despite their relatively well-defined gross appearance. On microscopic examination, they demonstrate infiltrative margins. Progressive growth may lead to bowel, ureteric, or vascular obstruction and occasionally fistulas. Computed tomography (CT) is an excellent imaging study to evaluate desmoid tumors and their relation to surrounding structures and in the follow-up of patients who undergo conservative medical therapy. Desmoid tumors are typically poorly enhancing solid lesions. They can usually be distinguished from postoperative fibrosis by the lack of mass effect of the fibrosis. On ultrasound, these appear as well-demarcated solid masses ( Fig. 66.3 ) containing low- to mid-level echoes. On magnetic resonance imaging (MRI), they are of low T1 and low T2 signal intensity with no or poor enhancement.
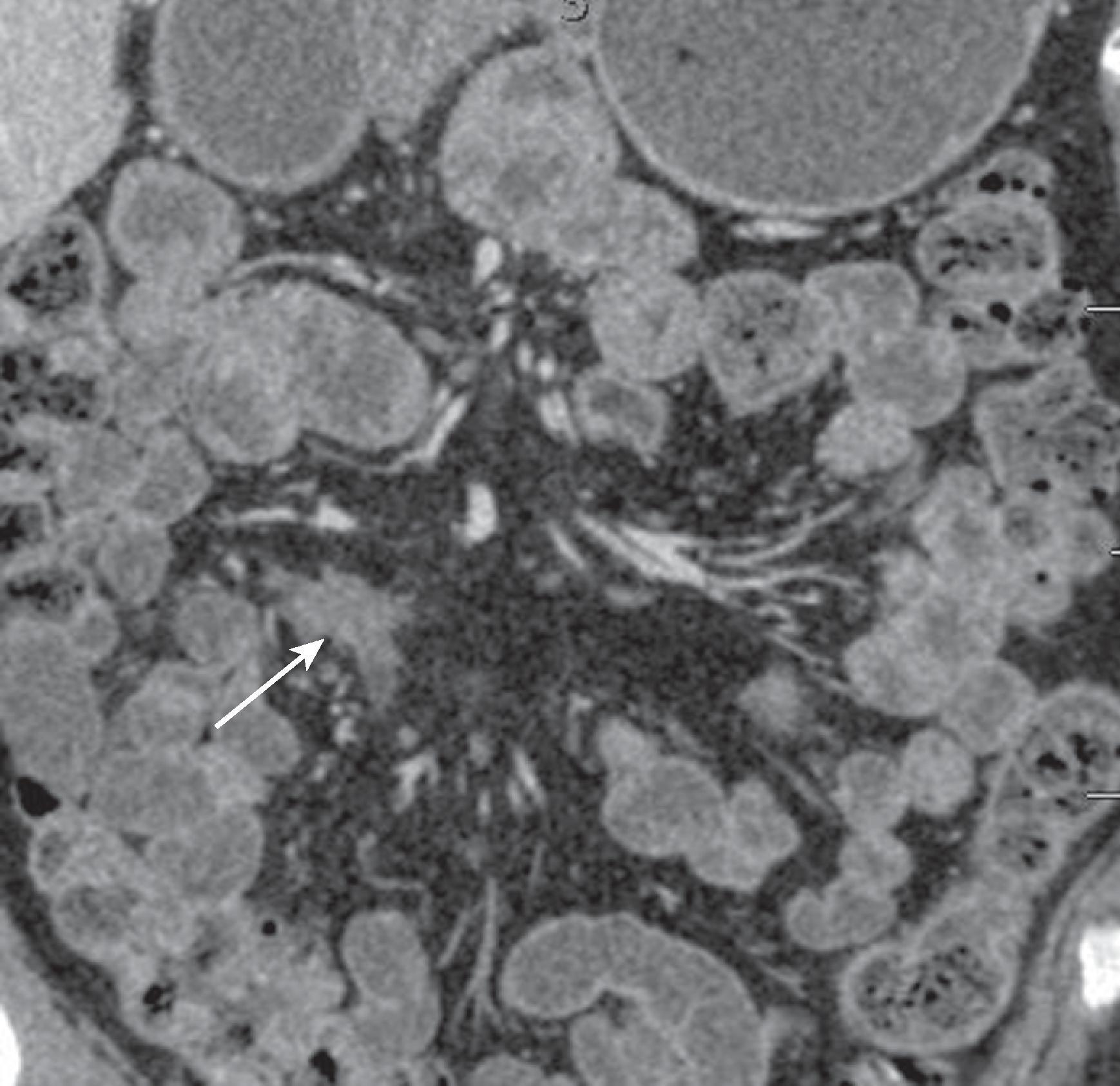
Malignant peritoneal mesothelioma (MPM) is a rare but aggressive tumor that arises from the serosa lining the pleura and the peritoneum. MPMs account for only 10% to 15% of all mesotheliomas. These may occur either alone or in conjunction with pleural mesothelioma. Most patients have a history of exposure to asbestos. , There is also a long latent period between exposure and appearance of peritoneal mesothelioma, typically 30 years after the exposure to asbestos. There is a slight male predominance, less than that of pleural mesotheliomas. It has been postulated that the patients with MPM have to have a higher cumulative exposure to asbestos than that of the patients with pleural mesothelioma.
Peritoneal mesotheliomas can be classified into diffuse versus localized mesothelioma. The diffuse type is aggressive as opposed to the localized form, which has a better prognosis with surgery.
Peritoneal mesotheliomas can be classified into epithelial, sarcomatoid, and mixed types on the basis of histologic features. There is great variability in the histologic appearance, making the diagnosis difficult. Sarcomatoid histology is associated with a poor prognosis. Immunohistochemical staining has been proved to be of great benefit in differentiating MPMs from secondary tumors affecting the peritoneum. MPMs produce large amounts of hyaluronic acid and stain with colloidal iron. In contrast, MPMs do not stain positive for the presence of mucin. Symptoms are nonspecific and include abdominal distention, pain, and malaise. Moreover, 55% of patients fail to demonstrate evidence of asbestosis on chest radiographs.
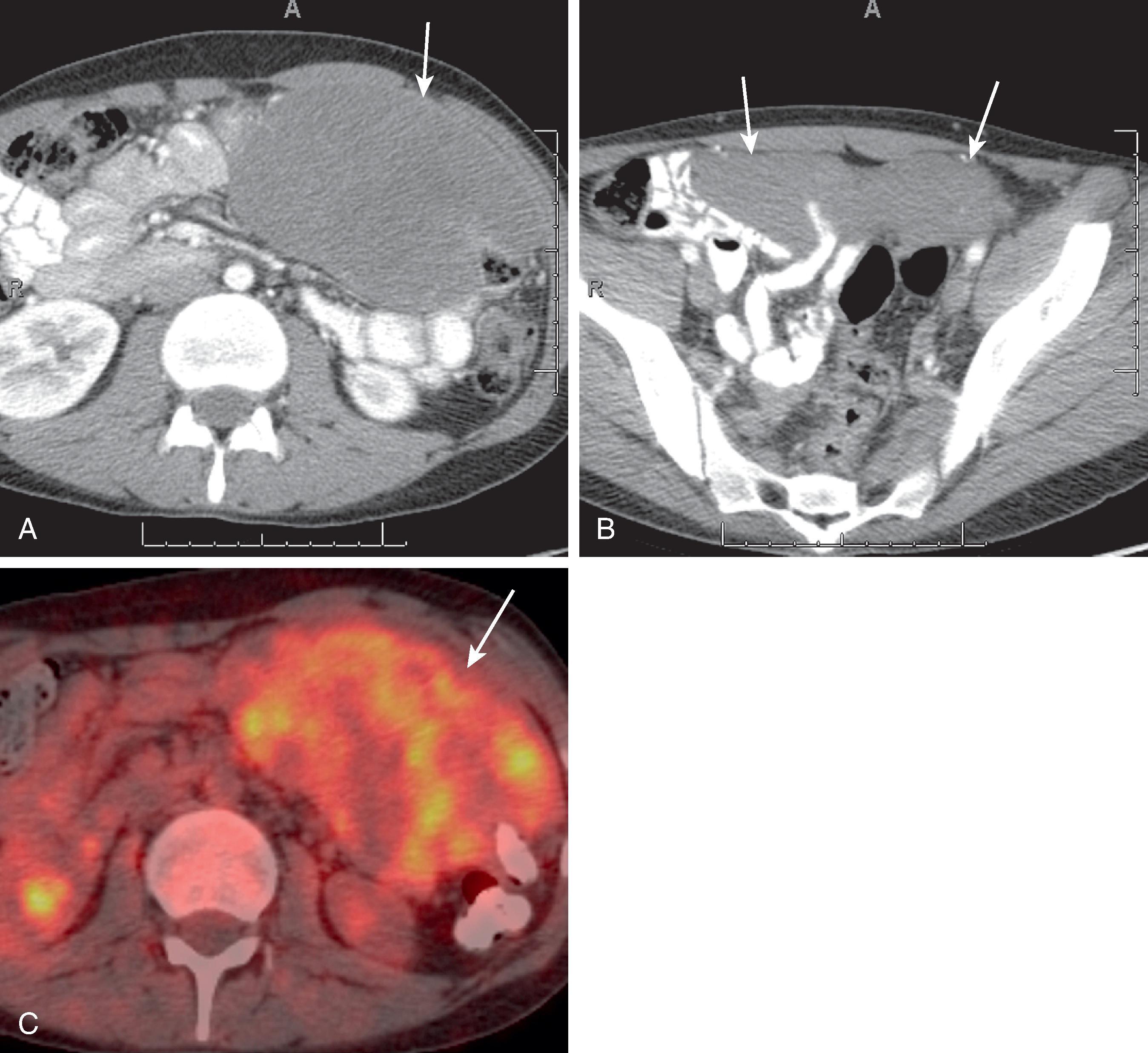
CT is the most useful test in the initial evaluation of patients with increasing abdominal girth and pain. The nodules and masses in mesothelioma enhance with intravenous administration of contrast material. These can be manifested as sites of peritoneal thickening, infiltration, or nodularity ( Fig. 66.4 ). A sheetlike pattern of growth with thickened appearance to the mesentery and bowel wall can also be seen. Bowel loops may be fixed from these infiltrative changes. Calcification is rare in MPMs. They can be associated with variable amounts of ascites. Scalloping or mass effect on adjacent organs is typically seen. On MRI, the nodules are of low T1 signal intensity and intermediate to high T2 signal intensity. The peritoneal nodules can also be seen on dynamic or diffusion-weighted imaging. Positron emission tomography (PET) has a limited role in exact anatomic evaluation of MPMs. All the imaging modalities are limited in their detection of peritoneal nodules less than 0.5 cm. Peritoneal mesothelioma is associated with poor survival; median survival time is 8 to 12 months after diagnosis.
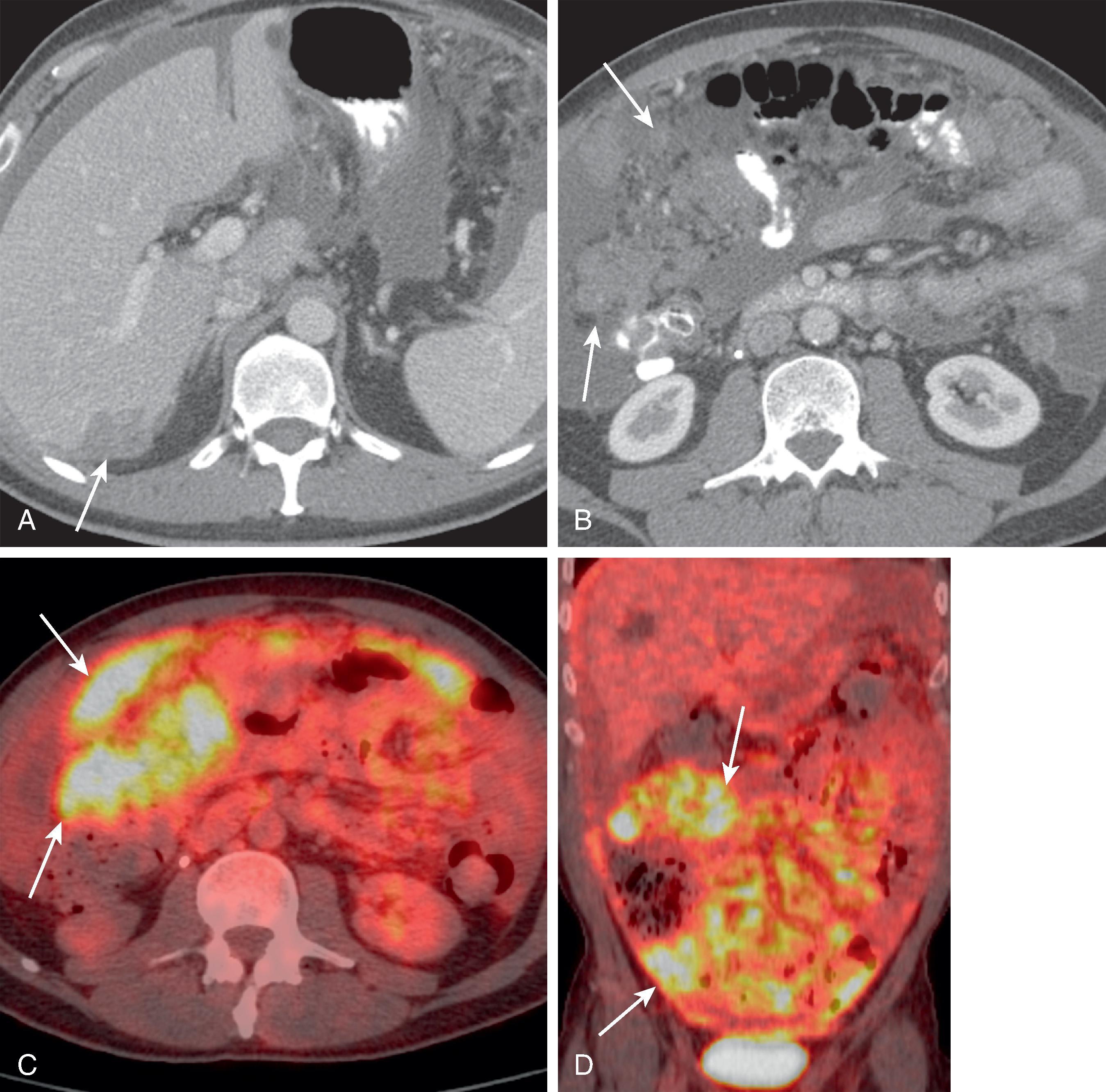
CT is useful in evaluating for a primary tumor elsewhere in the gastrointestinal or genitourinary tracts, breast, and pancreas. The differential diagnosis includes metastatic disease, primary papillary serous carcinoma of the peritoneum, lymphoma, and granulomatous disease. The presence of marked and enlarged lymphadenopathy favors lymphoma or metastatic disease as the most likely diagnosis. Similarly, calcification of the peritoneal thickening makes MPMs less likely.
The mesenteric infiltration results in a characteristic stellate and fixed appearance.
Primary peritoneal serous carcinoma is a rare malignant neoplasm that typically occurs in postmenopausal women. This is a primary tumor of the peritoneum, but the cell origin is thought to be the extraovarian mesothelium with Müllerian potential.
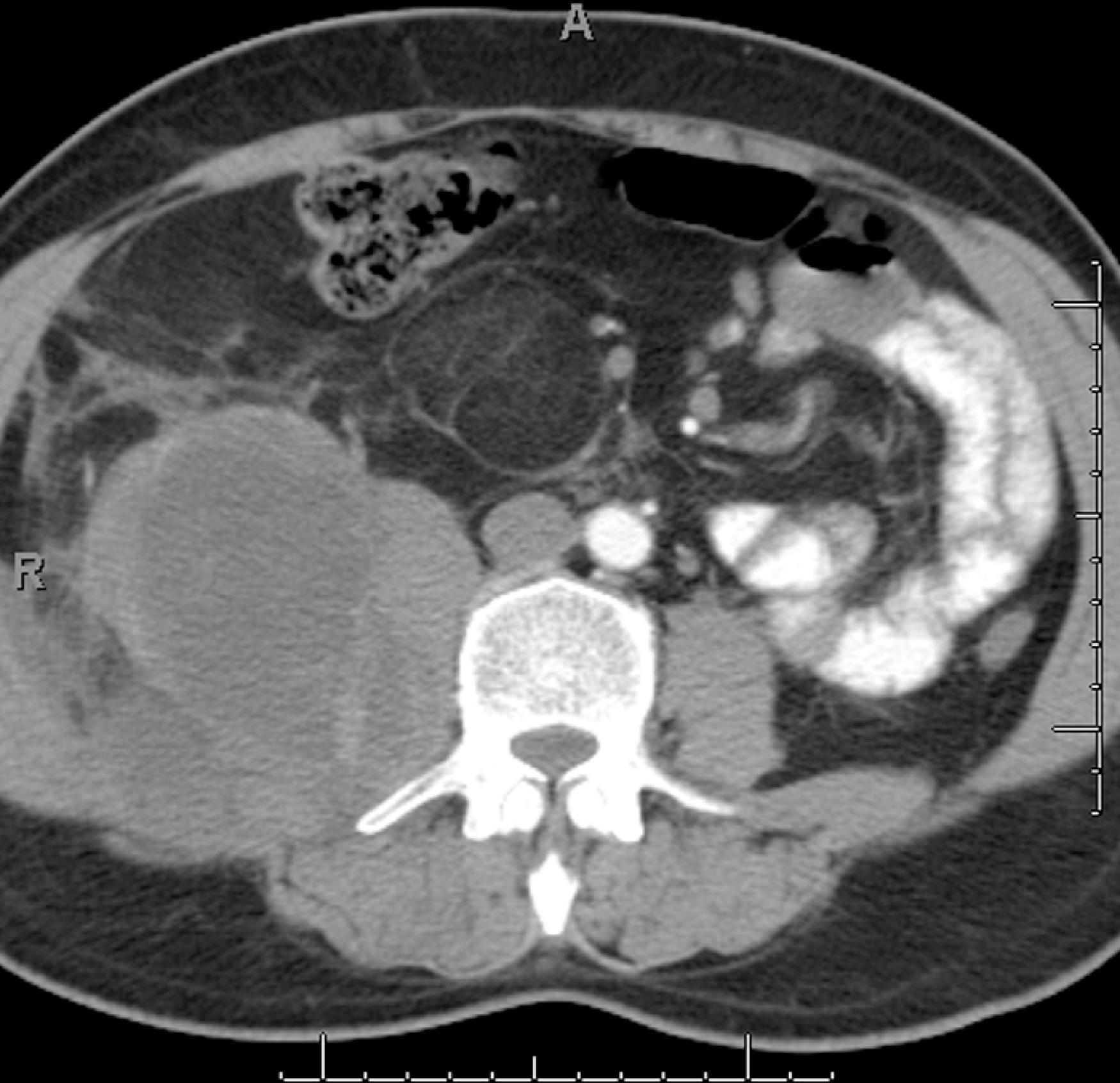
According to the Gynecologic Oncology group, for a diagnosis of primary peritoneal serous carcinoma to be made, the ovaries should be normal in size or enlarged from a benign process, and the involvement of the extraovarian sites should be greater than ovarian involvement. The prognosis and treatment are similar to those of serous ovarian carcinoma. Calcification can be seen with this tumor and can help differentiate MPM from this tumor ( Fig. 66.5 ). The CT and the pathologic appearance of primary peritoneal serous carcinoma is similar to that of carcinomatosis from serous ovarian cancer. Assessment for primary ovarian masses should be performed to exclude peritoneal carcinomatosis from ovarian cancer. Psammomatous calcifications can be seen in up to 30% of cases.
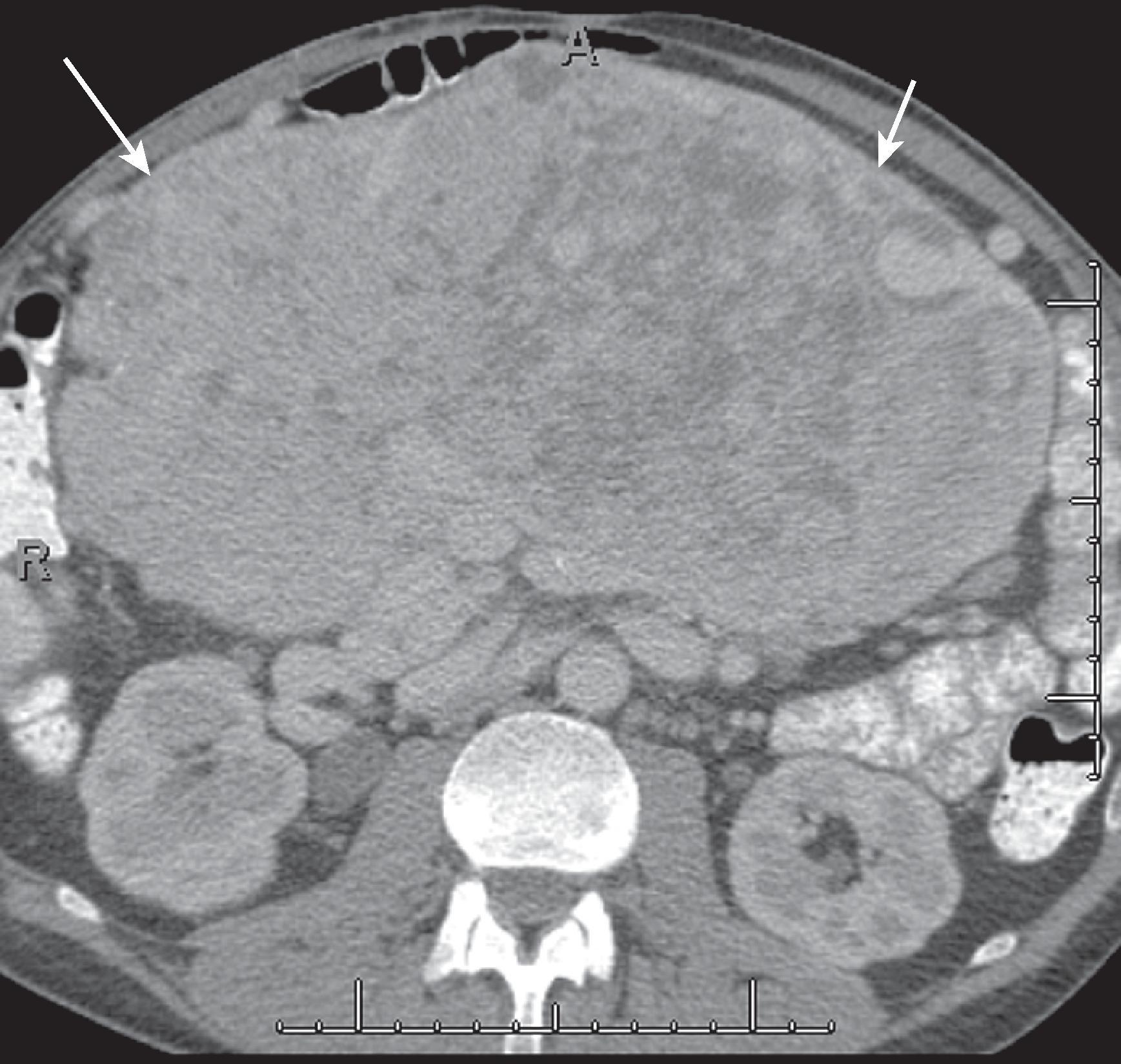
This is a rare subtype of mesothelioma that is clinically distinct from MPM. These tumors are seen typically in younger women and are not associated with asbestos exposure. They may be detected and removed incidentally. These tumors tend to be small. These tumors can be manifested as calcifications of the peritoneal surface without associated mass but can also be multiple peritoneal nodules. , The clinical course tends to be indolent after surgery. However, these tumors should be observed because of the small risk for development into MPMs.
This is another rare subtype of peritoneal mesothelioma occurring predominantly in young and middle-aged women along the pelvic peritoneal surface. There is no association with asbestos exposure in these patients. A higher incidence of prior abdominal surgery or pelvic inflammatory disease is seen in these patients. This tumor is composed of cysts that vary in size and are separated by septations or fibrous tissue. It can appear as a multicystic mass, multiple unilocular cysts adjacent to each other, or a unilocular cystic mass. On ultrasound, this tumor appears as a multiloculated lesion containing anechoic cystic spaces separated by echogenic septations. They can surround the ovaries, and the ovaries may appear entrapped within the cystic mass. On CT, this appears as a well-defined, noncalcified multiloculated cystic mass with enhancing internal septations. About 50% of these tumors tend to locally recur after surgical resection.
There is a slight risk for transformation into a malignant mesothelioma, and long-term follow-up should be performed in these patients.
This is a rare but extremely aggressive tumor that typically occurs in adolescents and young adults and more commonly in men. The most common clinical complaint is abdominal distention. The prognosis is poor, and the 3-year survival is less than 30%. CT shows multiple large solid intraperitoneal masses ( Fig. 66.6 ) without an apparent primary site. The tumor predominantly involves the omentum and paravesical space. Areas of central low attenuation may correspond to hemorrhage or necrosis. Ascites and hepatic metastases are associated findings. There is both hematogenous and intraperitoneal spread that can be seen with this tumor. Retroperitoneal and paratesticular involvement has been reported with this tumor. Malignant ascites is frequently seen with these tumors. They tend to be of low T1 and high T2 signal intensity and demonstrate heterogeneous contrast enhancement.
This is a very rare tumor that is typically discovered incidentally. Adenomatoid tumor can occur in conjunction with the multicystic mesotheliomas. It is typically small and characterized by epithelioid cells.
Tumors may arise from the mesenchymal structures and may be of fatty, vascular, lymphatic, or neurogenic tissue origin.
Tumors of fatty origin can range from benign lipoma to the malignant liposarcoma. Benign lipoma is usually a well-defined homogeneous lesion containing tissue of fat attenuation on CT. Liposarcomas can be ill-defined heterogeneous lesions with a variable soft tissue component ( Fig. 66.7 ). The diagnosis of liposarcoma can be made on CT if this mass contains fat interspersed with the soft tissue component or areas of enhancement.
Hemangiomas can occur in the mesentery. These are further divided into cavernous, capillary, and venous types. Cavernous hemangiomas containing large vascular sinusoids are the most common type of hemangioma to occur in the mesentery. They are typically of soft tissue attenuation and may contain phleboliths within them. , Hemangiopericytomas can also occur in the mesentery and are typically large, vascular masses ( Fig. 66.8 ).
Lymphangiomas are benign tumors of lymphatic origin. These can be either congenital tumors or benign tumors of mesenchymal origin. They are typically multiloculated cystic masses with thin enhancing walls ( Fig. 66.9 ). It can be difficult to differentiate them from cystic mesotheliomas.
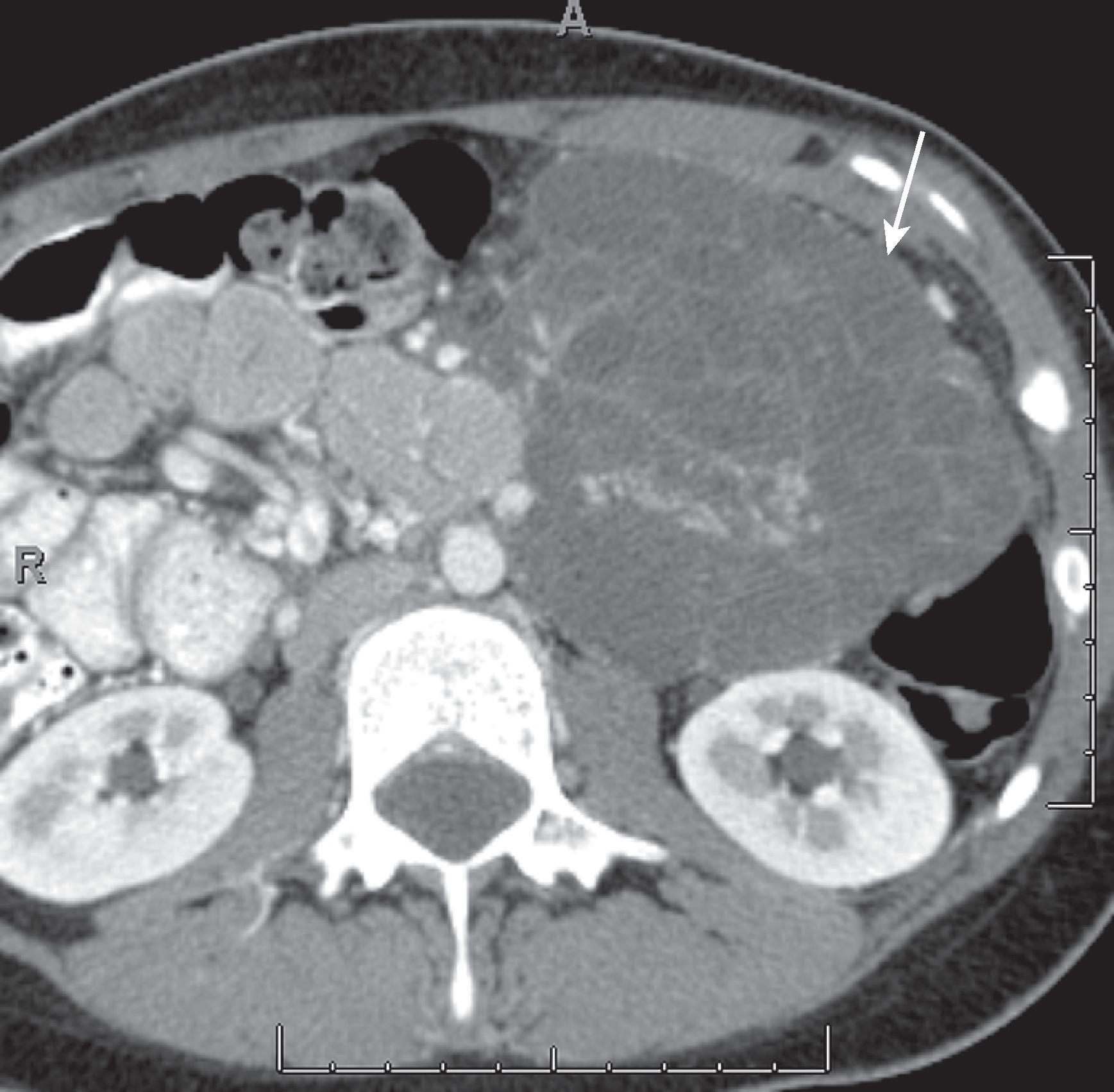
Tumors of neurogenic origin are typically benign and are more common in the retroperitoneum than in the mesentery. On CT, these tumors are solid low-attenuation masses ( Fig. 66.10 ) that show minimal contrast enhancement. They are of low T1 signal intensity and intermediate to high T2 signal intensity and show minimal contrast enhancement. They may show a tubular or elongated pattern. Uncommonly, they may show malignant degeneration.
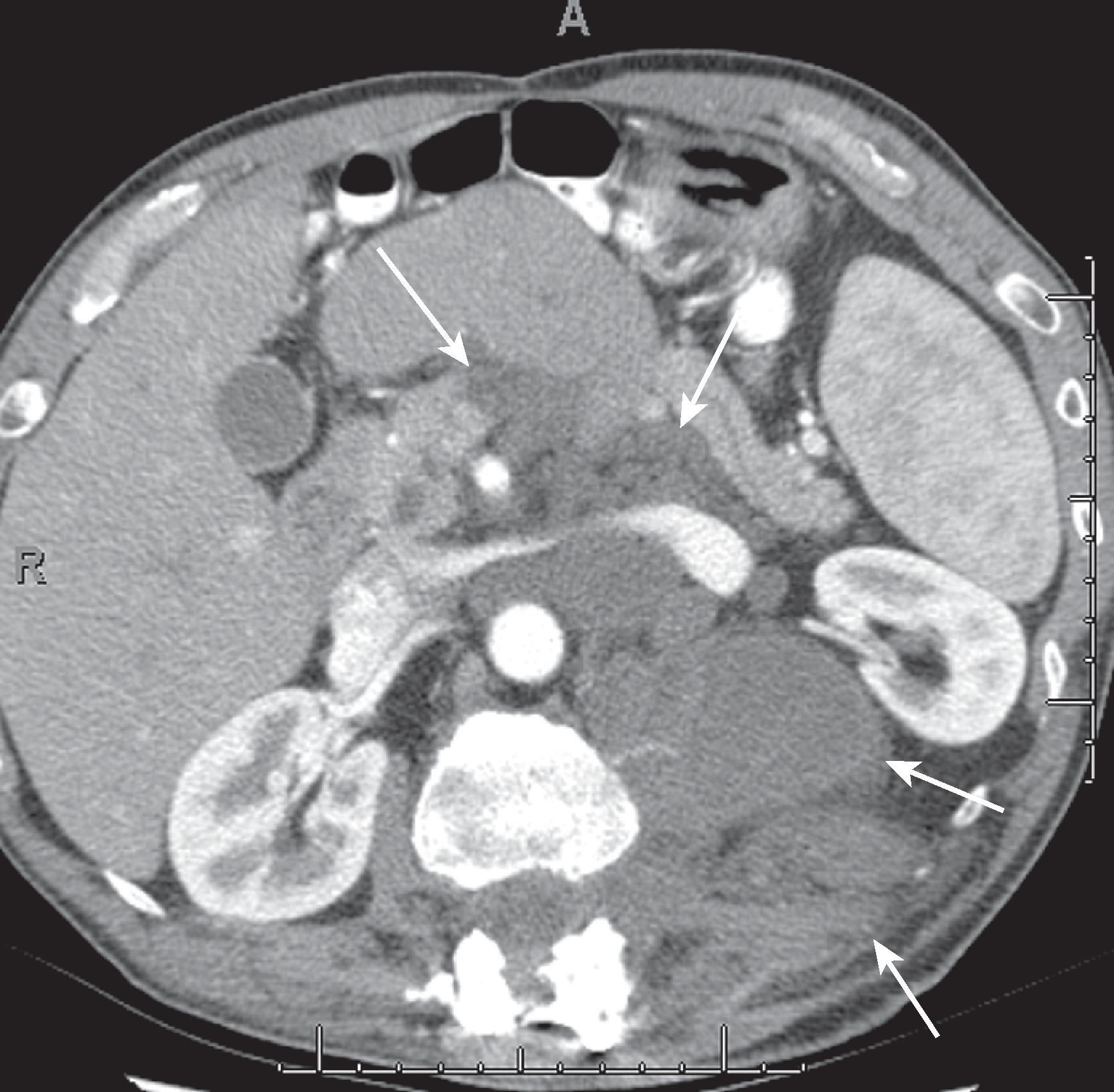
Leiomyomatosis peritonealis disseminata is a rare entity characterized by multiple smooth muscle tumor nodules throughout the peritoneum. This is typically found in young women with uterine fibroids and detected incidentally.
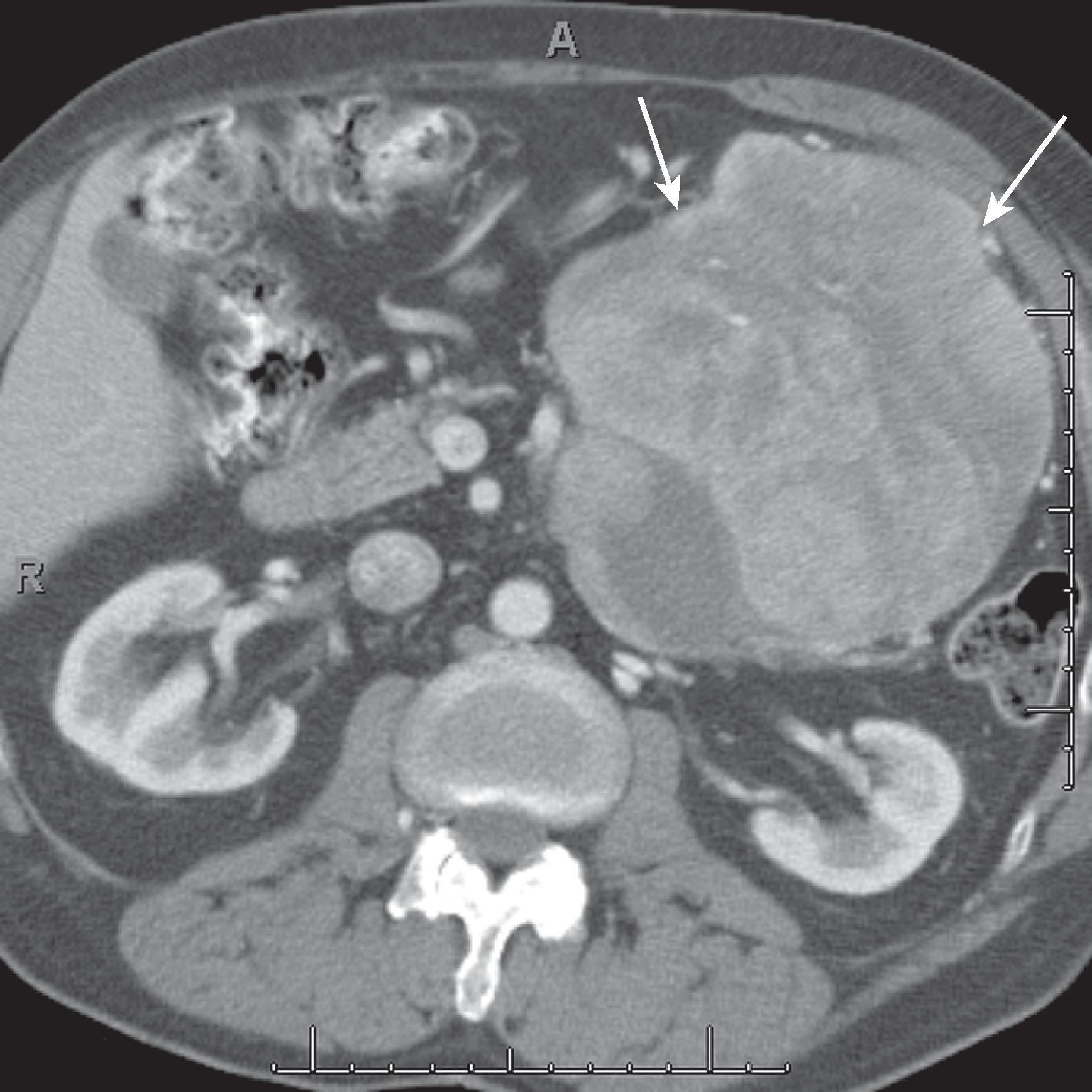
Sarcomas are more common in the retroperitoneum compared with the mesentery. The tumors often arise in the retroperitoneum and extend into the peritoneum. They are typically large at the time of diagnosis ( Fig. 66.11 ) and can show areas of central low attenuation related to necrosis or hemorrhage. Malignant fibrous histiocytoma is the most common peritoneal sarcoma.
The intra-abdominal spread of metastatic disease can occur by (1) direct extension along the mesentery and ligaments, (2) intraperitoneal seeding, (3) lymphatic extension, and (4) hematogenous dissemination.
Direct extension occurs most commonly in gastrointestinal and pancreatic tumors and can involve the various ligaments surrounding the site of primary tumor. Intraperitoneal seeding is related to the movement of malignant cells within the ascitic fluid, thereby being deposited in the peritoneal cavity. Lymphatic dissemination is most commonly seen with lymphoma. Hematogenous metastases typically occur on the antimesenteric border of the bowel loops by distal embolization. This is commonly seen in melanoma and breast and lung carcinoma.
Peritoneal carcinomatosis is usually seen in tumors arising from the gastrointestinal tract, pancreas, melanoma, breast, lung, and ovary. Any of the four mechanisms listed before can seed the peritoneum, and this can cause peritoneal carcinomatosis. A variable amount of fluid exists in the peritoneal cavity. This circulates from the superior abdomen caudally to the pelvis and then back again to the superior abdomen. Both gravity and respiration play a role in the fluid circulation within the peritoneal cavity. Peritoneal carcinomatosis is manifested on CT as nodularity to the peritoneum, which can progress to peritoneal masses. When the primary tumor is mucinous, such as in appendiceal or ovarian tumors, diffuse peritoneal involvement with cystic implants can be seen ( Fig. 66.12 ). This is called pseudomyxoma peritonei. Detection of peritoneal carcinomatosis is difficult, especially when the nodules are subcentimeter in size. Peritoneal nodules can often mimic unopacified small bowel loops. Accurate assessment for peritoneal carcinomatosis requires adequate bowel opacification and the intravenous administration of contrast material. The nodules create mass effect on the organs adjacent to them. They can cause scalloping of the liver and spleen; they can cause mesenteric infiltration and thickening, leading to a stellate mesentery; and they can involve the surface of the bowel, causing bowel wall thickening and potentially bowel obstruction. Common sites of involvement include the right hemidiaphragm, right paracolic gutter, cul-de-sac, and omentum.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here