Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mesenchymal lesions that occur in the vulva and vagina can be separated into two general categories: (1) those that are relatively site-specific, i.e., they may occasionally occur at extragenital sites; and (2) those that occur more commonly at other sites but which may also involve this region. The former group is described in detail in this chapter. Most entities in the latter group have the same clinical and pathologic characteristics as their extragenital counterparts, with exception of vulvovaginal smooth muscle tumors which have site-specific diagnostic criteria (and thus are also covered here). Certain entities important in the differential diagnosis are also discussed.
Fibroepithelial stromal polyps are common in women of reproductive age (most commonly during pregnancy) but may be seen in postmenopausal women on hormonal replacement therapy. They are often incidental findings discovered during routine gynecologic examination. Symptoms, when present, may include bleeding, discharge, or the sensation of a mass. Fibroepithelial polyps usually present as a solitary lesion; however, multiple polyps may occur during pregnancy, after which they typically regress.
The lesion is usually <5 cm, polypoid or pedunculated, and may project from a thin connecting stalk. Occasionally, they may have multiple finger-like projections, which can mimic a condyloma.
Fibroepithelial stromal polyps are exophytic lesions with a variably cellular stroma, central fibrovascular core, and overlying squamous epithelium. The most characteristic feature is the presence of stellate and multinucleate stromal cells, which are most commonly seen near the epithelial–stromal interface or adjacent to the prominent central vasculature ( Fig. 4.1 ). Sometimes the lesion displays high stromal cellularity, nuclear pleomorphism, and mitotic activity (including >10 mitoses/10 high power fields [HPFs] and atypical mitoses), thereby mimicking a sarcoma ( Fig. 4.2 ). Importantly, there is no clearly defined margin or sharp demarcation from the epithelial–stromal interface (absence of cambium layer).
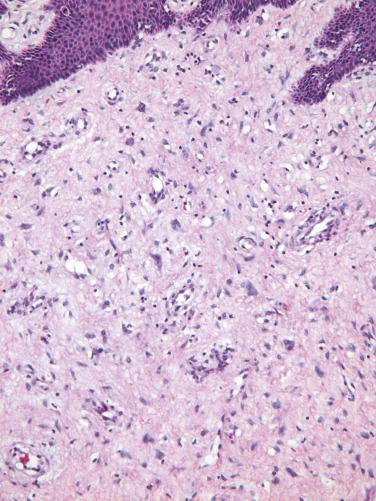
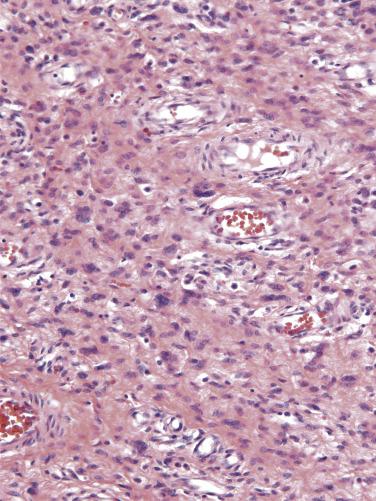
The stromal cells of these lesions are often positive for desmin, vimentin, and estrogen and progesterone receptors (ER and PR), and less frequently for actin. RB1 expression is intact.
Fibroepithelial stromal polyps, particularly those with increased cellularity and atypical stromal cells, must be distinguished from a malignant process. Sarcomas typically have an identifiable lesional margin, show homogeneous cellularity throughout, and lack the stellate and multinucleate stromal cells near the epithelial–stromal interface. In contrast, even the most floridly pseudosarcomatous examples of fibroepithelial stromal polyps characteristically show stellate multinucleated cells and tend to exhibit a greater degree of cellularity in the center of the lesion. Fibroepithelial stromal polyps are readily distinguished from botryoid embryonal rhabdomyosarcoma , as fibroepithelial stromal polyps are rare before puberty and lack the characteristic hypercellular subepithelial (cambium) layer and specific markers of skeletal muscle differentiation. When stromal polyps are edematous, a deep (aggressive) angiomyxoma may be considered; however, the latter is typically more deeply located, is uniformly hypocellular, has myxoid instead of edematous stroma, and lacks stellate and multinucleate cells.
These lesions have the potential to recur locally, particularly if incompletely excised or if there is continued hormonal stimulation (e.g., pregnancy).
Benign polypoid growth that arises from distinctive subepithelial stroma of distal female genital tract
Most common during pregnancy
May occur in vulva, vagina, and, rarely, cervix
If large, may cause pain and bleeding
Typically reproductive-aged women
May occur in postmenopausal women on hormone replacement therapy
There is an association with pregnancy
Typically polypoid or pedunculated with thin connecting stalk
Multiple polyps may occur during pregnancy
Benign lesion; may regress (following pregnancy)
Complete local excision treatment of choice
Potential for local recurrence if incompletely excised or if continued hormonal stimulation
Usually <5 cm
Polypoid or pedunculated with thin connecting stalk
Polypoid with variably cellular stroma, central fibrovascular core, and overlying squamous epithelium
Stellate and multinucleate stromal cells are characteristic; typically located at the epithelial–stromal interface and around blood vessels
Some polyps may exhibit stromal hypercellularity, stromal cell nuclear pleomorphism, and increased mitotic activity (so-called pseudosarcoma botryoides)
Desmin, vimentin, ER, and PR typically positive
Actin less frequently positive
Intact RB1 expression
Embryonal rhabdomyosarcoma
Deep (aggressive) angiomyxoma
This is a benign neoplasm that occurs in the vulvovaginal region of reproductive-aged women but it may also occur in the inguinoscrotal region in males. It is often thought to represent a cyst on clinical examination.
The tumors are typically small (usually <5 cm) and well circumscribed, and they display a tan/white and rubbery cut surface.
Angiomyofibroblastoma, although not encapsulated, is well demarcated from the surrounding vulvar soft tissues. It is composed of plump, ovoid (plasmacytoid) to spindle-shaped cells that may be multinucleated and tend to cluster around the numerous small- to medium-caliber blood vessels. The cell clusters are set within a variably edematous to collagenous matrix with alternating zones of cellularity ( Fig. 4.3 ). Mitotic activity is uncommon and occasionally intratumoral adipose tissue may be present.
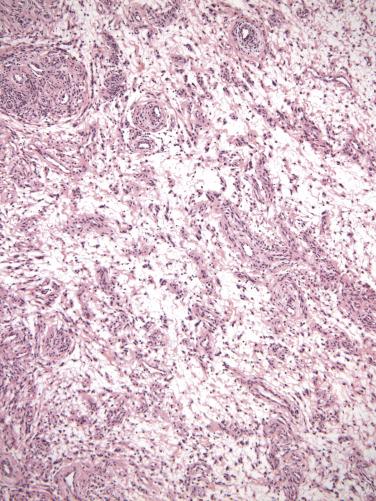
The tumor cells are typically positive for desmin, ER, and PR. Smooth-muscle actin positivity is variable but usually negative. CD34 is typically negative and RB1 expression is typically intact.
The most important differential diagnostic consideration is the distinction of angiomyofibroblastoma from deep (aggressive) angiomyxoma because of the differences in biologic behavior and treatment. Unlike deep angiomyxoma, angiomyofibroblastoma is well circumscribed and has alternating zones of cellularity and more prominent vascularity composed of small- to medium-sized vessels, in contrast to the infiltrative growth, homogeneous hypocellularity and medium- to large-sized vessels typically seen in deep angiomyxoma. Other entities to consider in the differential diagnosis with angiomyofibroblastoma include fibroepithelial stromal polyp, cellular angiofibroma, mammary-type myofibroblastoma and prepubertal vulvar fibroma. Fibroepithelial stromal polyp is polypoid, has an ill-defined interface with surrounding tissue, a different vascular pattern (with centrally located larger vessels) and stellate and mutlinucleated cells near the stromal–epithelial interface. Cellular angiofibroma is more uniformly cellular, contains larger and more commonly hyalinized vessels, and is typically diffusely positive for CD34. Mammary-type myofibroblastoma is morphologically and immunohistochemically identical to myofibroblastoma of the breast and compared to angiomyofibroblastoma, it lacks the prominent vascular component and perivascular distribution of epithelioid cells. In addition, as cellular angiofibroma and mammary myofibroblastoma fall within a family of genetically related tumors showing loss of RB1/13q14 , there is typically loss of RB1 immunoexpression in these tumors. Finally, prepubertal vulvar fibroma is distinguished from angiomyofibroblastoma by occurring in a younger age group and by its ill-defined margins and patternless proliferation of bland spindle cells; in addition, it lacks the prominent vascular component and is CD34 positive.
Angiomyofibroblastoma is a benign tumor without recurrent potential; therefore, local excision with clear margins is adequate treatment. Rarely, this tumor may undergo sarcomatous transformation.
Tumor composed of myofibroblasts and thin-walled capillaries, which principally occurs in vulvovaginal soft tissues
Uncommon
Vulvovaginal region
Benign, nonrecurring neoplasm
Reproductive-aged women
Often thought to represent a vulvar cyst
Excellent prognosis
Local excision with clear margins
Usually <5 cm
Well circumscribed
Tan-white, rubbery cut surface
Well circumscribed, non-encapsulated
Alternating zones of cellularity (cellular bundles alternating with edematous/fibrotic hypocellular areas)
Plump (plasmacytoid) to spindle-shaped cells
Numerous delicate thin-walled capillaries
Clustering of stromal cells around vasculature
Variably edematous to collagenous matrix
Desmin, ER, PR typically positive
CD34 and smooth muscle actin typically negative
Intact RB1 expression
Deep “aggressive” angiomyxoma
Fibroepithelial stromal polyp
Cellular angiofibroma
Mammary-type myofibroblastoma
Prepubertal vulvar fibroma
Cellular angiofibroma is a benign tumor that occurs in the vulva or perineum of middle-aged women (mean 54 years), although it also occurs in the inguinoscrotal region in men and at extragenital sites. In the vulva, they most commonly present as a small, well-circumscribed, painless, subcutaneous mass.
Tumors are typically small (average 2.7 cm) with a well-demarcated border. On cut section, they have a firm, rubbery gray-white surface.
Most cellular angiofibromas have a well-demarcated border on low-power examination; however, a few may exhibit focal limited infiltration of surrounding soft tissues. It is a cellular neoplasm composed of short, intersecting fascicles of bland spindle-shaped cells with scant pale-staining cytoplasm and ovoid nuclei ( Fig. 4.4 ). Scattered atypical nuclei or multinucleate cells may sometimes be seen. Rare examples of cellular angiofibroma have discrete foci of cytologically atypical cells or a sarcoma-like component, the latter as an abrupt transition to a discrete sarcomatoid component which in a subset closely resembles atypical lipomatous tumor or pleomorphic liposarcoma; the prognostic significance of these findings is uncertain. Mitoses are typically infrequent, but some tumors may show brisk mitotic activity. The vascular component is prominent with numerous, relatively homogeneous small- to medium-sized, thick-walled (and often hyalinized) blood vessels. Interspersed wispy collagen bundles are also characteristic and mast cells are a common associated finding. In addition, most tumors also have a small component of adipose tissue, often located at the periphery. Entrapped small nerves may also be seen at the periphery of the tumor.
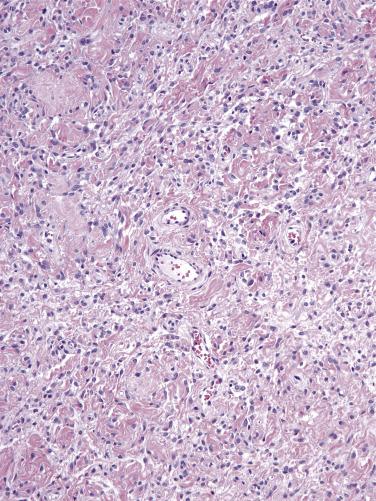
The tumor cells are positive for CD34 (in 60% of cases), smooth-muscle actin (20%), and desmin (8%). ER and PR may also be positive (∼50% of cases) whereas S-100 is negative. RB1 expression is deficient with loss of staining. In cases with sarcomatoid transformation having the appearance of a lipomatous tumor, MDM2 and CDK4 are negative, but p16 can be strongly positive.
Cellular angiofibroma harbors monoallelic deletions of RB1 and FOXO1 at the 13q14 locus, a shared feature with spindle cell lipoma and mammary-type myofibroblastoma.
Because of the presence of short intersecting fascicles, cellular angiofibroma may be confused with a benign smooth-muscle tumor. It is distinguished from a leiomyoma by its shorter fascicles, more prominent vascular component, less abundant eosinophilic cytoplasm, and lack of blunt-ended nuclei. Mammary-type myofibroblastoma and cellular angiofibroma show significant morphologic and immunohistochemical overlap (although the vessels of the former are not as prominent), and based on their shared molecular abnormality, may be related tumors on a morphologic spectrum. Less frequently, cellular angiofibroma may be confused with angiomyofibroblastoma (discussed previously), prepubertal vulvar fibroma, deep angiomyxoma, and solitary fibrous tumor. Prepubertal vulvar fibroma occurs more commonly in young girls, has ill-defined margins, a hypocellular appearance, and patternless proliferation of spindle cells. Deep angiomyxoma more commonly involves deep soft tissue and has an infiltrative margin. In addition, it is myxoid and hypocellular, and RB1 immunoexpression is intact. In contrast to cellular angiofibroma, solitary fibrous tumor is characterized by a “patternless” architecture, heterogeneous cellularity, hemangiopericytoma-like vasculature, and frequent presence of keloid-type collagen; it is also typically positive for STAT6.
Cellular angiofibroma is a benign tumor. Incomplete excision may lead to regrowth of tumor; therefore, local (conservative) excision with negative margins appears to be adequate treatment.
Tumor composed of bland spindle cells admixed with a prominent vascular component
Relatively uncommon; located in the vulvovaginal region (can occur at extragenital sites)
Benign, nonrecurring tumor
Middle-aged women (mean 54 years)
Small, well-circumscribed subcutaneous mass
Patients may present with pain
Local excision with clear margins
Excellent prognosis
Typically small (<3 cm) and well circumscribed
Firm, rubbery consistency
Gray-white cut surface
Usually well-demarcated border
Short intersecting fascicles of bland spindle-shaped cells
Cells with ovoid nuclei and scant, pale eosinophilic cytoplasm with indistinct borders
Typically no cytologic atypia but mitotic activity may be brisk; occasionally scattered atypical nuclei, zones of atypical cells, and rarely sarcomatous transformation
Prominent vascular component with small- to medium-sized vessels, often thick-walled and hyalinized
Interspersed wispy collagen bundles
Entrapped fat and nerves at the periphery
Abundant mast cells
CD34 positive in 60%, smooth muscle actin in 20%, and desmin in 8% of cases
ER and PR positive in ∼50% of cases
RB1 loss of expression
S-100 negative
Leiomyoma
Mammary-type myofibroblastoma
Angiomyofibroblastoma
Prepubertal vulvar fibroma
Deep (aggressive) angiomyxoma
Solitary fibrous tumor
Mammary myofibroblastoma was first described in the breast with a predilection for older men; since its initial description, it is now known to occur in extramammary sites, particularly the inguinal/groin area and the term “mammary-type myofibroblastoma” is the designated terminology. In the largest series to date, this tumor more commonly affects males (66% vs. 34%) and anatomic locations include in order of frequency: inguinal/groin region, breast, chest wall/axilla, trunk, and extremities. The tumors occur in individuals over a wide age range (from first to tenth decade) with a mean age of 54 years. Most patients are asymptomatic but some present with a painless swelling or mass. Most tumors occurring in the groin are subcutaneous but it is important to note that outside this location, they may occasionally be deep-seated (intramuscular, pelvis, retroperitoneum, or abdomen).
Mammary-type myofibroblastomas are usually pink or tan to brown, firm and well-circumscribed masses that often have a whorled, nodular, and occasionally mucoid cut section ( Fig. 4.5 ). Tumors can measure up to 22 cm with a mean size of 6.6 cm.
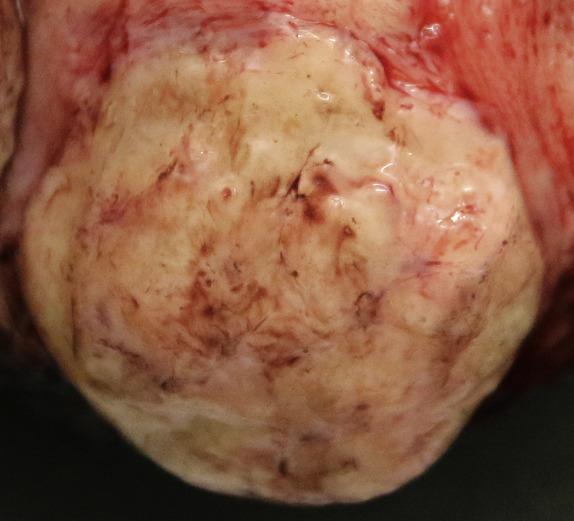
These tumors histologically resemble mammary myofibroblastoma. They are well-circumscribed, unencapsulated tumors composed of haphazardly arranged variably sized fascicles or a patternless proliferation of bland spindle cells with oval nuclei, pale cytoplasm, and indistinct cell borders ( Fig. 4.6 ). A variable amount of adipose tissue is present ranging from little/none to predominant ( Fig. 4.6 ). Stromal collagen can sometimes have a ropy appearance reminiscent of that seen in spindle cell lipoma. Less commonly, focal cytologic atypia often seen as “bizarre” degenerative appearing nuclei, epithelioid morphology, or neurilemmoma-type nuclear palisading can be seen.
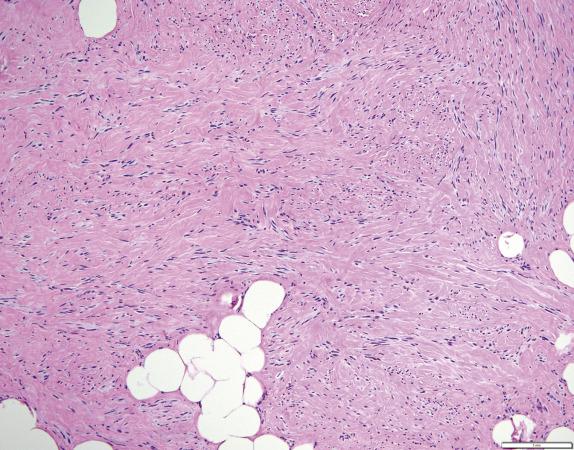
The spindle cells are frequently positive for desmin and CD34; only rare cases are negative for these markers. RB1 expression is lost in the vast majority (>90%).
Mammary-type myofibroblastoma can show loss of 13q14 similar to cellular angiofibroma and spindle cell lipoma. As these tumors can also have significant morphologic and immunophenotypic overlap, it is thought that they likely represent a spectrum of neoplasia within a genetically related family of tumors.
The differential diagnosis includes cellular angiofibroma and angiomyofibroblastoma (discussed previously), prepubertal vulvar fibroma and deep (aggressive angiomyxoma). Prepubertal vulvar fibroma more commonly occurs at a younger age, is more infiltrative and less cellular, and lacks a fascicular growth. Deep (aggressive) angiomyxoma is uniformly myxoid and hypocellular, has infiltrative margins, lacks fascicular growth, and shows intact RB1 expression.
Mammary-type myofibroblastoma is benign. There appears to be a very low risk of recurrence as there have been two documented recurrences 17 and 20 years after initial excision.
Benign mesenchymal neoplasm composed of a patternless proliferation of spindle cells and variable amounts of adipose tissue, histologically resembling mammary myofibroblastoma
Uncommon
Inguinal/groin region>breast>chest wall/axilla>trunk>extremities
Low risk of (often late) recurrence
M>F
Wide range (mean 54 years)
Most asymptomatic
Slow growing painless mass/swelling
Local excision
Benign neoplasm with low risk of recurrence
Pink, tan-brown firm, well-circumscribed mass
Whorled, nodular, occasionally myxoid cut surface
Mean 6.6 cm
Well circumscribed and unencapsulated
Haphazardly arranged fascicles or patternless growth
Bland spindle cells with ovoid nuclei and pale indistinct cytoplasm
Variable amounts of adipose tissue
Stromal hyalinization and/or myxoid change common; ropy collagen may be seen
Unusual features: focal cytologic atypia, epithelioid morphology, neurilemmoma-type appearance
Desmin and CD34 frequently positive
RB1 loss of expression (>90%)
Cellular angiofibroma
Angiomyofibroblastoma
Prepubertal vulvar fibroma
Deep (aggressive) angiomyxoma
Prepubertal vulvar fibroma typically occurs in young girls (range 4–12 years) as a gradual vulvar swelling; it is typically unilateral, painless, and most commonly involves the labium majus.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here