Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Meibomian gland dysfunction is the major cause of evaporative dry eye and often occurs in conjunction with aqueous deficiency dry eye.
Expression of the meibomian gland with examination of the expressed secretion is critical to clinical diagnosis and monitoring of meibomian gland disease.
Obstruction of the meibomian gland orifices is due to abnormally thickened meibum or hypertrophic keratinization of the orifices, while senescence of the meibocytes with advancing age can result in hyposecretion.
Treatment of meibomian gland dysfunction relies predominantly on expression of the glands and modification of the meibum secretion by use of topical azithromycin or the oral tetracycline family of antibiotics.
Treatment of evaporative dry eye includes therapy of meibomian gland dysfunction but also treatment of any associated aqueous tear insufficiency, including use of tear supplements, lubricants, and topical antiinflammatory therapy.
Meibomian gland dysfunction (MGD) is an extremely common, yet often overlooked, chronic condition of the posterior eyelids. A consensus workshop has defined MGD as a chronic, diffuse abnormality of the meibomian glands commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease. It is important to recognize its presence in a patient complaining of dry eye–like symptoms because MGD is the most common cause of evaporative dry eye disease, although it is not known how many functioning meibomian glands are necessary to maintain the antievaporative effects of a normal lipid layer. Recent clinical studies identified MGD either alone or in combination with aqueous tear deficiency as the most commonly occurring form of dry eye, suggesting that MGD be considered as a part of dry eye disease. , Lacrimal insufficiency and dermatologic conditions are often concomitant and must be identified and addressed in concert with the treatment of the MGD for therapy to be effective.
This chapter reviews recent developments in the understanding of the pathophysiology, role of bacteria, and changes in meibomian secretion lipid composition and behavior seen in MGD. Effective treatment options for patients with this condition are described.
The meibomian glands, which number 30–40 in the upper lid and 20–30 in the lower lid, are arranged in a single row perpendicular to the lid margin. They occupy the full-thickness of the tarsal plate. Their orifices emerge posterior to the eyelashes and just anterior to the mucocutaneous junction ( Fig. 33.1 ). The glands are grape-like clusters and yellowish in gross appearance with a main duct with which up to 30–40 saccular acini communicate.
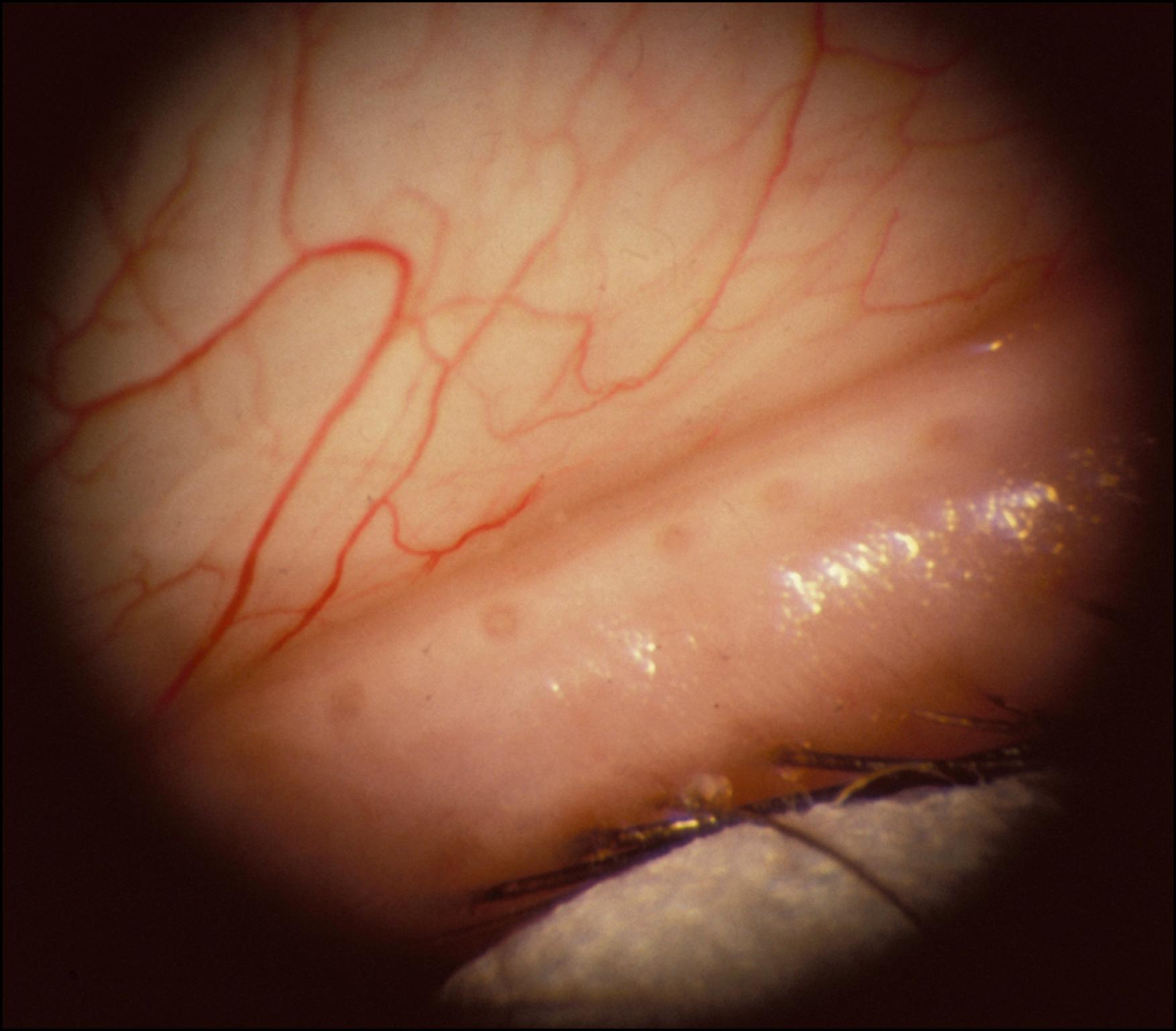
The ducts are lined by four to six layers of at least partially keratinized cells. The acinar cells are nonkeratinized and differentiate in a centripetal direction, with the innermost cells degenerating to form the holocrine lipid secretion. The glands are surrounded by the dense collagen of the tarsus, fibroblasts, lymph spaces, and a network of nerves and blood vessels. Elastic tissue, smooth muscle fibers, and portions of the orbicularis oculi are intimately associated with the glands.
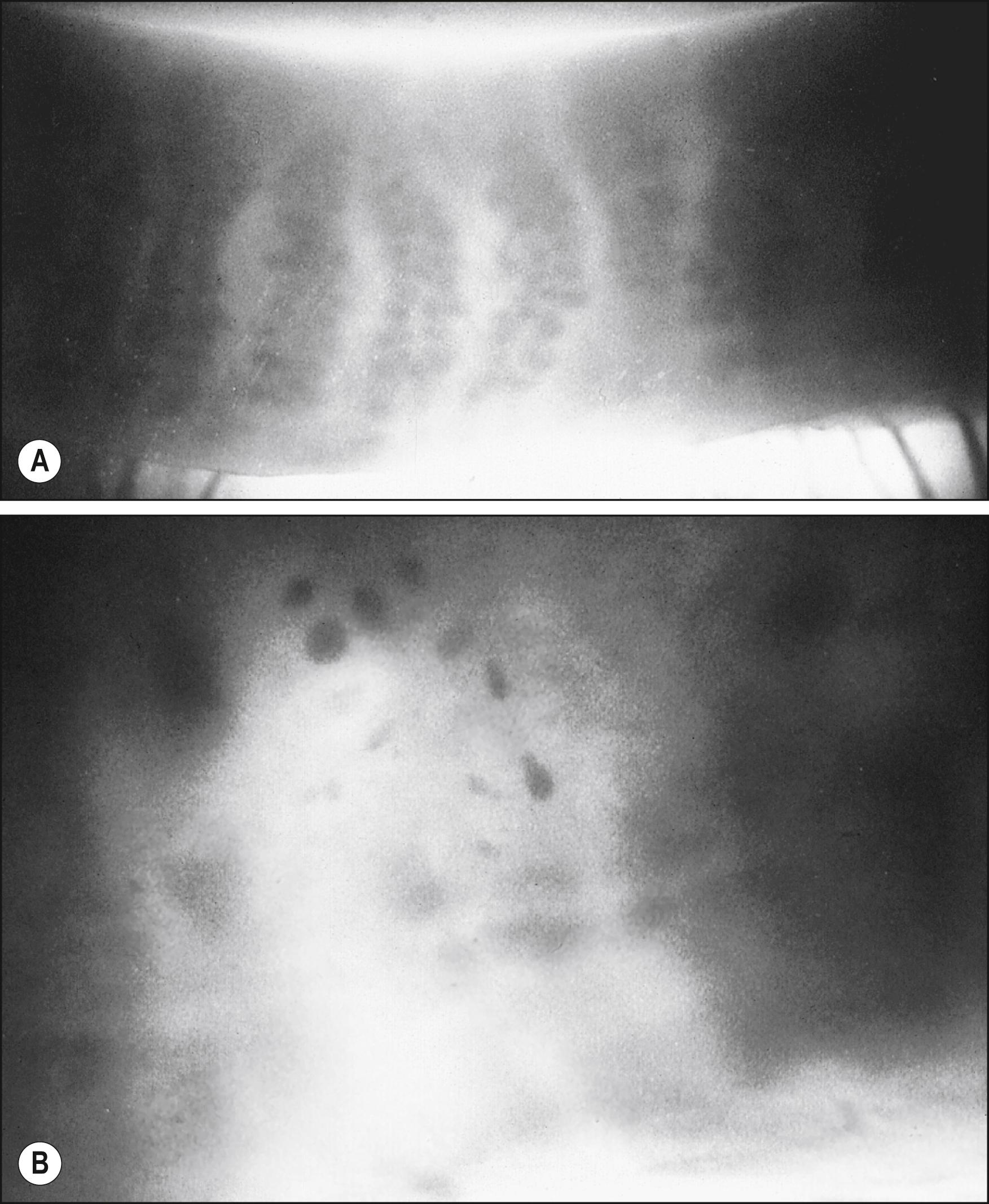
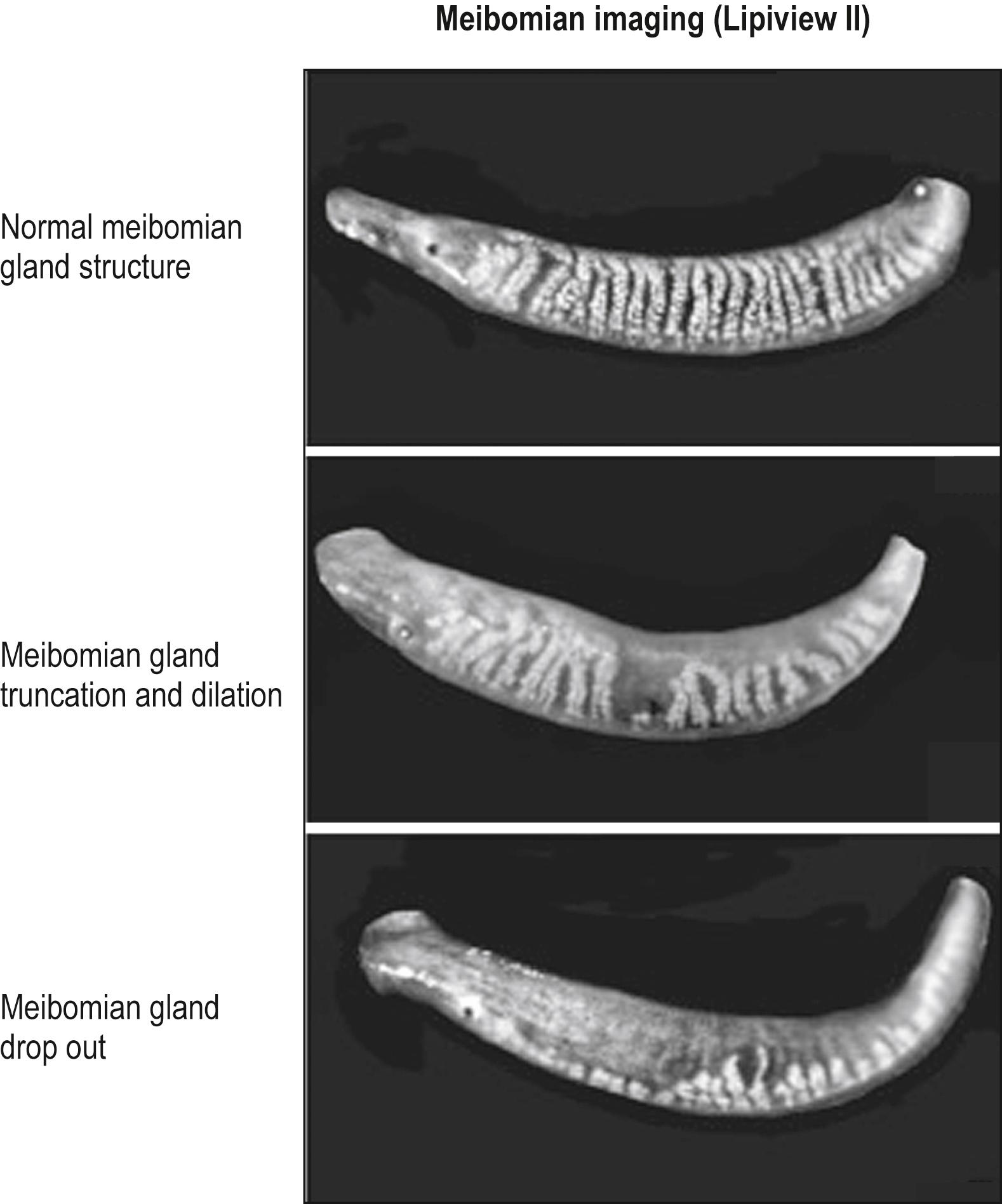
Meibography is a technique that uses transillumination biomicroscopy of the everted eyelid with infrared photography or video imaging. The normal appearance of the meibomian glands using this technique is shown in Fig. 33.2A . The glands can also be imaged by in vivo confocal microscopy. Imaging of both normal and abnormal meibomian glands is depicted in Fig. 33.3 .
The term meibomian gland dysfunction was first suggested by Korb and Henriquez. It is more appropriate than the terms meibomianitis or meibomitis , as inflammation is not necessarily present. The meibomian gland secretion, which is distinct from sebum (cutaneous sebaceous gland secretion) in its composition, has been termed meibum by Nicolaides et al.
The lipid layer of the preocular tear film, produced by the meibomian glands, has several important functions as listed in Box 33.1 . Compromise of the lipid layer leads to increased evaporation with resultant dry eye–like symptoms, despite normal aqueous production.
Retards evaporation from the tear film.
Prevents contamination of the tear film by providing a barrier to cutaneous sebum.
Lowers the surface tension of tears, allowing water to be drawn into the tear film, thereby thickening it.
Provides a seal between the lid margins during sleep.
Provides a smooth optical surface.
Enhances spreadability and stability of the tear film.
Studies have shown that the superficial lipid layer retards evaporation of underlying water approximately fourfold in rabbits. In the presence of meibomian gland dropout (see Fig. 33.2B ), there is a threefold increase in evaporative rate in humans. MGD increases tear electrolytes uniformly, consistent with a purely evaporative effect, in contrast to lacrimal gland disease in which sodium ions rise disproportionately in secretion at low flow rates. Both, however, result in a concentrated tear film characterized by hyperosmolarity, a major hallmark of dry eye disease. Increased concentrations of electrolytes are thought to be responsible for changes in conjunctival goblet cell density and corneal epithelial glycogen levels. These changes undoubtedly contribute to the conjunctival and corneal manifestations of meibomian gland disease.
Increasing the concentration of polar lipids in the lipid layer ruptures the tear film. Contamination by a drop of sebum results in immediate tear film dispersal. Thus meibomian gland secretion may provide an important barrier to contamination of the tear film by the highly polar cutaneous sebum. At eye surface temperature, meibomian lipid is more viscous than sebum and able to form a barrier to its entry onto the ocular surface. The melting point of human meibomian secretion is approximately 32°C–34°C. Normal eye surface temperatures range between 32°C and 36°C. Any decrease in the melting point secondary to abnormal synthesis or the influence of bacterial lipases would result in liquefaction of the meibomian fluid and the loss of the barrier mechanism. Conversely, an increase in melting point would lead to meibomian gland inspissation and a compromised barrier secondary to decreased secretion. A report has linked oleic acid content in meibum with changes in viscosity.
The lipid layer produced by the meibomian glands represents the outermost layer of the tear film and protects the hydrated gel covering the ocular surface. The tear film is thought to be approximately 7 μm thick in the open eye, but a recent study indicates that the tear film may be as thick as 40 μm. The lipid layer is about 40–100 nm thick. Thickness is influenced by the width of the interpalpebral opening.
Blinking is important for the excretion of meibomian lipid. Jetting of the oil onto the tear film has been observed with blinking. It has been suggested that the orbicularis muscle milks the glands by contracting during blinking. Meibometry is used to measure the amount of lipid on the lid margin by determining the degree of translucency induced on plastic tape applied to the lid margin. In the absence of blinking, the meibomian glands become engorged, and the measurable amount of lipid on the lid margin decreases; it is readily restored by the first few blinks. Levels are greater 1 hour after waking than later in the day and are thought to be due to decreased secretion from the glands during sleep, with glandular engorgement and then discharge of the excess lipid on awakening when blinking resumes. The thickness of the lipid layer increases after forceful blinking. Thus blinking appears to be important in the release of meibomian gland lipid into the tear film. It is probable that blinking abnormalities contribute to the formation of MGD, and studies of those performing prolonged computer work confirm a higher prevalence of MGD.
Tear film break-up time is reduced in MGD. Various models have been proposed to explain this phenomenon. One model suggests that with a deficient aqueous subphase, the lipid layer contaminates the hydrophilic mucin layer, making it hydrophobic with resultant disruption of the tear film. Alternatively, it has been postulated that a key step in tear film break-up is the rupture of the mucin layer by van der Waals expulsion forces. Tear film break-up time rapidly returns to normal when the meibomian glands are expressed.
Although the meibomian glands are richly innervated, the direct neural control is not well understood. Neurostimulation of the intranasal passage may, however, increase release of meibomian gland secretion by activation of intrinsic muscles in the eyelid. Hormones are known significantly to affect the secretion of sebaceous glands of the skin and the meibomian glands. Production is increased by androgens and decreased by antiandrogens and estrogens. Recent studies have demonstrated that the meibomian gland is an androgen target organ and that decreased androgens alter the lipid profile and may lead to MGD and evaporative dry eye.
MGD is found with increasing frequency with age. In some cases, it may represent a senescent degeneration of the meibomian glands or be the result of an abnormal blink mechanism secondary to aging changes affecting the lids. In a large population-based study, Viso et al. reported that not only is MGD ubiquitous in the elderly population, but also there are more asymptomatic subjects with MGD than those with symptoms.
The pathophysiology of MGD early on has been attributed to hyperkeratinization of the ductal tissue near the orifice on the lid margin or thickened secretion from the gland resulting in obstruction. Recent evidence of age-related MGD may involve altered PPARγ signaling and/or loss of stem cell renewal resulting in acinar atrophy and development of an age-related MGD leading to hyposecretion of meibum. On histopathologic examination, obstruction of the meibomian gland orifices is probably the most common cause of MGD in younger patients. Studies indicate that hyperkeratinization is important in the pathogenesis of obstruction. , , Narrowing of the ductule lumen with desquamation of epithelial cells leads to glandular obstruction. Acini may dilate or atrophy.
Obstruction of the glandular contents results in increased pressure on the cuboidal cells lining the acini, flattening these cells. Stagnated meibomian secretion leads to lid inflammation and often to the development of chalazia. Cystic degeneration of the glands not associated with inflammation may be seen.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here