Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term megaloblastic anemia is used to describe a group of disorders characterized by a distinct morphologic pattern in hematopoietic cells. A common feature is a defect in deoxyribonucleic acid (DNA) synthesis, with lesser alterations in ribonucleic acid (RNA) and protein synthesis, leading to a state of unbalanced cell growth and impaired cell division. Most megaloblastic cells are not resting but vainly engaged in attempting to double their DNA, with frequent arrest in the S phase and lesser degrees of arrest in other phases of the cell cycle. An increased percentage of these cells have DNA values between 2 N (where N is the amount of DNA in the haploid genome) and 4 N because of delayed cell division. This increased DNA content in megaloblastic cells is morphologically expressed as larger-than-normal “immature” nuclei with finely particulate chromatin, whereas the relatively unimpaired RNA and protein synthesis results in large cells with greater “mature” cytoplasm and cell volume. The net result of megaloblastosis is a cell whose nuclear maturation is arrested (immature) while its cytoplasmic maturation apparently proceeds normally independently of the nuclear events. The microscopic appearance of this nuclear-cytoplasmic asynchrony (or dissociation) is morphologically described as megaloblastic. Each cell lineage has a limited but unique repertoire of expression of defective DNA synthesis. This is significantly influenced by the normal patterns of maturation of the affected cell line. Additional variables that affect RNA and protein synthesis can lead to the attenuation or modification of megaloblastic expression (see Masked Megaloblastosis).
Megaloblastic hematopoiesis commonly manifests as anemia, but this feature is only a manifestation of a more global defect in DNA synthesis that affects all proliferating cells. The peripheral blood picture is characteristic and reflective of megaloblastic hematopoiesis within the bone marrow. The diagnosis is therefore usually straightforward, but because any condition that specifically perturbs DNA synthesis may lead to megaloblastosis, determination of the precise cause is necessary before institution of therapy. Inappropriate therapy can lead to disastrous consequences for the patient. The biochemical basis for megaloblastosis needs to be understood within the context of evaluation of potential and real variables affecting DNA, RNA, and protein synthesis in a given patient. The most common causes of megaloblastosis are true cellular deficiencies of vitamin B 12 (cobalamin) or folate, vitamins that are essential for DNA synthesis.
Because of the imperative for conservation of cobalamin within the body, there is a finely tuned mechanism in place to ensure a sequential handover of this precious cargo from one protein to another—from the point of its entry into the mouth through the gut, across the enterocyte, into the circulation with specialized uptake into cells, passage through lysosomes into cytoplasm, and even into mitochondria. Throughout this odyssey, cobalamin is accompanied by several chaperones that sequentially bind, sequester, and thereby ensure that cobalamin does not participate in side reactions. This ensures its fitness for service for critical enzymes.
Despite the greater abundance of folate in the diet relative to cobalamin, there are also specialized means to ensure that the natural folates in food are first chopped and diced before being ushered across the enterocyte through specialized pathways. After passage from the portal blood into the general circulation, folate is extracted by cell surface folate receptors, undergoes endocytosis, and is then shunted together with a proton across another channel into the cytoplasm. It is then received by an overabundance of high-affinity multifunctional enzymes that channel the folate across set pathways to support critical synthesis of thymidine for DNA and other key cellular functions including methylation reactions.
A general principle is that the preexisting store of these vitamins will dictate the speed with which overt deficiency develops; this is particularly relevant to pregnant women and children in resource-limited countries with preexisting borderline stores of folate and cobalamin.
The pathophysiology of cellular cobalamin and folate deficiency is most readily discerned by the clinician who approaches megaloblastosis with a clear understanding of the physiology of these vitamins. A detailed discussion of cobalamin and folate therefore follows.
The term cobalamin refers to a family of compounds with the structure shown in Fig. 40.1 . Details of the chemistry, nomenclature, and in vivo substitutions of cobalamin are shown in Figs. 40.1–40.3 , and excellent reviews are available on the colorful history, chemistry, and biology of cobalamin.
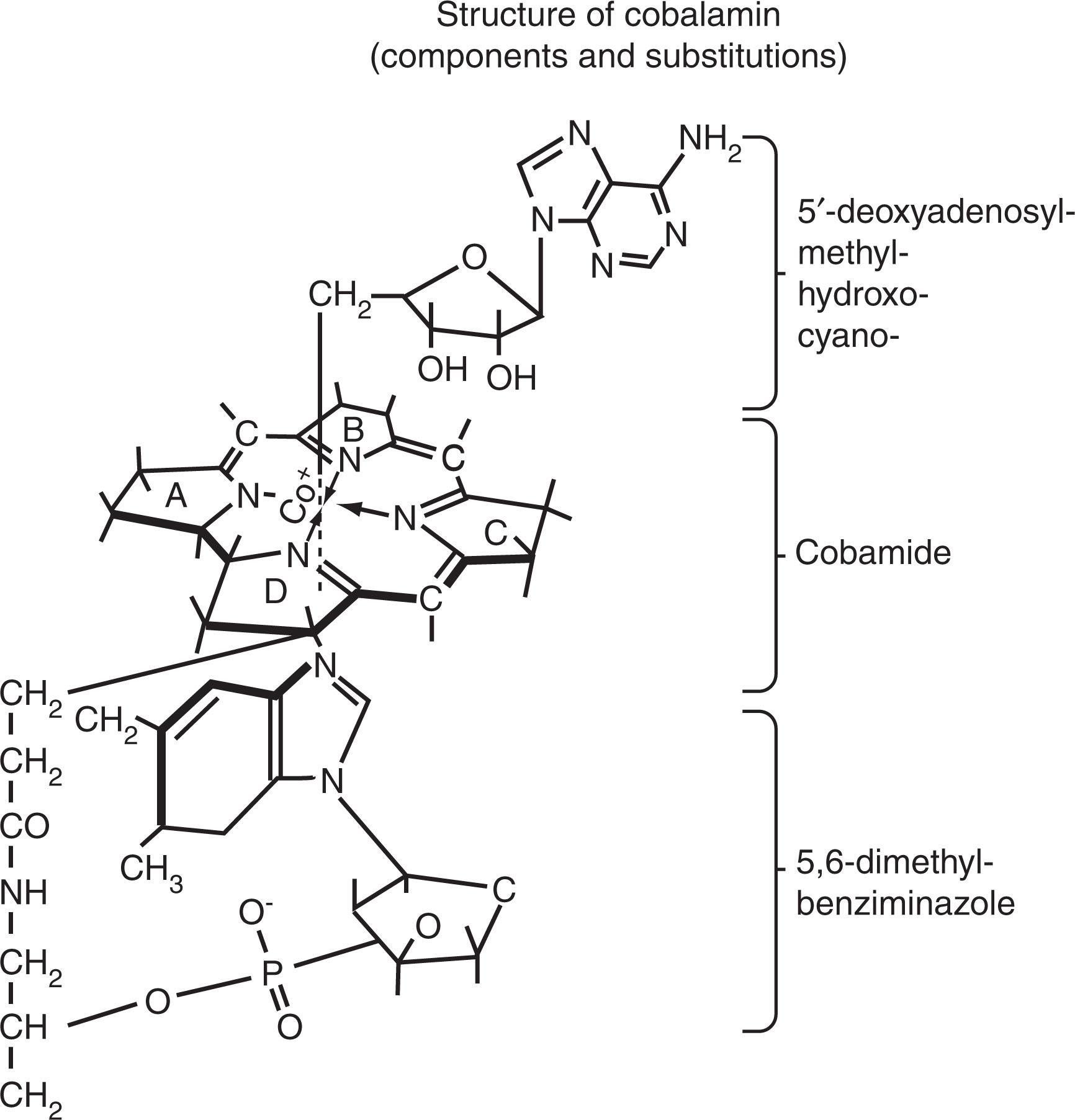
Cobalamin is produced in nature only by microorganisms, and humans receive cobalamin solely from the diet. Cobalamin is synthesized and used by some microorganisms (e.g., bacteria, fungi). Some strains (such as Pseudomonas denitrificans ) produce cobalamin during fermentation, making them excellent and cheap commercial sources for cobalamin used in therapy. Herbivores obtain their dietary quota of cobalamin from plants contaminated with cobalamin-producing soil bacteria (rhizobia) that grow in roots and nodules of legumes. Because rhizobia-related organisms are also found in the large intestine of animals (and humans), volitional or inadvertent coprophagy can lead to intake of cobalamin by herbivores; however, cobalamin from manure that contaminates plants is not a significant source for humans. Nevertheless, colonic cobalamin-producing bacteria—like Klebsiella pneumoniae that are related to rhizobia—can be found in the small intestine of some individuals from which cobalamin can be absorbed. For all practical purposes, there is no unfortified plant food, including fermented soy products, tempeh /tempe , seaweed (which are actually multicellular algae, such as nori [red algae], chlorella [green algae], spirulina [blue-green algae]), or other organic produce that can consistently provide a sufficient amount of active cobalamin to support daily requirements.
Animal protein is the major dietary cobalamin source for nonvegetarians. Meats from parenchymal organs are richest in cobalamin (over 10 μg/100 g wet weight); fish and muscle meats, milk products, and egg yolk have 1 to 10 μg/100 g of wet weight. An average nonvegetarian Western diet contains 5 to 7 μg/day of cobalamin, which adequately sustains normal cobalamin equilibrium. A vegetarian diet only supplies between 0.25 and 0.5 μg/day of cobalamin, so all vegetarians do not receive adequate dietary cobalamin and are at risk for cobalamin deficiency. Even a Mediterranean diet with modest intake of animal-source proteins places mothers and babies at risk for cobalamin deficiency. The current recommended daily allowance is 2.4 μg for men and nonpregnant women, 2.6 μg for pregnant women, 2.8 μg for lactating women, and 1.5 to 2 μg for children 9 to 18 years old. However, reevaluation of cobalamin requirements within a university community (aged 18 to 50 years) from the USA suggests that a higher intake of 4 to 7 micrograms of cobalamin each day is optimum for adequate cobalamin status. This is in line with earlier studies from the USA and Europe.
Food cobalamin is stable to high-temperature cooking but is readily converted to inactive cobalamin analogues by ascorbic acid. Cobalamin is exceptionally well stored in tissues in its coenzyme forms. Of the total-body content of 2 to 5 mg in adults, about 1 mg is in the liver. There is an obligatory loss of 0.1% per day (1.3 μg) regardless of total-body cobalamin content. It takes about 3 to 4 years to deplete cobalamin stores when dietary cobalamin is abruptly malabsorbed, but it may take longer to develop nutritional cobalamin deficiency, because of an efficient enterohepatic circulation, which accounts for turnover of 5 to 10 μg/day of cobalamin.
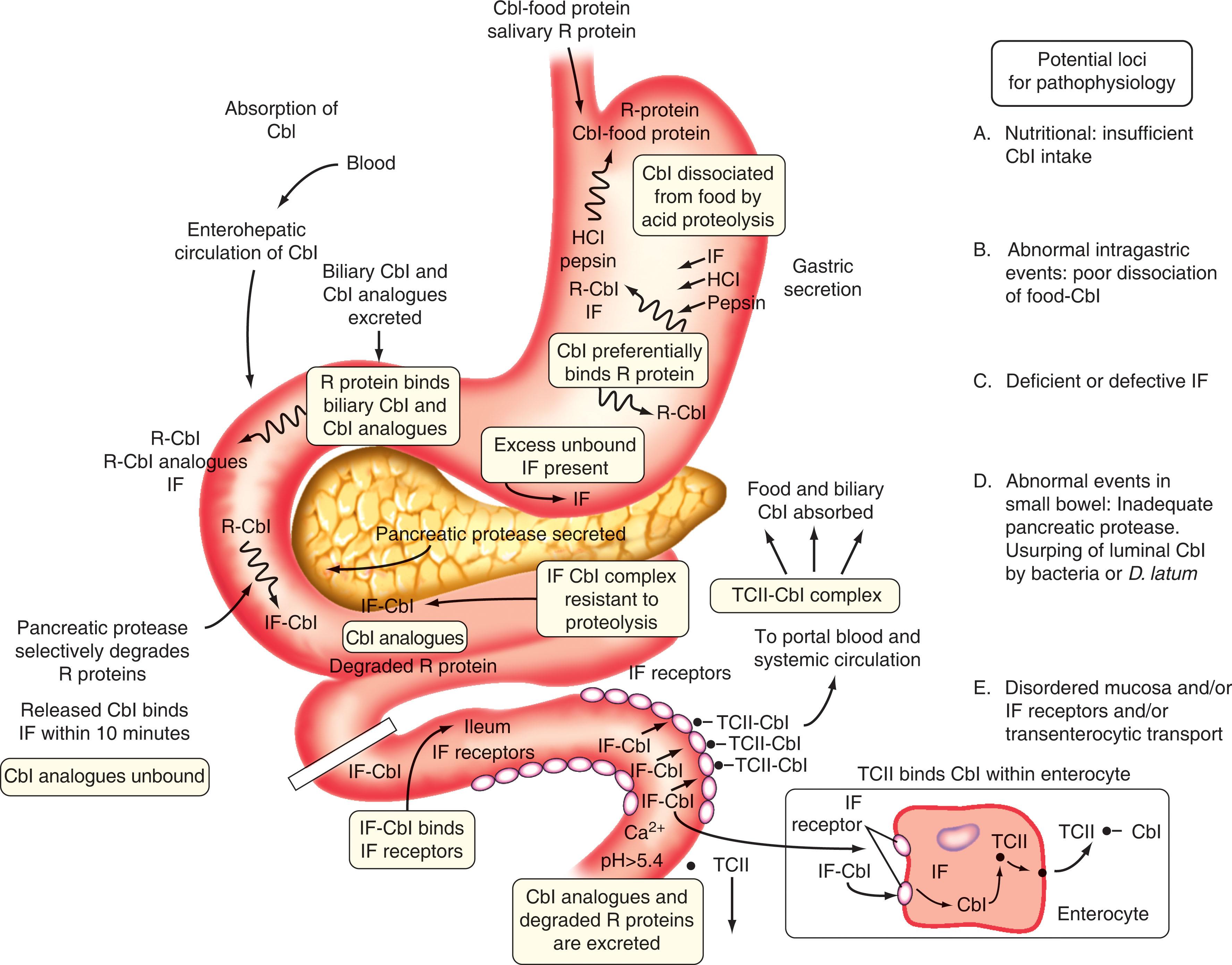
Cobalamin in food is usually in coenzyme form (5′-deoxyadenosylcobalamin [adenosylcobalamin] and methylcobalamin), nonspecifically bound to proteins (see Fig. 40.2 ). In the stomach, peptic digestion at low pH is a prerequisite for cobalamin release from food protein. Once released by proteolysis, cobalamin preferentially binds a high-affinity, 150-kDa, cobalamin-binding protein called R protein (a haptocorrin) from gastric juice and saliva that has an even higher affinity for cobalamin than gastric intrinsic factor (IF). The cobalamin-bound R protein complex (holo-R protein), along with excess unbound (apo)-R protein and IF, pass through into the second part of the duodenum, where pancreatic proteases degrade holo-R and apo-R proteins (but not IF). This results in the transfer of cobalamin to IF, a 45-kDa glycoprotein with high-affinity binding (K a = 1.5 × 10 10 M −1 ), 1 : 1 molar stoichiometry, stability, and resistance to proteolysis over a pH range of 3 to 9. Failure to degrade holo-R proteins by pancreatic protease precludes the involvement of IF in cobalamin absorption because the downstream ileal IF-cobalamin receptors only interact with IF-bound cobalamin. Although R proteins bind cobalamin and most cobalamin analogues with comparably high affinity, IF only binds cobalamin.
IF is produced in parietal (oxyntic) cells in the fundus and cardia of the stomach. IF has two binding sites: one for cobalamin and another for the ileal IF-cobalamin receptor. IF is produced in far greater excess than is actually required for absorption; indeed, the IF in only 2 to 4 mL of normal gastric juice can reverse cobalamin deficiency in adults who lack IF. In the absence of IF, less than 2% of ingested cobalamin is absorbed, whereas in its presence, approximately 70% is absorbed.
IF is secreted in response to food in the stomach in a manner analogous to secretion of acid (i.e., by vagal and hormonal stimulation). IF binds biliary cobalamin and newly ingested cobalamin following its transfer from R protein. Because biliary cobalamin analogues are not transferred from R protein to IF, this is an efficient method for fecal excretion of cobalamin analogues while allowing for reabsorption of biliary cobalamin. The stable IF-cobalamin complex passes through the jejunum to the ileum, where specific membrane-associated IF-cobalamin receptors for IF-cobalamin are concentrated on microvilli of terminal ileal mucosal cells.
The functional IF-cobalamin receptors are composed of a complex of two proteins collectively known as cubam, composed of cub ilin (CUBN) and am nionless (AMN), that is essential to complete transport of the IF-cobalamin complex from the intestinal lumen into the ileal enterocyte. CUBN is a large (400-kDa) peripheral membrane protein, which requires the smaller transmembrane protein, AMN, for its expression at the brush border, and vice versa. Dysfunction of cubam because of mutation in either CUBN or AMN is the basis for Imerslund-Gräsbeck syndrome. These IF-cobalamin receptors (cubam) require Ca 2+ for binding at pH above 5.4; they do not bind free IF, cobalamin, or R protein–bound cobalamin; therefore these receptors are highly specific and have a high affinity for IF-cobalamin (K a = 1 × 10 9 M −1 ). The human ileum contains enough IF-cobalamin receptors [cubam] to bind up to 1 μg of IF-bound cobalamin; this is the rate-limiting factor in cobalamin absorption.
After the cobalamin-IF complex is internalized by cubam for subsequent processing, these receptors are recycled back to the cell surface, whereas cobalamin enters the cytoplasm. Subsequent physiologic events are unclear. The multidrug resistance–associated protein (MRP) 1 mediates the cellular export of cobalamin across the basolateral membrane of intestinal epithelium. Then cobalamin apparently binds transcobalamin II (TCII) within or at the basal surface of the ileal enterocyte. TCII is abundant in the microvascular endothelium and cobalamin, which appears in portal blood largely (over 90%) bound to TCII, reaches peak levels in about 8 hours.
By contrast, therapeutically administered cobalamin in large doses can also passively diffuse through nasal, buccal, gastric, and jejunal mucosa so that less than 1% of a large dose of oral cobalamin appears in the circulation in minutes. This property is used to advantage in individuals with cobalamin malabsorption in lieu of parenteral replacement (discussed later).
More than 90% of recently absorbed or injected cobalamin is bound to TCII, which is the specific serum transport protein for delivery of cobalamin to tissues. TCII, a 38-kDa polypeptide synthesized in many tissues, preferentially binds cobalamin with 1 : 1 molar stoichiometry and high affinity (K a = 1 × 10 11 M −1 ). The TCII-cobalamin complex is rapidly cleared from the circulation in less than an hour. TCII-bound cobalamin binds to specific cell surface 58-kDa TCII-cobalamin receptors (encoded by the CD320 gene) present on several cells. High-affinity TCII-cobalamin binding to TCII-cobalamin receptors is specific only for holo- and apo-TCII (K a = ~5 × 10 10 M −1 ). However, because some cobalamin analogues can bind TCII with high affinity, these also have the same potential for cellular uptake as cobalamin.
Circulating cobalamin, which is predominantly in the form of methylcobalamin, is not found free in plasma. Binding to TCII accounts for only 10% to 30% of the total serum cobalamin, with the majority (approximately 75%) of remaining cobalamin being bound to another protein, transcobalamin I (TCI). TCI (another haptocorrin) binds biologically active cobalamins as well as biologically inactive cobalamin derivatives. Because TCI is not a cellular transport protein, it is best viewed as a plasma-storage form of cobalamin; indeed, cobalamin-bound TCI has a slow clearance rate (half-life of 9 to 12 days). A third transport protein, transcobalamin III (TCIII), is closely related to TCI but has a half-life in minutes because it is an asialoglycoprotein. TCIII binds a wide spectrum of cobalamin analogues with high affinity and delivers them via hepatic asialoglycoprotein receptors to hepatic cells, and thence into bile for fecal excretion. Between 0.5 and 9 μg of cobalamin taken up by hepatic TCII receptors is secreted into bile, of which approximately 75% is reabsorbed, analogous to food cobalamin, reflecting an efficient enterohepatic circulation of cobalamin.
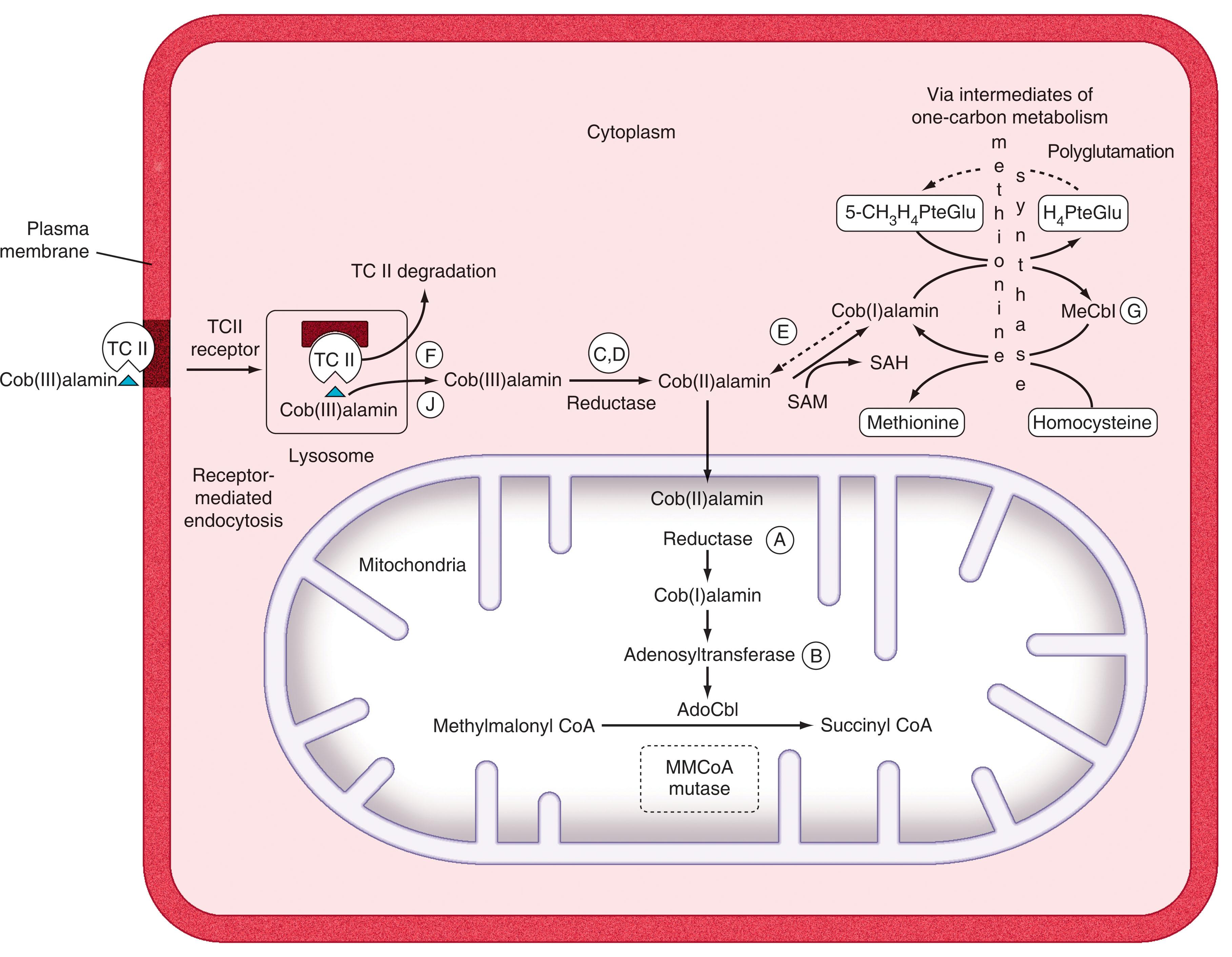
Once bound to TCII receptors, the TCII-cobalamin complex is internalized by conventional receptor-mediated endocytosis (see Fig. 40.3 ). At the low pH extant in lysosomes, TCII dissociates from cobalamin and is degraded, whereas transport of cobalamin across lysosomes into the cytosol requires two distinct membrane proteins, LMBRD1 and ABCD4, which act synergistically (see Fig. 40.3 ). Cob(III)alamin, the most “oxidized” form of cobalamin, must be converted to cob(II)alamin and cob(I)alamin by two sequential reductase steps (see Fig. 40.3 ). Once in the cytoplasm, cobalamin is bound to CblC, an enzyme that catalyzes removal of the cyano or methyl or adenosyl groups that are bound to cobalt in the cobalamin molecule. From here, cobalamin is moved to target enzymes in the mitochondria or the cytoplasm.
Over 95% of intracellular cobalamin is bound to two intracellular enzymes, methylmalonyl-CoA mutase and methionine synthase. When cobalamin interacts with its target enzymes, it exists in a “base-off/His-on” conformation, which reflects a very close relationship with these enzymes (see legend for Fig. 40.1 ).
In mitochondria, cob(I)alamin is converted to its coenzyme form, adenosylcobalamin, which acts as a coenzyme with methylmalonyl-CoA mutase to mediate the intramolecular exchange of a hydrogen atom attached to one carbon atom with a group attached to an adjacent carbon atom; in this way, methylmalonyl-CoA is converted to succinyl-CoA (methylmalonyl-CoA is normally generated during the catabolism of branched-chain amino acids, odd-chain fatty acids, and cholesterol). When formed, succinyl-CoA can then enter the Krebs tricarboxylic acid cycle.
In the cytoplasm, cobalamin, as methylcobalamin, functions as a coenzyme for methionine synthase, a critical enzyme for which both folates and cobalamin are required for normal one-carbon metabolism (see Fig. 40.3 ). Methionine synthase is a modular protein with four distinct and separate regions for binding homocysteine, 5-methyl-tetrahydrofolate (5-methyl-THF; 5-methyl-H 4 PteGlu), the cobalamin prosthetic group, and S -adenosylmethionine (SAM). The reaction proceeds by methyl transfer from 5-methyl-tetrahydrofolate to methionine synthase–bound cob(I)alamin to form methylcobalamin, followed by transfer of this methyl group to homocysteine to form methionine and regeneration of cob(I)alamin. In this process, 5-methyl-tetrahydrofolate is converted to tetrahydrofolate, which is subsequently polyglutamylated by folyl polyglutamate synthase; this addition of multiple glutamic acid moieties to tetrahydrofolate facilitates both its retention within cells and participation in one-carbon metabolism. During this reaction, spontaneous oxidation of cob(I)alamin (which has no axial ligand) to the catalytically inactive cob(II)alamin form requires reduction back to cob(I)alamin before it can accept a methyl group. There is a specific redox regulator known as methionine synthase reductase that restores enzyme activity in the presence of SAM and NADPH ; this enzyme is mutated in patients with cblE mutations.
The physiologic importance of the key cofactor roles of the two forms of cobalamin (i.e., adenosylcobalamin and methylcobalamin) in methylmalonyl-CoA mutase and methionine synthase, respectively, is that the products and by-products of these enzymatic reactions are critical for DNA, RNA, and protein biosynthesis.
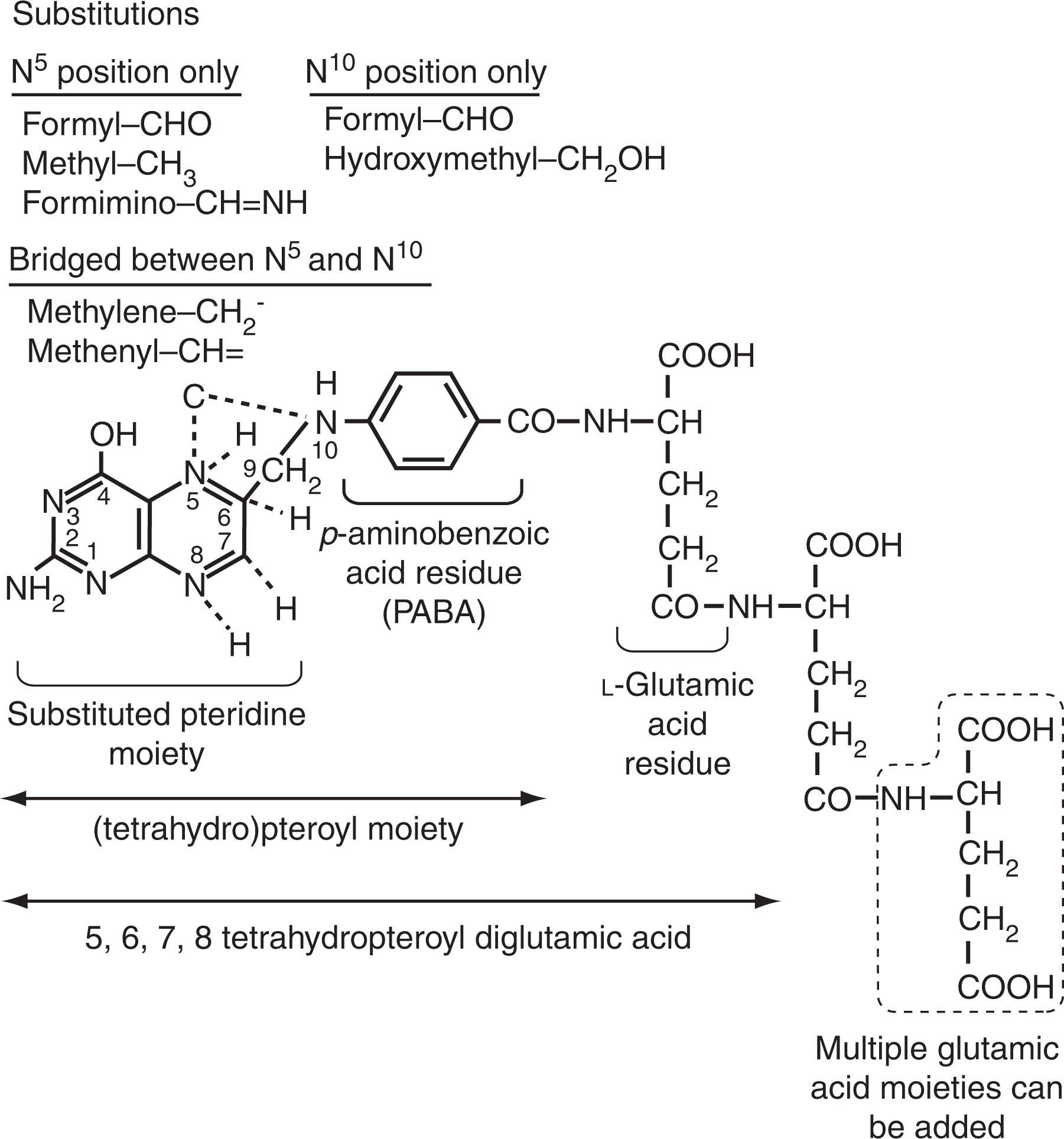
Folates (the anionic forms of folic acid, also called vitamin B 9 ) are synthesized by microorganisms and plants, including leafy vegetables (spinach, lettuce, broccoli), beans, fruits (bananas, melons, lemons), yeast, and mushrooms, and are also found in animal meats ; see Fig. 40.4 for chemistry and nomenclature. Among natural folates, which are predominantly in polyglutamylated form, only one-half are bioavailable; by contrast, 85% of folic acid that is added to food or ingested as a supplement is bioavailable. Several factors can influence the bioavailability of folates. These include: (1) The stability of the food folate. Natural reduced folates are labile and susceptible to oxidative cleavage by nitrates or light exposure, but folic acid is much more stable. Prolonged boiling or cooking over 30 minutes reduces natural folates by 50% to 80%, whereas ascorbate increases bioavailability, and refrigeration of leafy foods exposed to fluorescent light in supermarkets can double the folate content. (2) Pureed foods allow easier access to the glutamate carboxypeptidase II (also known as folate-polyglutamate hydrolase), which converts folate polyglutamates to simpler folate monoglutamates to facilitate absorption ; so perturbation of this enzyme by organic acids (orange juice) or drugs (sulfasalazine, ethanol) can preclude absorption; conversely, high-affinity folate-binding proteins in human or cow’s milk can increase folate absorption for infants and women, respectively. (3) Interference with the proton-coupled folate transporter (PCFT)-mediated folate absorption across the proximal jejunum from either intestinal diseases or drugs (proton-pump inhibitors, sulfasalazine, pyrimethamine) will affect the bioavailability of food folate.
The recommended daily allowance of folate is as follows: adult men and nonpregnant women, 400 μg; pregnant women, 600 μg; lactating women, 500 μg; children 9 to 18 years, between 300 and 400 μg. A balanced Western diet contains adequate amounts of folate, but the net dietary intake of folate in many resource-limited countries is often insufficient to sustain folate balance.
After dietary folate polyglutamates are converted to folate monoglutamates at the enterocyte brush border, they are transported through the duodenal and jejunal brush border by physiological, high-affinity, luminal surface–facing membrane PCFTs, which are most efficient in an acidic milieu. At pH 5.5, there is equivalent affinity for transport of physiologic reduced folates and folic acid, but at pH 6.5, reduced 5-methyl-tetrahydrofolate is transported more efficiently. PCFT is a folate-hydrogen symporter, so with each folate molecule transported, there is a net translocation of positive charge. Loss-of-function mutations in PCFT in the enterocyte and choroid plexus result in hereditary folate malabsorption, where there is an inability to transport folate across the intestine and the choroid plexus. Within the enterocyte, folates are reduced to tetrahydrofolate and then methylated before release into plasma as 5-methyl-tetrahydrofolate. Most of the folic acid taken up by the PCFT in the proximal small intestine is also converted within the enterocyte to 5-methyl-tetrahydrofolate. Folate production by bacteria in the small intestine can also enter the circulation.
The flux of folate from the basolateral membrane of the enterocyte to the portal blood is mediated through MRP3. MRP proteins have low affinity but high capacity and are best viewed as cellular “sump pumps” that eject excess folates (and antifolates) out of cells. Together with MRP2, which mediates folate transport into bile, these MRPs maintain an efficient enterohepatic circulation, which helps retain folate.
Some bacterially produced folates, especially those produced by probiotic bacteria (genus Bifidobacterium ), can be absorbed across the large intestine, sufficient to raise the serum folate levels, but normally this accounts for no more than about 5% of the average folate requirement.
Passive diffusion of folic acid (which is formally referred to as pteroylmonoglutamate [and abbreviated as PteGlu]) is probably the primary mechanism of intestinal mucosal folate absorption at high pharmacologic concentrations. The small intestine has a large capacity to absorb folate, with peak folate levels in plasma achieved 1 to 2 hours after oral administration.
The normal serum folate level is maintained by dietary folate and a substantial enterohepatic circulation that amounts to about 90 μg/day of folate. Biliary drainage results in a dramatic fall in serum folate (to about 30% of basal levels in 6 hours), whereas abrupt interruption of dietary folate leads to a fall in serum folate levels in about 3 weeks. In the plasma, one-third of the folate is free, two-thirds are nonspecifically bound to serum proteins, and a small fraction binds soluble folate receptors. In contrast to cobalamin uptake, there is no specific serum transport protein that enhances cellular folate uptake.
Plasma 5-methyl-tetrahydrofolate and folic acid are rapidly transported into proliferating cells by specialized, high-affinity, glycosyl-phosphatidylinositol-anchored (membrane) folate receptor-α, which takes up these folates at physiologic (nanomolar) concentrations in serum. The plasma membrane containing the folate–folate receptor complex then invaginates and forms an endosomal vesicle that moves into the cytoplasm along microtubules. The perinuclear endosomal compartment then gets acidified to pH 6, which dissociates folate from folate receptors. The released folate then passes across the acidified endosome into the cytoplasm by a transendosomal pH gradient, which is mediated by either the PCFT, which closely co-localized with folate receptors, or a related moiety ( Fig. 40.5 ).
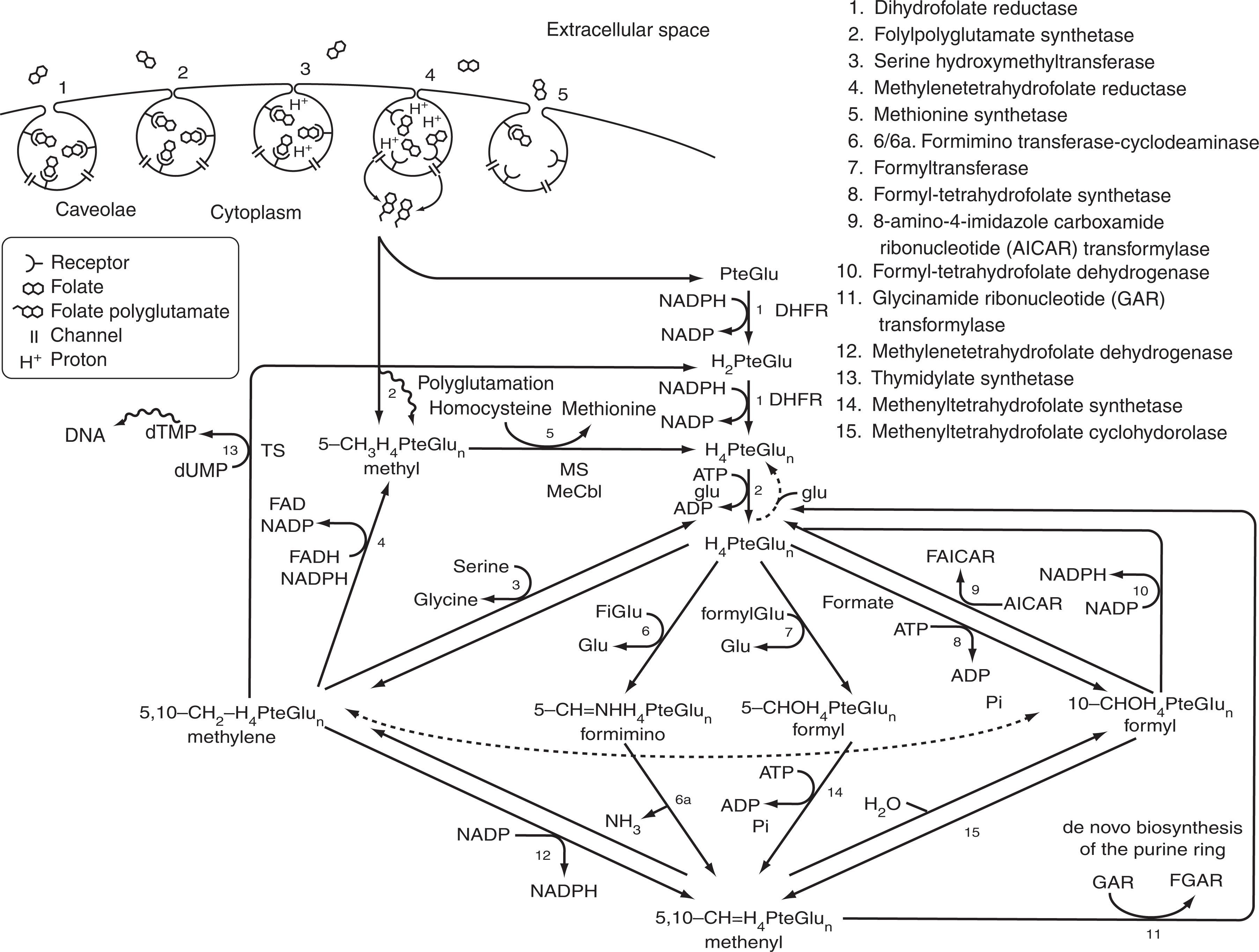
Folate receptor-α is critical to mediating the cellular uptake of folates in proliferating normal and malignant cells, in transport of folate across the placenta to the fetus, across the choroid plexus into the brain, and in renal conservation of folates. Folate receptor-α is expressed in several types of cancer cells, whereas folate receptor-β is expressed most in monocytes and macrophages ; hence these two forms of folate receptors are under investigation for potential clinical use in detecting (and treating) occult malignancy and inflammation, respectively.
The physiologic role of reduced-folate carriers is less clear; it is a low-affinity but high-capacity system that can also mediate the uptake of 5-methyl-tetrahydrofolate and pharmacologic folates (like methotrexate and folinic acid well, but folic acid poorly) into a variety of cells at physiologic pH. Induction of the upregulation of reduced-folate carriers via the blood-brain barrier is being investigated as a means to improve folate transport into the brain when there is either hereditary or acquired perturbation of the physiological components of brain folate transport (folate receptors and PCFT).
Cell surface folate receptor-α is upregulated in response to low extracellular and intracellular folate concentrations through transcriptional, translational, and posttranslational mechanisms. During cellular folate deficiency the accumulation of intracellular homocysteine leads to its covalent binding to a cytoplasmic protein known as heterogeneous nuclear ribonucleoprotein-E1 (hnRNP-E1), which mediates translational upregulation of folate receptor-α. Homocysteinylation of hnRNP-E1 at specific cysteine–cysteine disulfide bonds leads to the unmasking of an underlying messenger RNA (mRNA)-binding pocket for which folate receptor-α mRNA has a high affinity. This RNA-protein interaction then triggers the biosynthesis of folate receptors, which can then bind available folate and normalize cellular folate levels; in turn, this reduces the intracellular homocysteine concentration to baseline. In this context, hnRNP-E1 fulfills criteria as a cellular sensor of physiologic folate deficiency ( Fig. 40.6 ) because this protein is able to sense folate deficiency by interacting with homocysteine—a sensitive indicator of folate deficiency—and then respond by increasing RNA-protein interaction that triggers the biosynthesis and upregulation of folate receptors and restores folate status to normal.
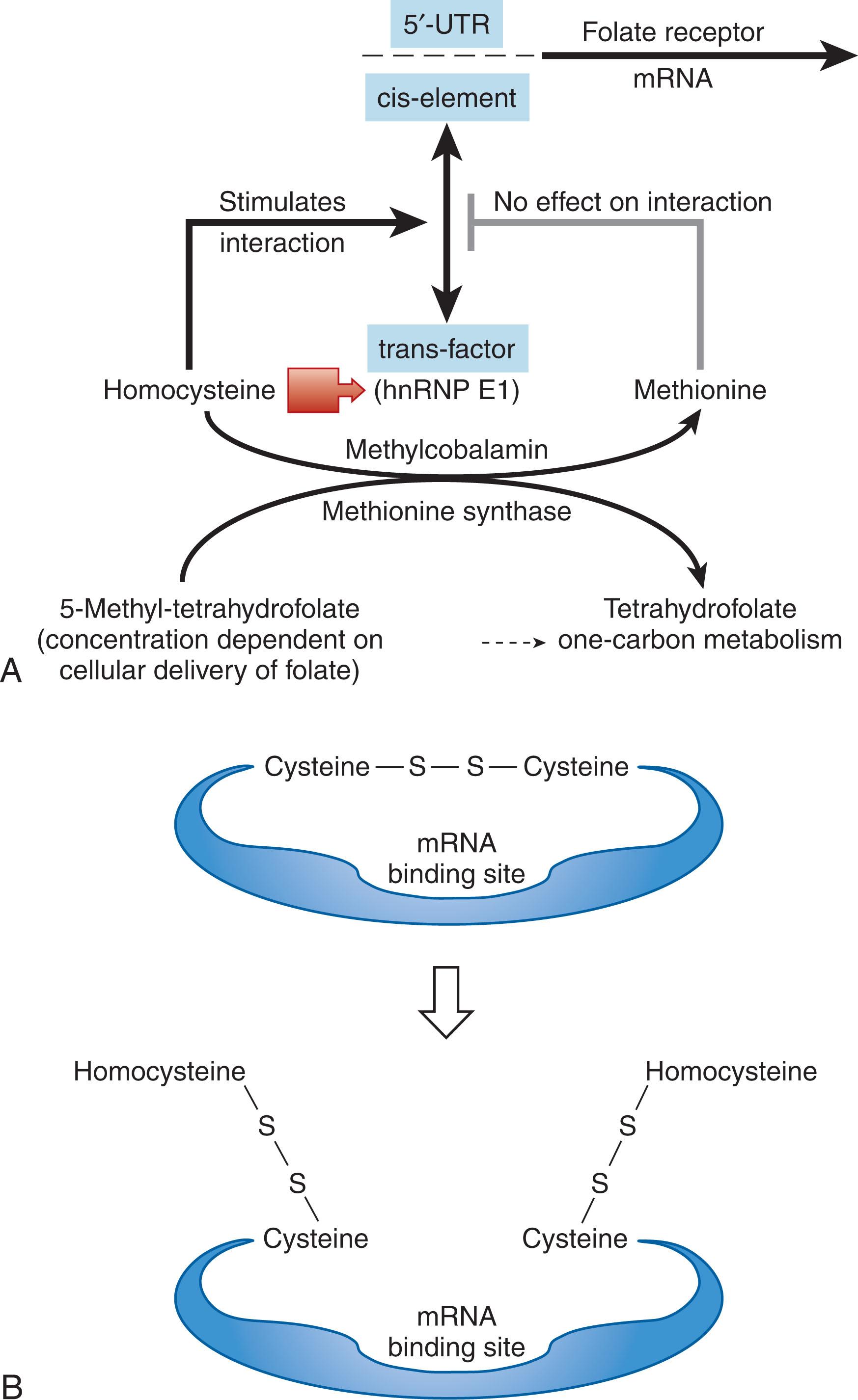
The broader significance of this mechanism is that homocysteinylated hnRNP-E1 actually orchestrates a nutrition-sensitive posttranscriptional RNA operon during folate deficiency . This is because the unmasked mRNA-binding pocket within homocysteinylated-hnRNP-E1, which is highly promiscuous, allows for binding of a variety of very diverse mRNAs (perhaps over 100), all of which have a common password composed of short RNA sequences; these RNA-protein interactions can, in turn, trigger the modulation (up or down) of a variety of several otherwise entirely unrelated proteins that likely contribute to the biologic features of reduced cell proliferation, differentiation, and apoptosis, which are a hallmark of folate deficiency.
In the nucleus, folate receptors can also function as a transcription factor by binding to cis-regulatory elements at promoter regions of Fgfr4 and Hes1 to regulate their expression.
Human placental folate receptors that are abundant and polarized only on the maternal-facing microvillous membrane of the syncytiotrophoblast are critical to transplacental maternal-to-fetal folate transport. Physiologic transplacental folate transport relies on the continued provision of adequate dietary folate intake by the mother. Following capture of maternal folate by placental folate receptors, the displacement of this pool by incoming dietary folates results in an intervillous blood concentration that is three times that of maternal blood. This allows for subsequent transfer of the folate to the fetal circulation along a downhill concentration gradient and ensures continued unidirectional transplacental folate transport. Thus a suboptimum intake of folate by the mother can reduce maternal-to-fetal folate transfer and predispose the embryo/fetus to serious developmental defects.
Because PCFT colocalizes with folate receptor-α, it is suggested that following binding and internalization of folate into low-pH endosomes, the folate dissociates from folate receptors and presumably passes via PCFT into the cytoplasm. However, because both PCFT and reduced-folate carriers are uniformly distributed in microvillous membrane, cytoplasm, and the fetal-facing basal plasma membrane, the following events are all still unclear in human transplacental folate transport to the fetus: the precise handover of folate following endocytosis into the cytoplasm, the potential role of transcytosis of vesicles containing folate, the transport of folate across the syncytiotrophoblast basement membrane into the fetal vasculature, and the role of MRPs.
Folate receptor-α is among the earliest genes activated in embryonic stem cells when there is the need for increased folate requirements to support DNA synthesis during spectacular bursts of intense cell proliferation—ranging as short as 2 to 3 hours during brief windows within the proliferative zone in the epiblast. Thus maternal folate deficiency compromises embryonic and fetal development—and probably even accounts for early miscarriages, as suggested by experimental studies in mice.
Both early-stage neural tube cells and neural crest cells abundantly express folate receptor-α. Experimental perturbation of folate receptor-α can lead to profound abnormalities in neural-tube closure leading to neural-tube defects (NTDs), as well as in heart, facial, and eye development which are collectively referred to as neurocristopathies. These neurocristopathies derive from abnormal proliferation and/or migration and/or differentiation of neural crest cells and result in cleft lip or cleft palate, endocardial cushion defects, and other midline defects. Such studies are consistent with a physiologic role for folate receptor-α and the importance of its functioning normally to prevent the development of NTDs and neurocristopathies. Thus congenital heart defects, comprising approximately 1% of live births, and NTDs, among the most common congenital neurologic birth defects, are both dependent on the fidelity of folate receptor-α expression, precisely at the right time and in the right cells during early cardiac and neurologic development. The finding of a significant increase in blocking autoantibodies against placental folate receptor-α in women with pregnancy complicated by NTDs is a striking human correlate of experimental studies in mice using antibodies, but the causal relationship warrants additional study. Folate receptor-α also provides folate during neuronal regeneration and repair after injury where DNA methylation is also involved.
The pathway of folate receptor–α dependent basolateral-to-apical transcytosis within vesicles across the choroid plexus cells followed by exosome-mediated folate delivery across the cerebrospinal fluid (CSF) into the brain parenchyma has been characterized. Briefly, 5-methyl-THF is bound by glycosyl-phosphatidyl inositol (GPI)-anchored folate receptor-α on the basolateral surface of choroid plexus cells. Following endocytosis there is sequential transfer of folate receptor-α-bound folate from an early- to a later-endosomal compartment called multivesicular bodies. The inward budding of the limiting membrane of multivesicular bodies leads to formation of several intraluminal vesicles that now contain outward-facing folate receptor-α. Following transcytosis of multivesicular bodies (containing their cargo of intraluminal vesicles) and eventual fusion with the apical membrane, there is discharge of these intraluminal vesicles as 40 to 100 nm exosomes into the CSF. These exosomes (containing outward oriented folate receptor-α-bound folates) then cross the ependymal cell layer and enter the brain parenchyma.
Some of the endocytosed folate receptor-α-bound folate is transported out of acidified endosomal compartments via PCFTs into the choroid plexus cell cytoplasm. However, more information on the cooperative function of folate receptor-α and PCFT in folate transport to the human brain is required, particularly because loss of PCFT-mediated function in hereditary folate malabsorption profoundly compromises choroid plexus transport of folates.
After glomerular filtration, luminal folate binds folate receptor-α in the brush border membranes of proximal renal tubular cells and is internalized rapidly by folate receptor-α–mediated endocytosis; in the low pH of endocytotic vesicles, there is dissociation of folates and slow transport across basolateral membranes into the blood, with recycling of apo-folate receptor-α back to the luminal brush border membrane. A large 550-kDa membrane protein called megalin, which interacts with CUBN and is found in renal proximal epithelial cells, functions as a multiligand receptor for a variety of macromolecules. Megalin also binds to and mediates endocytosis of TCII-cobalamin complexes as well as filtered folate bound to soluble folate-binding proteins in kidney proximal tubules.
Polyglutamylation of folate by the enzyme folylpolyglutamate synthase catalyzes the addition of multiple glutamate equivalents to their γ-carboxyl residue (see Fig. 40.4 and 40.5 ). In most eukaryotic cells, the pentaglutamate and hexaglutamate forms predominate. Polyglutamylation retains folates within cells; in addition, polyglutamylated folates are more efficient substrates for folate-dependent enzymes. In human erythrocytes (red blood cells [RBCs]), folate is accumulated at earlier stages within the marrow by folate receptors ; on maturation, more than 90% of H 4 PteGlu (n) molecules interact with hemoglobin, which, because of its high capacity, assists in intracellular folate retention.
In an elegant example of conservation of resources, metabolic pathways involving folate are compartmentalized within cells as multienzyme complexes that shuttle one-carbon units along set paths toward key reactions leading to pyrimidine and purine biosynthesis. The major form of folate transported into the cell is 5-methyl-tetrahydrofolate (5-methyl-THF; 5-methy-H 4 PteGlu) (see Fig. 40.5 ); by contrast, folic acid (pteroylmonoglutamate (PteGlu) requires reduction to tetrahydrofolate by dihydrofolate reductase in a two-step reaction (PteGlu to dihydro pteroylglutamate [H 2 PteGlu] to tetrahydro pteroylglutamate [H 4 PteGlu, or THF]). After cellular uptake, 5-methyl-THF must first be converted to THF via methionine synthase (in the methylation cycle). This is a key reaction because THF is the preferred physiologic substrate for folylpolyglutamate synthase, which adds multiple glutamate moieties to THF (see Fig. 40.4 ). Only then can the polyglutamylated form of THF participate in one-carbon metabolism where it can be converted to either 10-formyl-THF—used in de novo biosynthesis of purines—or to 5,10-methylene-THF—used for synthesis of thymidylate. Moreover, 5,10-methylene-THF and 10-formyl-THF can be interconverted by intermediates (see Fig. 40.5 ).
Folate metabolism and folate-dependent enzymes are compartmentalized: approximately 40% are in the mitochondrial matrix, 50% in the cytoplasm, and 10% in the nucleus. The methylation reaction occurs in the cytosol whereas thymidylate synthesis occurs in the nucleus. The mitochondrial compartment, which is not shown in Fig. 40.5 , contains its complement of folate cofactors, and homologues of the major cytosolic enzymes. For example, cytoplasmic 5-methyl-THF and 5-formyl-THF can enter mitochondria by a mitochondria-specific reduced-folate carrier, whereas SAM, which is also required for mitochondrial methylation reactions, enters mitochondria by a specific transporter. Other one-carbon donors like serine, glycine, dimethylglycine, and sarcosine also enter mitochondria and ultimately generate formate that crosses back into the cytoplasm. In the cytoplasm, C1-THF synthase, a trifunctional enzyme, uses this mitochondria-derived formate with THF to form 10-formyl-THF, which is required for the de novo synthesis of purines (see Fig. 40.5 ); this enzyme can also catalyze the interconversion of THF, 10-formyl-THF, 5,10-methenyl-THF, and 5,10-methylene-THF. In this way, the continued delivery of mitochondrial formate helps perpetuate cytoplasmic one-carbon metabolism. Another major entry point of one-carbon units into cytoplasmic folate metabolism is through the formation of 5,10-methylene-THF from serine (which is derived from glycolytic intermediates); here the enzyme serine hydroxymethyltransferase catalyzes the addition of carbon 3 from serine to THF to give rise to the key intermediate coenzyme 5,10-methylene-THF. After 5,10-methylene-THF is converted to 5-methyl-THF by the enzyme methylenetetrahydrofolate reductase, it can be used in the methylation cycle, which involves methylation of homocysteine via methionine synthase to form methionine and tetrahydrofolate. After 5,10-methylene-THF is converted (via intermediates) to 10-formyl-THF, it can be used for purine nucleotide biosynthesis involving de novo synthesis of purine nucleotides for DNA and RNA. 5,10-Methylene-THF can also be used in the thymidylate cycle via the enzyme thymidylate synthase, which generates thymidylate (by converting deoxyuridine monophosphate [dUMP] to deoxythymidine monophosphate [dTMP]) for DNA synthesis (see Fig. 40.5 ). Parenthetically, this nuclear one-carbon metabolism compartment is activated during S phase of the cell cycle, following a posttranslational modification of folate-dependent enzymes like thymidylate synthase, serine-hydroxymethyltransferase, and dihydrofolate reductase by specialized small ubiquitin-like modifier (or SUMO) proteins; this modification allows the entry and enrichment of these sumoylated enzymes into the nucleus to provide a buffer against the stress of imminent folate deficiency.
After methionine is generated, it can be converted to a methyl donor through its adenosylation to SAM. SAM is a universal donor of methyl groups for critically important biologic methylation reactions involving over 80 proteins, membrane phospholipids, the synthesis of neurotransmitters, RNA, DNA, and histones. Among these, DNA methylation is a major epigenetic mechanism that is central to the regulation of several cellular functions such as gene transcription, chromatin structure, imprinting, development, and genomic instability. DNA is highly methylated in CpG sequences (over 50%); here a methyl group is targeted to the DNA base cytosine in the context of a CpG dinucleotide by DNA methyltransferases. Methylation confers a condensed structure and transcriptional repression, whereas hypomethylation does the opposite. Altered patterns of DNA methylation, particularly hypomethylation involving growth-promoting genes, or hypermethylation of tumor suppressor genes, are popular contemporary themes in our understanding of the epigenetic changes in DNA and the genesis of cancer. In addition, histone hypomethylation can alter gene expression. After these transmethylation reactions that use SAM, the immediate product of these reactions is S -adenosylhomocysteine (SAH), which is converted to homocysteine by SAH hydrolase. The SAM to SAH ratio regulates the balance of such cellular methylation reactions.
Dietary folate deficiency can lead to hypomethylation in animals, whereas folate consumption supports normal patterns of methylation. However, analysis in humans has not yielded consistent results, nor has the correlation of folate status and DNA methylation been sufficiently rigorously studied.
Because of the critical role of methylcobalamin for methionine synthase, a deficiency of cobalamin inactivates methionine synthase and results in the accumulation of the substrate 5-methyl-THF; this so-called methyl-folate trap results because the upstream enzyme reaction involving methylenetetrahydrofolate reductase (which converts 5,10-methylene THF to 5-methyl-THF in preparation for the methionine synthase reaction) is also irreversible. Therefore when 5-methyl-THF accumulates and cannot be converted to THF, it readily leaks out of the cell. (This explains why patients with cobalamin deficiency can have normal to high serum folate values even in the face of tissue folate deficiency). The ensuing intracellular THF deficiency compromises one-carbon metabolism and initiates the pathophysiologic cascade leading to perturbed DNA synthesis and megaloblastosis.
With either cobalamin or folate deficiency, there will be a net decrease in 5,10-methylene-THF that interrupts the thymidylate synthase–mediated conversion of dUMP to dTMP. (Although salvage pathways for purine synthesis can compensate for reduced generation of purines through one-carbon metabolism, salvage pathways cannot compensate for reduced thymidine.) This results in a high dUMP/dTMP ratio and an increase in deoxyuridine triphosphate (dUTP), which can get misincorporated into DNA. At this juncture, an editorial enzyme recognizes this faulty misincorporation and excises dUTP. However, with a continued inadequate supply of deoxythymidine triphosphate (dTTP) there is a continued cycle of uracil misincorporation into DNA in folate deficiency, its removal by uracil-DNA glycosylase, and refilling of the missing base by DNA polymerase β. However, with repetition over several cycles, multiple single-strand nicks are introduced into DNA; this predisposes to chromosome breaks that can contribute to an increased risk of cancer associated with folate deficiency. In addition, folate deficiency can also lead to double-strand breaks in DNA, which are difficult to repair when the two nicks are close to one another (within 12 bp of each other) on opposite strands. Collectively, such double-strand DNA breaks in folate-deficient cells predispose to the development of acentric chromosomes, DNA fragments, and micronuclei. This can also render folate-deficient epithelial tissues more permissive to the genomic integration of HPV16 DNA, and trigger HPV16-induced cancer.
Defective DNA synthesis caused by folate deficiency is reflected by numerous chromosomal abnormalities, including abnormalities in telomeres, which correlate with biomarkers of chromosomal instability and mitotic dysfunction. There is excessive chromosomal elongation with despiralization associated with random breaks and exaggerated centromere constriction, expression of folate-sensitive fragile sites in hematopoietic cells, and reduced biosynthesis, acetylation, and methylation of arginine-rich histone. All this leads to perturbation of the cell cycle with an increased proportion of cells in prophase of the mitotic cycle and G 2 that leads to apoptosis of erythroid precursors and anemia.
There is widening disparity in nuclear-cytoplasmic asynchrony as a cobalamin- or folate-deficient cell divides, until the more mature generations of daughter cells die in the marrow or are arrested (as megaloblastic cells) at various stages of the cell cycle. All proliferating cells exhibit megaloblastosis, including the luminal epithelial mucosal cells of the entire gastrointestinal tract, cervix, vagina, and uterus. However, megaloblastic changes are most striking in the blood and bone marrow. Ineffective hematopoiesis extends into long bones, and the bone marrow aspirate—which is superior to the biopsy for observing megaloblastosis—exhibits trilineal hypercellularity, especially of the erythroid series. Indeed, the plethora of bone marrow morphologic changes can lead an untrained observer to the diagnosis of erythroleukemia. The appearance of exuberant cell proliferation with numerous mitotic figures is misleading because these cells are actually proliferating very slowly (see box on Morphology in Megaloblastosis From Cobalamin and Folate Deficiency).
Erythroid hyperplasia reduces the myeloid-to-erythroid ratio from 3:1 to 1:1. Proerythroblasts are not as obviously abnormal as later forms; they may simply be larger (promegaloblasts). Megaloblastic changes are most strikingly displayed in intermediate and orthochromatic stages, which are larger than their normoblastic counterparts. In contrast to the normally dense chromatin of comparable normoblasts, megaloblastic erythroid precursors have an open, finely stippled, reticular, and sieve-like pattern ( Fig. 40.7 ). The orthochromatic megaloblast, with its hemoglobinized cytoplasm, continues to retain its large sieve-like immature nucleus, in sharp contrast to the clumped chromatin of orthochromatic normoblasts. The nucleus is often eccentrically placed in these large oval or oblong cells, and lobulation or indentation of nuclei with bizarre karyorrhexis is often seen. In cells destined for the circulation as macro-ovalocytes, the nucleus may occasionally not be completely extruded. Of the potential progeny of proerythroblasts that develop into later megaloblastic forms, 80% to 90% die in the bone marrow. Marrow macrophages effectively scavenge dead or partially disintegrated megaloblasts. This is the basis for ineffective erythropoiesis (intramedullary hemolysis).
Increased mean corpuscular volume (MCV) with macro-ovalocytes (up to 14 μm), which is variously associated with anisocytosis and poikilocytosis
Nuclear hypersegmentation of polymorphonuclear neutrophils (PMNs) (1% PMN with six lobes or 5% with five lobes)
Thrombocytopenia (mild to moderate)
Leukoerythroblastic morphology (from extramedullary hematopoiesis)
General increase in cellularity of all three major hematopoietic elements
Abnormal erythropoiesis—orthochromatic megaloblasts
Abnormal leukopoiesis—giant metamyelocytes and “band” forms (pathognomonic), hypersegmented PMNs
Abnormal megakaryocytopoiesis—pseudo hyperdiploidy
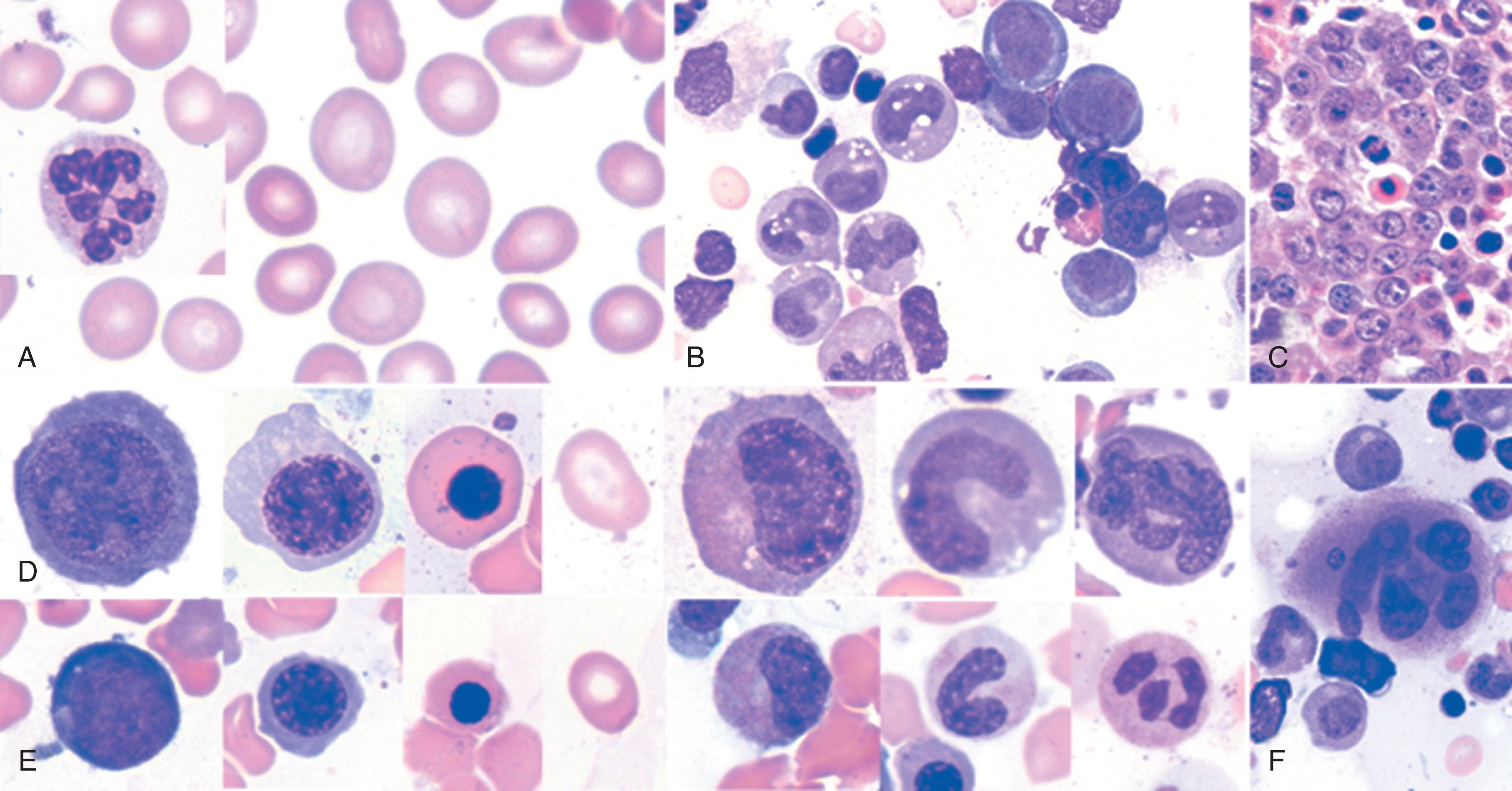
Leukopoiesis is also abnormal. There is an absolute increase in these cells, which are large and have similar sieve-like chromatin. Spectacular giant (20 to 30 μm) metamyelocytes and “band” forms that are often seen are pathognomonic for megaloblastosis (see Fig. 40.7 ). There may be bizarre nucleoli with small cytoplasmic vacuoles. It is probable that giant metamyelocytes cannot easily traverse marrow sinuses, and their maturation into circulating hypersegmented polymorphonuclear neutrophils (PMNs) is unlikely. Granulation of the cytoplasm remains unaffected.
Megakaryocytes may be normal or increased in numbers and may exhibit additional complexities in megaloblastic expression (see Fig. 40.7 ). Complex hypersegmentation (i.e., pseudohyperdiploidy) is associated with liberation of fragments of cytoplasm and giant platelets into the circulation. The net output of platelets is decreased in severe megaloblastosis, and abnormal but reversible platelet dysfunction has been documented.
In early cobalamin or folate deficiency, normoblasts may dominate the marrow with only a few megaloblasts seen. Complete transformation to megaloblastic hematopoiesis is observed in florid cases and is reflected by various degrees of pancytopenia.
The earliest manifestation of megaloblastosis is an increase in mean corpuscular volume (MCV) with macro-ovalocytes (up to 14 μm) (see Fig. 40.7 ). Because these cells have adequate hemoglobin, the central pallor, which normally occupies about one-third of the cell, is decreased. By contrast, thin macrocytes have larger than normal central pallor ( Table 40.1 ). In severe anemia, poikilocytosis and anisocytosis are evident. Cells containing remnants of DNA (i.e., Howell-Jolly bodies), arginine-rich histone, and nonhemoglobin iron (i.e., Cabot rings) may be observed. Extramedullary megaloblastic hematopoiesis may also result in a leukoerythroblastic picture.
| Macrocytosis a Without Megaloblastosis b |
|
| Spurious Increases in MCV Without Macro-Ovalocytosis c |
|
a The central pallor that normally occupies about one-third of the normal red blood cell is decreased in macro-ovalocytes. This contrasts with the finding of thin macrocytes, in which the central pallor is increased.
b Although megaloblastosis implies that a bone marrow test has been performed, with the addition of highly sensitive tests for the specific diagnosis of cobalamin and folate deficiency, the need for a bone marrow test is often dictated by the urgency to make the diagnosis.
c When the Coulter counter readings of a high MCV are not confirmed by looking at the peripheral smear.
Nuclear hypersegmentation of DNA in PMNs strongly suggests megaloblastosis when associated with macro-ovalocytosis (see Fig. 40.7 ). Normally fewer than 5% of PMNs have more than five lobes, and no cells have more than six lobes in the peripheral blood; megaloblastosis is suggested when greater than 5% PMNs have more than five lobes or 1% PMN have more than six lobes. (See box on Diagnostic Issues: Information From the Peripheral Smear and Bone Marrow Aspirate.)
Ineffective use of iron results in an increased percentage of saturation of transferrin and increased iron stores. If there is associated iron deficiency, the MCV may be normal, and only iron therapy can unmask the megaloblastic manifestations in the peripheral blood. In thalassemia, the entire erythrocyte morphology normally expected in megaloblastosis is masked ; however, megaloblastic leukopoiesis is still observed. Significant intramedullary hemolysis (ineffective erythropoiesis) involving more than 90% of megaloblastic precursors is reflected by a lowered absolute reticulocyte count, increased bilirubin (up to 2 mg/dL), decreased haptoglobin, and increased lactate dehydrogenase (LDH) often above 1000 units/mL. There is also a modest decrease in the circulating RBC life span. (See box on Masked Megaloblastosis.)
Megaloblastosis in rapidly proliferating cells of the gastrointestinal tract leads to a variable degree of morphologic changes and atrophy of luminal epithelial cells. This leads to functional defects, which can include malabsorption of cobalamin and folate in some patients. A vicious cycle whereby megaloblastosis begets more megaloblastosis is established that can be interrupted only by specific therapy with cobalamin or folate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here