Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
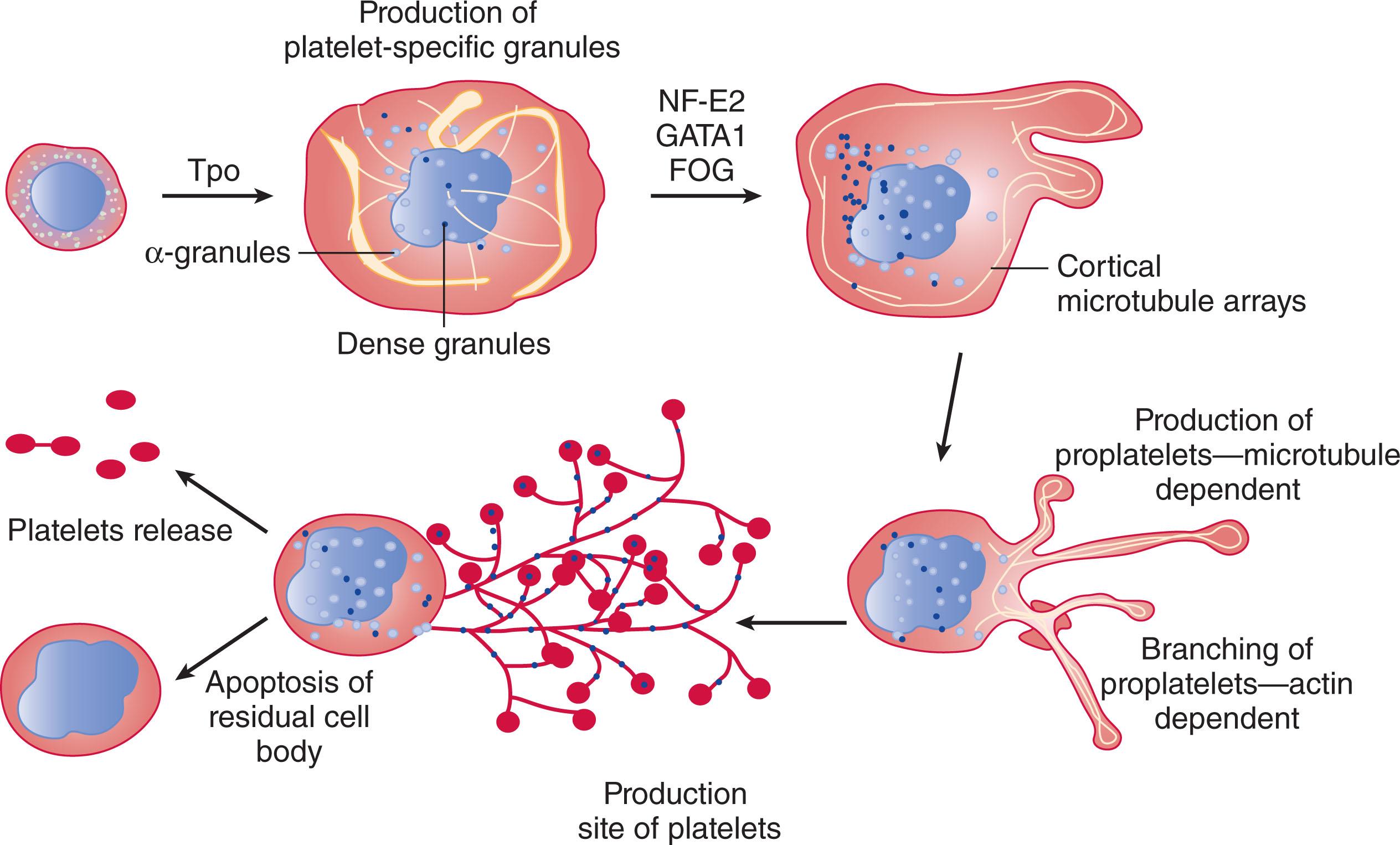
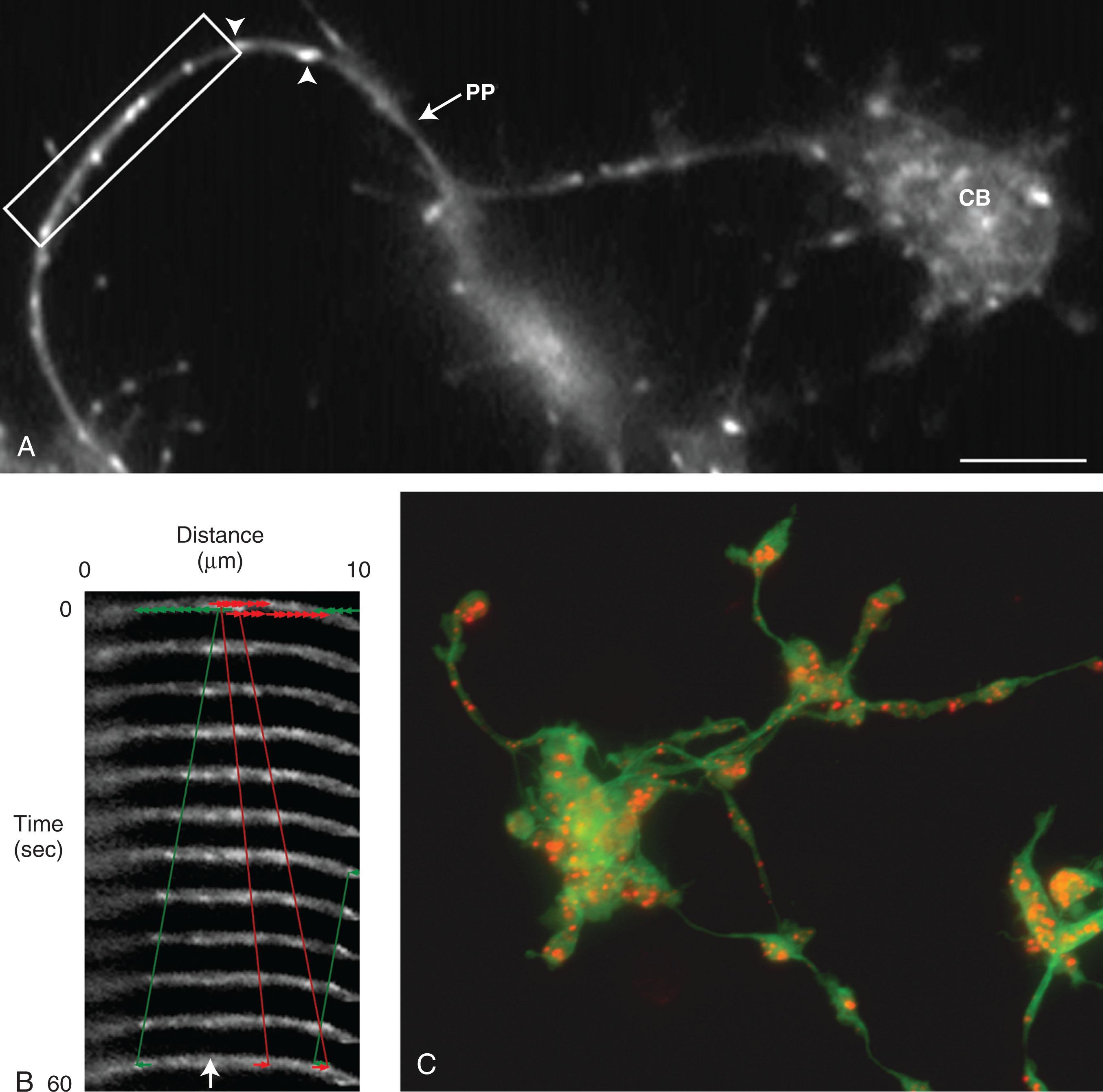
Platelets are small anucleate fragments that are formed from the cytoplasm of megakaryocytes and have a characteristic discoid shape. To assemble and release platelets, megakaryocytes become polyploid by endomitosis and follow a maturation program that results in the conversion of the bulk of their cytoplasm into multiple long processes called proplatelets . To produce its quota of 1000 to 2000 platelets, a megakaryocyte may release as many as 10 to 20 proplatelets. Platelets then form selectively at the ends of proplatelets. As platelets develop, their content of granules and organelles is delivered to them in a stream of individual particles moving from the megakaryocyte cell body to the nascent platelet buds at the proplatelet tips. Platelet formation can be arbitrarily divided into two phases. The first phase of megakaryocyte differentiation and maturation takes days to complete and requires megakaryocyte-specific growth factors such as thrombopoietin (TPO). Massive nuclear proliferation without cell division (endomitosis) to 16 to 32 N and enlargement of the megakaryocyte cytoplasm occur because the megakaryocyte is filled with cytoskeletal proteins, platelet-specific granules, and sufficient membrane to complete the platelet assembly process. The second phase is relatively rapid and can be completed in hours. During this phase, megakaryocytes generate platelets by remodeling their cytoplasm first into proplatelets, then preplatelets, which are released into the circulation, where they undergo repeated fission to generate discoid platelets.
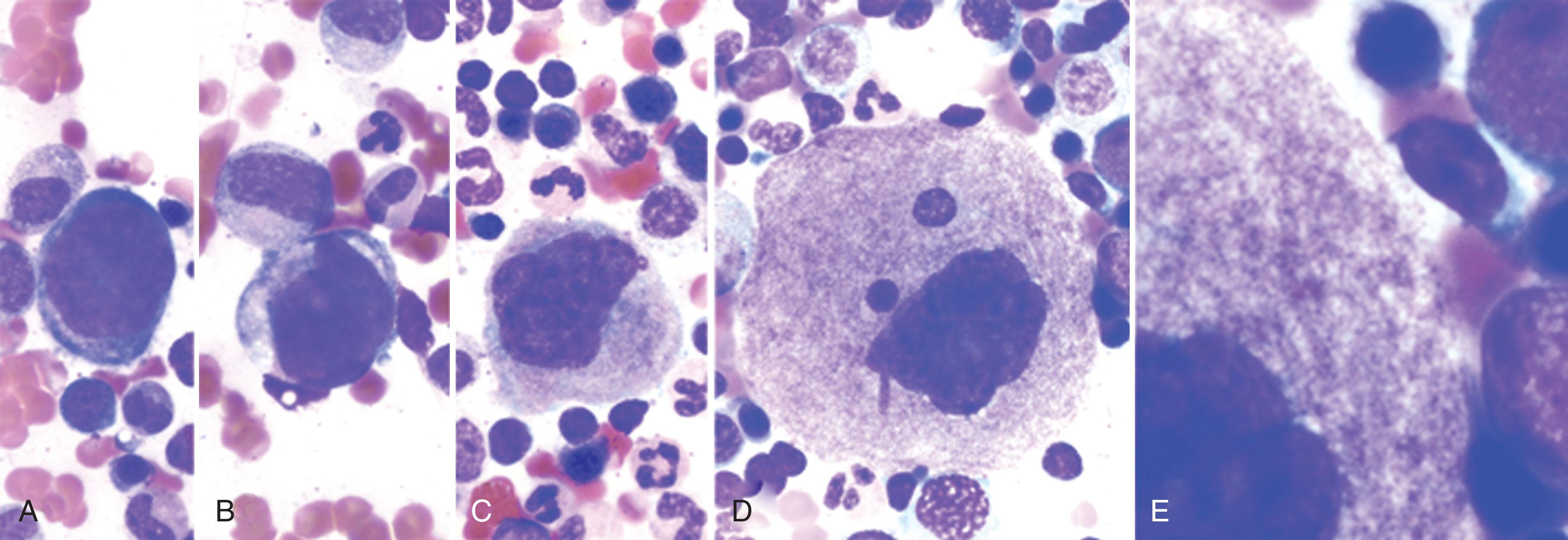
Hematopoietic stem cells, which are endowed with the genetic capacity to differentiate into all blood cell lineages, are induced down the pathway to become megakaryocytes by their exposure to growth factors such as TPO. Megakaryocytes become polyploid (i.e., 4 N, 16 N, 32 N, 64 N) through repeated cycles of deoxyribonucleic acid (DNA) replication without cell division. Normal ploidy ranges from 4 to 64 times the haploid DNA complement, but the majority of cells fall within three ploidy classes (8 N, 16 N, and 32 N), with 16 N being dominant ( Fig. 123.1 ). Ploidy number appears to be a predetermined event, possibly signifying genetic diversity among megakaryocyte populations. Megakaryocyte polyploidization results in a functional gene amplification whose likely function is an increase in protein synthesis. This process, called endomitosis , is a shortened mitosis caused by a block in late anaphase, thereby bypassing cytokinesis.
Cells undergoing diploid mitoses proceed through cytokinesis and complete abscission division, whereas megakaryocytes exhibit regression of the cleavage furrow and reenter G 1 as polyploid cells. During polyploidization of megakaryocytes, the nuclear envelope breaks down, and an abnormal spherical mitotic spindle forms. The spindle has attached chromosomes that align from a position equidistant from the spindle poles. Sister chromatids segregate and move toward their respective poles (anaphase A). However, the spindle poles fail to move apart and do not undergo the microtubule-driven separation typically observed during anaphase B. Individual chromatids are not moved to the poles, and subsequently a single nuclear envelope encapsulates the entire set of sister chromatids. In most cell types, checkpoints and feedback controls ensure that DNA replication and cell division are tightly coupled. Megakaryocytes appear to be an exception to this rule, indicating they have managed to deregulate this process. Proposed mechanisms for regulating endomitosis include a reduction in mitosis-promoting factor or decreased expression of cyclin B. Cyclins appear to play a critical role in directing endomitosis. Cyclin D3 is overexpressed in the G 1 phase of maturing megakaryocytes, but triple knockout of cyclins D1, D2, and D3 in mice does not appear to affect megakaryocyte development. In contrast, cyclin E–deficient mice exhibit a profound defect in megakaryocyte development. The molecular programming involved in endomitosis is characterized by the mislocalization or absence of at least two critical regulators of mitosis: the chromosomal passenger proteins Aurora-B/AIM-1 and Survivin. AIM-1 (absent in melanoma 1), a serine/threonine kinase in the Aurora family that is implicated in mitosis, is downregulated as megakaryocyte polyploidization occurs, suggesting its loss may lead to the abortive mitosis and polyploidization. However, while Aurora kinase A is required for hematopoiesis, it is dispensable for mouse megakaryocyte endomitosis and differentiation. Whereas deletion of the anaphase promoting complex (APC)/C cofactor Cdc20 causes mitotic arrest and severe thrombocytopenia, lack of the kinases Aurora-B, Cdk1, or Cdk2 does not affect endomitosis of megakaryocytes or platelet counts. Deletion of Cdk1 forces a change to endocycles without mitosis, whereas polyploidization in the absence of cdk1 and cdk2 occurs in the presence of aberrant replication events. Notably, ablation of these kinases rescues defects in Cdc20-null megakaryocytes. These observations suggest that endomitosis can be functionally replaced by alternative polyploidization mechanisms in vivo. One explanation for endomitosis could be inhibition of microtubule-based forces in anaphase B. Spindle pole separation during anaphase B is thought to be powered by the sliding of antiparallel interdigitating microtubules past each other mediated by the mitotic kinesin-like protein 1. This protein localizes at regions of overlapping microtubules during anaphase B, and studies in vitro indicate that it can slide microtubules past each other. Therefore lack of spindle pole separation during endomitosis may result from failure of megakaryocytes to undergo normal spindle orientation and/or the absence of signals that localize or activate a kinesin motor molecule that provides force for sliding.
Megakaryocytes, the largest of the hematopoietic cells, undergo a pronounced cytoplasmic maturation to attain their large volumes. Cytoplasmic maturation begins during endomitosis and increases considerably after all DNA amplification has ended ( Fig. 123.2 ). Megakaryocytes enlarge dramatically as they mature, reaching sizes of 100 to 150 μm in diameter in culture and in bone marrow. During this process, the megakaryocyte cytoplasm rapidly fills with platelet-specific proteins, organelles, and membrane systems that ultimately are subdivided and packaged into platelets ( Fig. 123.3 ). Their cytoplasmic space expands and, except for the most cortical regions, becomes densely filled with internal membranes that subsequently serve as the repository for the plasma membrane to be regurgitated for coating proplatelets as they extend. This internal membrane system, which is one of the most striking features of mature megakaryocytes, has been referred to as the demarcation membrane system (DMS) or, more recently, the invaginated membrane system (IMS). The DMS, first described by Yamada in 1957, consists of an extensive, tortuous, branching network of membrane channels composed of flattened cisternae and tubules. Initially, the DMS was proposed to play an essential role in platelet formation by defining preformed “platelet territories” or “platelet fields” within the megakaryocyte cytoplasm. Release of individual platelets was postulated to occur by massive fragmentation of the megakaryocyte cytoplasm along DMS fracture lines between these fields. However, studies demonstrating that platelets are primarily assembled and released from proplatelet ends (see Platelet Formation later in this chapter) are inconsistent with this notion and indicate instead that the DMS functions predominantly as a membrane reserve for proplatelet formation. Direct visualization of mature DMSs containing phosphatidylinositol 4,5-bisphosphate suggests that it is the source of proplatelet membranes. Studies by Eckly et al. have begun to provide insights into how the DMS forms and matures. To develop the DMS, the megakaryocyte plasma membrane enfolds at specific sites to generate a perinuclear pre-DMS. Next, the pre-DMS is expanded into its mature form by material added from Golgi-derived vesicles and by endoplasmic reticulum–mediated lipid transfer. This structural description is in line with studies on platelet glycosyltransferases, which arrive early in the forming DMS and eventually make their way to the megakaryocyte and platelet surface. Thus far, only a handful of proteins that participate in DMS formation have been identified based on alteration in DMS structure in certain knockout mouse models. Membrane-deforming proteins that use F-BAR (Family of Bin-Amphysin-Rvs) domains to curve membranes or use guanosine triphosphate (GTP) as an energy source to bud vesicles from membranes appear to be necessary for normal megakaryocyte maturation and platelet release. Gross disruptions in DMS structure are found in megakaryocytes isolated from either filamin A–knockout, pascin2-knockout, dynamin 2–knockout, or Cdc42-interacting protein 4 (CIP4)-knockout mice. CIP4 is an F-BAR protein that induces membrane tubulation and localizes to membrane lipids via its BAR domain and interacts with the Wiskott-Aldrich syndrome protein (WASp). CIP4 −/− mice have mild thrombocytopenia, with a 25% decrease in the platelet count. While megakaryocyte numbers and ploidy are normal in CIP4 −/− knockout mice, megakaryocytes isolated from these mice are less effective in producing proplatelets in vitro.
Dynamins are highly conserved, large mechanochemical GTPases involved in endocytosis and vesicle transport, and mutations in dynamin 2 have been associated with thrombocytopenia in humans. Dynamin 2–dependent endocytosis is required for megakaryocyte development in mice. Filamin connects glycoprotein (GP)Ibα to the actin cytoskeleton and binds pacsin2, a molecule that deforms membranes. Pacsin2 has an F-BAR and SH3 domain that binds dynamin and N-WASp. Pacsin2 plays an important role in organizing internal membranes during megakaryocyte development and platelet production.
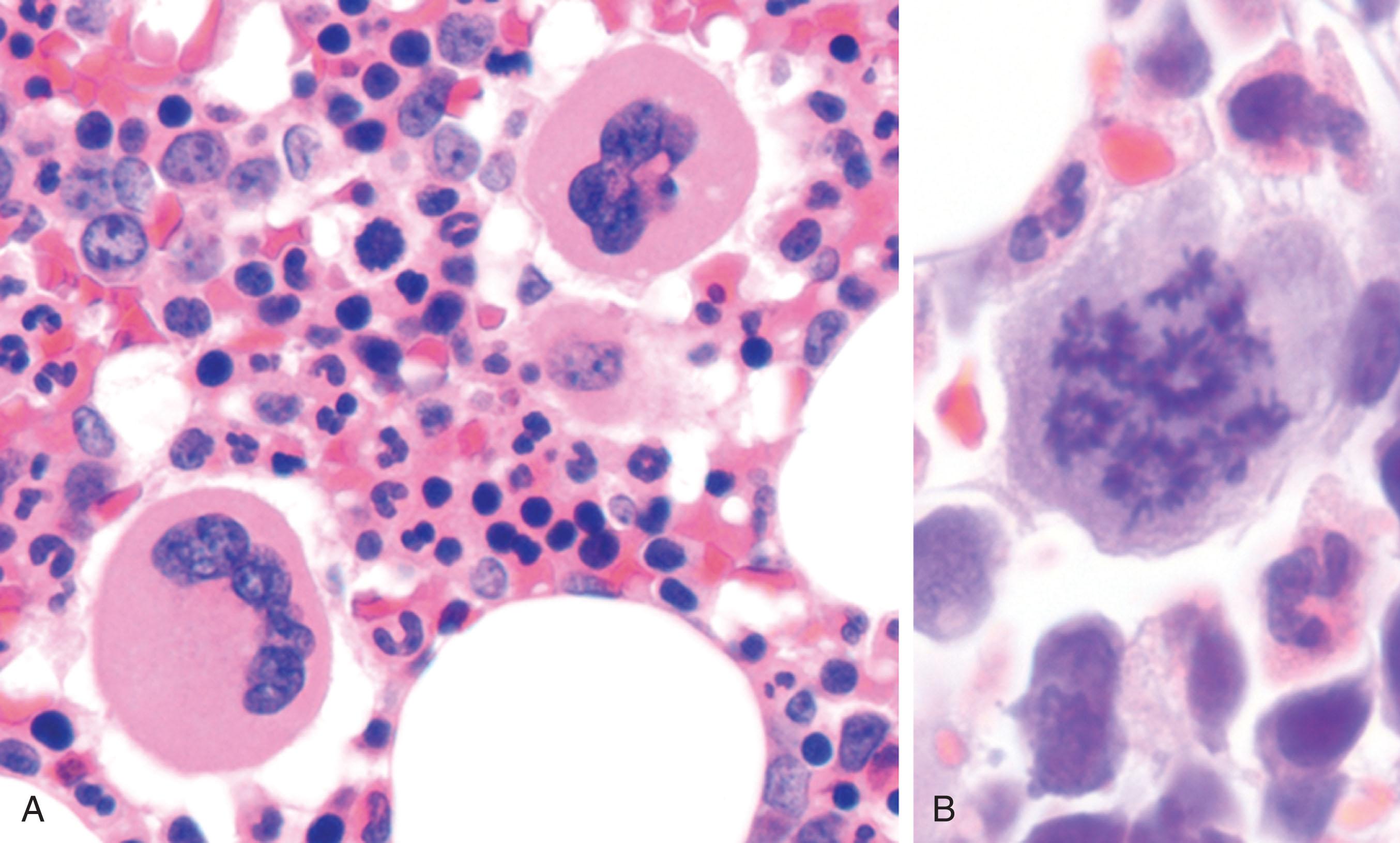
Maturing megakaryocytes, like other granulated cells, contain an abundance of ribosomes and rough endoplasmic reticulum, where protein synthesis occurs. During this phase of megakaryocyte development, the cytoplasm fills with cytoskeletal proteins, platelet-specific receptors and secretory granules, and normal cellular organelles such as mitochondria and lysosomes. A hallmark of mature megakaryocytes is their abundance of platelet-specific secretory granules. The two specific granules destined for platelets are α-granules and dense granules. α-Granules, the more abundant (approximately 40 to 80 per platelet) and larger of the two (200 to 500 nm in diameter), contain proteins that enhance platelet adhesion, promote cell-cell interactions, regulate angiogenesis, and stimulate vascular repair. α-Granules store matrix proteins and contain GP receptors in their membranes ( Fig. 123.4A ). The bulk of cellular P-selectin and a portion of α IIb β 3 and the GPIb/IX/V complex, a receptor for von Willebrand factor (vWF), are expressed in the membranes of α-granules. Adhesion molecules within the granules include, but are not limited to, vWF, fibrinogen, fibronectin, vitronectin, and thrombospondin. α-Granule proteins can be derived from different sources. Some proteins, such as α-thromboglobulin and vWF, are synthesized by megakaryocytes. However, fibrinogen, also a major component of α-granules, is not synthesized by megakaryocytes and is instead taken up from plasma by an endocytic mechanism requiring fibrinogen binding to α IIb β 3 . Although little is known about the intracellular trafficking of proteins in megakaryocytes, experiments using cryosectioning and immunoelectron microscopy suggest that multivesicular bodies are an essential intermediate stage in the formation of platelet α-granules. During megakaryocyte development, large (≈0.5 μm) multivesicular bodies undergo a gradual transition from granules containing 30 to 70 nm internal vesicles to granules containing secretion concentrates.
The second and smaller type of platelet granule is the dense granule. Platelets contain relatively few dense granules (approximately 4 to 6 per platelet), which are approximately 150 nm in diameter. Dense granules have electron opaque cores, which give them a bull’s-eye appearance, and function primarily to recruit additional platelets to sites of vascular injury. Dense granules contain soluble activating agents, such as serotonin and adenosine diphosphate (ADP), as well as divalent cations. A novel electron-dense tubular system–related compartment called the T-granule has been described in platelets. The T-granules contain Toll-like receptor 9 (TLR9), which links the innate and adaptive immune response during infectious inflammation. TLR9 colocalizes with protein disulfide isomerase, a vascular thiol isomerase, and is associated with either vesicle-associated membrane protein (VAMP) 7 or VAMP 8. T-granules are believed to be a remnant of the endoplasmic reticulum and are derived from the dense tubular system.
When the megakaryocyte reaches a certain point of maturation, proplatelet production begins, and granules are sent into the proplatelets, destined for platelets.
The development of megakaryocytes and the process of platelet biogenesis occur within a complex bone marrow environment where both cytokines and adhesive interactions play an essential role. Megakaryocytes are imprisoned within the subendothelial layer of the bone marrow sinuses, where development and platelet biogenesis are regulated at multiple levels by several cytokines. TPO, which is synthesized in the bone marrow and liver, is the principal regulator of thrombopoiesis. TPO also plays a central role in hematopoietic stem cell survival and proliferation. Circulating levels of TPO induce proliferation and maturation of megakaryocyte progenitors by binding to the c-Mpl (myeloproliferative leukemia protein) receptor and signaling induction. TPO regulates all stages of megakaryocyte development, from the hematopoietic stem cell stage through cytoplasmic maturation. TPO increases platelet production by increasing both the number and size of individual megakaryocytes. However, it is important to note that TPO plays no role in triggering megakaryocytes to make proplatelets or to release platelets. c-Mpl activation is regulated by a complex array of signaling molecules that turn on specific transcription factors (see Transcriptional Regulation of Platelet Formation later in this chapter) to drive megakaryocyte proliferation and maturation. Although TPO appears to function as the main regulator of megakaryocyte development, it is not exclusive in this action. The cytokine stem cell factor, granulocyte-macrophage colony-stimulating factor, FLT (fms related receptor tyrosine kinase 1) ligand, interleukin (IL)-1, IL-3, IL-6, IL-11, CCL5, and erythropoietin also can regulate megakaryocyte development but appear to function mainly in concert with TPO. Mice that lack TPO or its receptor c-Mpl have approximately 15% of the normal platelet count. The discovery of TPO in 1994 and the development of primary megakaryocyte or mouse embryonic stem cell cultures that can be induced to faithfully reconstitute platelet formation has provided systems for studying megakaryocytes in the act of making platelets in vitro. Megakaryocytes isolated from mouse fetal liver and incubated with TPO for 4 to 5 days mature into large polyploid cells that are capable of generating and releasing large numbers of platelets. In a similar fashion, mouse embryonic stem cells can be induced to mature into polyploid megakaryocytes in the presence of stromal cells and TPO, IL-6, and IL-11. This process requires 10 to 12 days, during which the conversion of embryonic stem cells into hematopoietic stem cells very likely occurs in the first half of the time and the maturation of hematopoietic stem cells into proplatelet-producing megakaryocytes in the second half. Human embryonic stem cells can be coaxed to differentiate into mature megakaryocytes, although the process takes several more days in culture. Recently, platelets have been generated from induced human pluripotent stem cells in culture using a doxycycline-controlled c-MYC expression vector.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here