Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The complement system is a group of circulating proteins that promote inflammation and host defense.
Unregulated tissue damage is a possible complication of complement activation; a large variety of circulating and membrane-bound proteins exist to regulate complement activity.
Complement component C3 is the central convergence point for all complement activation pathways.
It is frequently necessary to measure serum complement levels to track disease activity. However, it is important to recognize that serum complement measurements are snapshots of a dynamic process involving variable rates of complement consumption and production.
Methods are available that allow accurate determination of serum levels of complement components.
Functional complement assays are sensitive and precise tools that provide information about the activity and integrity of a complement component or pathway.
The simplest functional assay of the classical pathway (CH50) measures total hemolytic complement activity.
The complement system comprises a group of greater than 30 circulating or membrane-bound regulatory glycoproteins that control parts of the innate immune system. Like other parts of the immune system, it protects the host against pathogens as well as facilitating clearance of apoptotic cells, counterbalancing the response of the immune system. There are a number of regulatory glycoproteins, either circulating or membrane-bound, that work to create the balance needed for an appropriate response. As will be demonstrated in this chapter, aberrant action of the complement system can lead to the damage of a large variety of organ systems, the severity of which can be catastrophic.
The existence of a group of host defense proteins that would later become the complement system was theorized in the late 19th century. Eventually, with improved lab testing, the many variable glycoproteins were identified and the complement system was further elucidated. Despite their relatively recent discovery, the proteins involved in the complement system are much older than the adaptive immune response. Even very primitive organisms have components of complement similar to the alternative and mannose binding lectin (MBL) pathways, which do not rely on immunoglobulin for activation. Immunoglobulin is utilized for activation in the classical pathway and provides more targeted, specific host defense. Further information on discovery of the complement system is available elsewhere ( ).
The purpose of the complement system is to identify foreign cells or microbes and destroy them, which can be accomplished through several different methods. These include direct cell lysis via the membrane attack complex, phagocytosis via C3b opsonization, and inflammation wherein phagocytes, chemotactically attracted to an inflamed site by complement components C5a or C3a, ingest and destroy microbes.
There are three complement pathways in humans: the classical pathway, alternative pathway, and mannose-binding lectin pathway (MBL pathway). Figure 48.1 demonstrates the reaction sequence. The nomenclature for the proteins of the complement system has been set forth by the World Health Organization ( ; ). The original naming convention from 1968 indicates that in the future there might be typical biochemical scientific naming given to these enzymes and that the current system could be viewed as a placeholder until this occurs. However, this has not happened yet, and the current naming system remains in place. The classical pathway has nine proteins, which are identified by the uppercase letter C. These are named in order of appearance in the complement cascade, though a notable exception is C4, which acts before C2. Proteins that act solely in the alternative pathway are termed factors and have a corresponding uppercase letter to identify them, such as factor B and factor D. In the complement cascade, there are several proteins that require cleavage before they can become active enzymes. The term for this precursor inert enzyme is zymogen . There are multiple reactions that result in cleavage of complement proteins, at which point the remnants are renamed to include a lowercase letter. For example, after cleavage of C4, the two fragments become C4a and C4b. The suffix “a” denotes the smaller fragment of the pair and “b” is assigned to the larger piece. The exception to this is C2, whose larger split product is termed C2a , and the smaller one C2b . If the original cleavage product is broken down further, the new suffix will be lowercase letters in alphabetic order, for example, C3c and C3d. Proteins that have become inactive will be renamed with a lowercase i attached as a prefix to the beginning of the compound. (e.g., iC3b). The polypeptide chains of native complement proteins are labeled with Greek lowercase letters (α, β, γ). There is an exception to this rule in the case of C1, which should be thought of as a group of three molecules named C1q , C1r , and C1s. In the case of C1q, the polypeptide chains are designated A, B, and C.
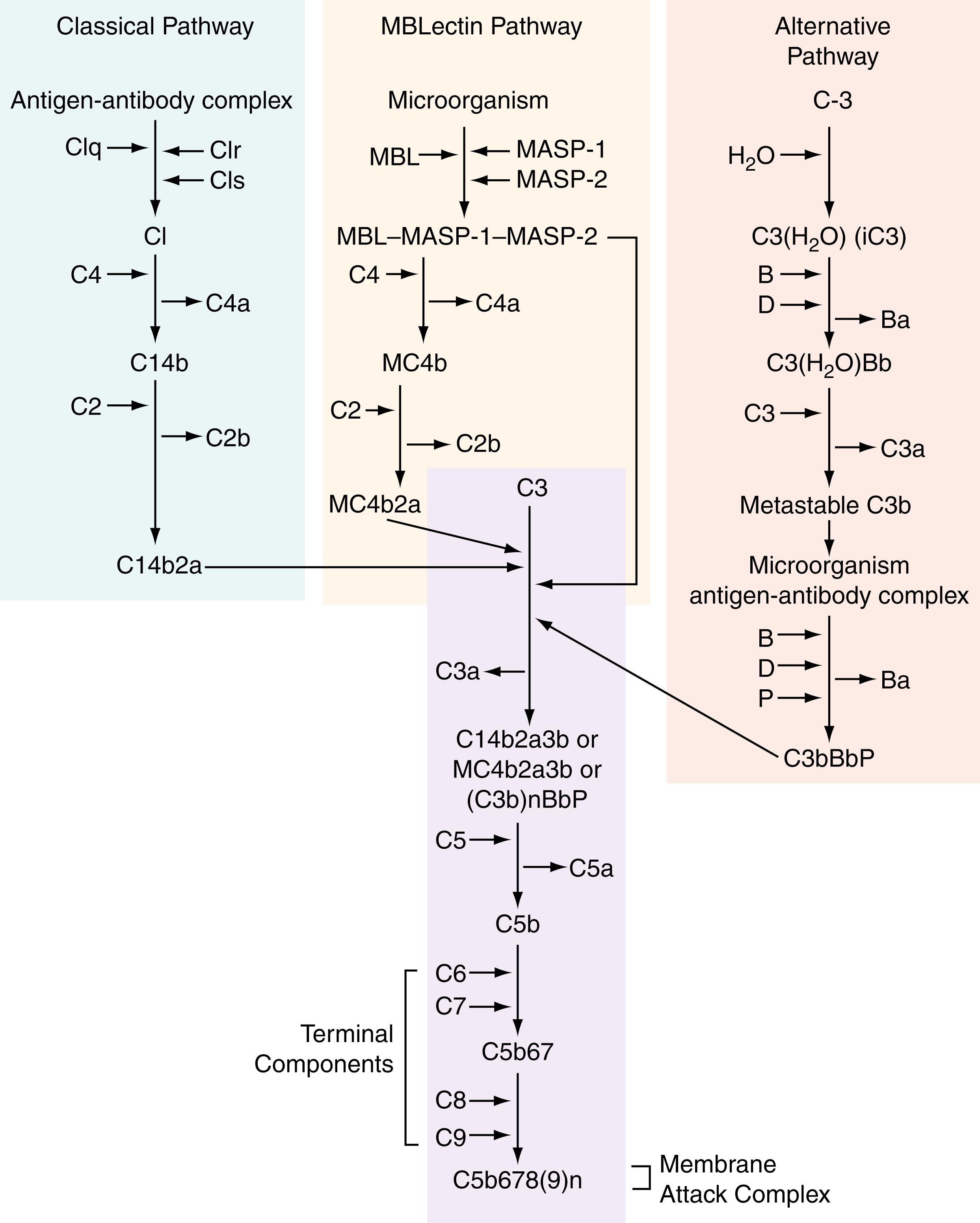
If a component—whether it be single or a multicomponent complex—has enzymatic activity, there is a bar over the named compound(s). Abbreviations are used for proteins of the MB Lectin pathway (e.g., MBL for mannan-binding lectin, MASP for MBL-associated serine protease). Multiple soluble regulatory proteins exist throughout the complement cascade and are designated by a letter or illustrative title, for example, C4-binding protein. The regulatory proteins that are membrane bound have a different nomenclature, which includes a CD number and descriptive title (e.g., CD46 is membrane cofactor protein). Complement receptors are denoted by two different naming systems: the first is the abbreviation CR with a number designation added as a suffix (for complement receptors 1–4); the second applies to the remaining complement receptors, for which the component’s name has an uppercase letter R added as a postfix.
The protein known as C3 is the central convergence point for the three complement activation pathways (the MBL pathway, alternative pathway, and classical pathway), which all proceed to lysis, phagocytosis, or regulation of the inflammatory response farther down the cascade.
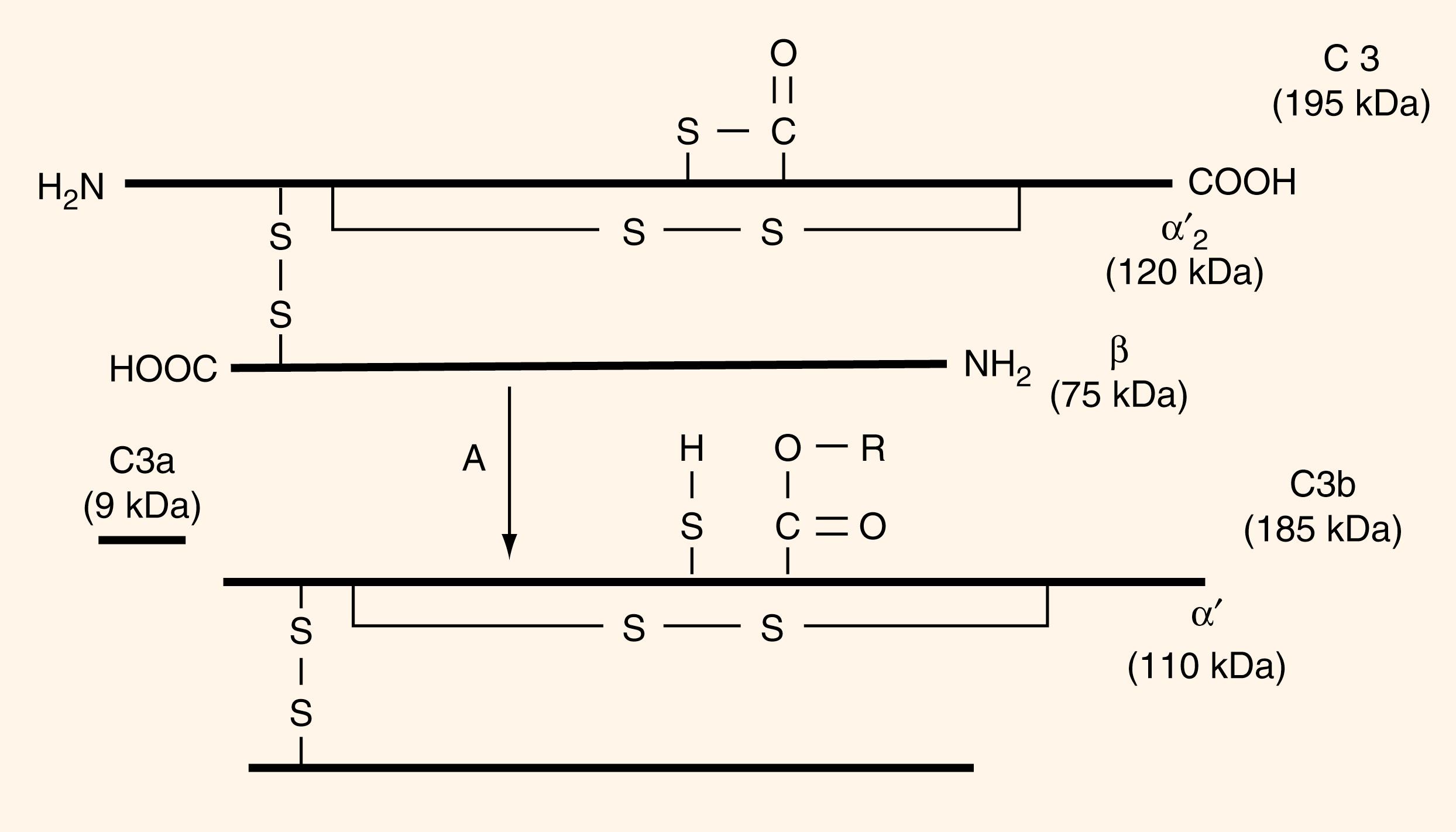
The C3 molecule itself is a two-chain peptide made in many cells, but mainly in hepatocytes. It begins as a single-chain molecule, pro-C3, and is then released into the circulation, where it will bind to the surface of a target. Intrachain disulfide bonds stabilize the α and β chains, while an interchain disulfide bond stabilizes the α and β connections ( Fig. 48.2 ). The α chain has a thioester in the middle that undergoes hydrolysis as water seeps into the molecule, resulting in cleavage of the α and β chain, which continuously activates the alternative complement pathway at a low level. The C3 molecule can also be cleaved via C3 convertase, resulting in the 9-kDa C3a fragment being freed from the larger C3b. The two fragments go on to perform separate functions: C3b coating target proteins to flag them for destruction and C3a to act as an anaphylatoxin signaling other cells and promoting inflammation ( ). Once the C3 molecule is cleaved, C3b can interact with C3b receptors, which native C3 is unable to do, such as CR1 (C3b/C4b, CD35). The C3b fragment will covalently bind to the surface of its target, for example, a microorganism. The bound target can then interact with the C3b receptors expressed on immunocytes, such as the phagocytes, B lymphocytes, and certain T lymphocytes.
This was the first of the three complement pathways to be discovered around 1900; thus, it was given the name classical . Despite its name, the classical pathway is the newest pathway in terms of an evolutionary perspective. It triggers complement activation via interaction with antibody-sensitized cells. It is made up of nine numbered proteins. More detailed information on this pathway can be found in other sources ( ; ).
Classical pathway activation most often occurs after interaction between an antigen and antibody, more specifically a C1-binding antibody. Immunoglobulin (Ig) M and IgG, with the exception of IgG4, are able to activate the classical pathway in humans. The initial protein in the classical pathway sequence is C1 (made of three molecules— C1q, C1r, and C1s ). While antibody is the most common way to activate this pathway, there are other molecules that can interact with C1q directly and trigger the cascade. Examples of these molecules include C-reactive protein, serum amyloid P component, β-amyloid, and a number of microorganisms, such as gram-negative bacteria, viruses, mycoplasmas, protozoa, and intracellular components such as deoxyribonucleic acid (DNA), cytoskeletal filaments ( ), and apoptotic cells ( ).
Certain infectious agents have been known to usurp the purpose of complement and use it to invade the host. This is the case in prion disease, which might use complement components such as C3 and C1q via the classical pathway to help facilitate its targeting lymphoid follicles ( ). In an experimental murine model, it was shown that deficiency in C3 and C1q actually leads to a delay in disease development after peripheral inoculation, potentially providing some degree of protection ( ).
C1 is the first protein in the classical pathway cascade that becomes activated by binding to an immunoglobulin already bound to its target. The C1 protein is 740 kDa, made up of one C1q molecule bound together with two C1r and two C1s chains stabilized by the presence of calcium ions and has been previously described as having a “bouquet of flowers” appearance. When it binds to immunoglobulin, C1q undergoes a conformational change that leads to autoactivation of the two C1r chains. Next, activated C1s2 will proteolytically cleave C4. C4 is cleaved into C4a and C4b, with C4b adhering to the activator surface or becoming hydrolyzed and remaining unbound in the fluid phase. The bound C4b molecules will group around the antigen-antibody-C1 site, which can attract multiple C4b molecules. The smaller product, C4a, is unbound in the fluid phase and acts as an anaphylatoxin (further discussed in the section on anaphylatoxins).
C4b in conjunction with C1s2 will then proteolytically cleave C2 in the company of magnesium ions into C2a and C2b. C2a is the larger of the two fragments and will remain bound to C4b, while C2b is released in the fluid phase. The C4b2a molecular complex is the C3 convertase for the classical pathway, which is chemically unstable and has a short half-life, as the C2a molecule may dissociate from the compound in an inactive form. The classical pathway C3 convertase (C4b2a complex) is necessary for binding C3 and continued cleavage through the complement pathway (see Fig 48.2 ). C2a, while complexed as part of C4b2a, will bind and cleave C3 into a larger C3b piece and C3a, with C3a remaining unbound in the fluid phase and acting as an anaphylatoxin. C3b will bind to the activator surface, forming C4b2a3b, which is the C5 convertase of the classical pathway and which prompts deposition of the remaining complement proteins on the surface of the target. It is again C2a that will leave C5 in the later steps.
Both IgM and IgG are able to activate the classical complement pathway though they are unequal in their capabilities to do so. A single IgM antibody, which has multiple antigen binding sites, once bound to antigen will undergo a conformational change that reveals a C1q binding site. Once IgM is bound to C1q, this interaction will trigger classical pathway complement activation.
On the other hand, IgG activation of the classical complement pathway relies on a continually exposed C1q binding site. However, it binds with a low affinity to C1q, thus mandating multiple IgG molecules in proximity to each other to prompt activation ( ). Eventually, with enough IgG antibodies, it can create a C1-binding site. Not only does IgG–antigen complex activate the classical pathway but it can also prompt the alternative pathway, which will be discussed further in the next section ( ).
The fact that congregated immunoglobulin binds C1q makes C1q a useful target when testing for soluble immune complexes in serum. Radiolabeled C1 is introduced into a patient’s serum or plasma sample. Using one of several separation techniques, unbound C1q can be separated from bound C1q. The part that is bound indicates the presence of immune complexes in the sample ( ). Alternatively, the enzyme-linked immunosorbent assay (ELISA) technique may be employed to detect and measure soluble immune complexes by identifying C1q- or C3-bound immune complexes ( ). C1q deposition in tissues can be directly observed in biopsy samples by immunofluorescent labeling of anti-C1q antibodies.
In the mid-1950s, another complement pathway was proposed by Pillemer and colleagues, although its validity would not be established until nearly 2 decades later. From an evolutionary standpoint, this pathway predates the classical pathway and is on the frontier of immunologic defense ( ). The alternative pathway functions with low-level constant activity by way of a “tick over” mechanism, an unprompted hydrolysis of C3 to C3(H 2 O). This reaction occurs at anywhere from 0.2% to 0.4% of the plasma pool per hour ( ). After the addition of a water molecule, the thioester domain undergoes a structural transformation so that factor B can bind in a magnesium-dependent fashion. The C3(H 2 O)–factor B complex can then be bound by factor D, leading to the formation of the initiation C3 convertase, C3(H 2 O)Bb. The complex C3(H 2 O)Bb will go on to cleave C3 into the anaphylatoxin C3a and the opsonin C3b. The initiation C3 convertase is an unstable structure that can be stabilized by the protein properdin, which will bind it and prolong its half-life from 1 to 18 minutes ( ). Once cleavage occurs, the serine protease factor D will revert back to an inactive form ( ). In this way, the cycle will continue and generate more C3b.
C3b is a rapidly decaying molecule that requires its target to be close by. The carbonyl group of its thioester will intermingle with a hydroxyl or amide group on a protein or carbohydrate on the exterior of its target ( ).
The spontaneous hydrolysis of C3 may be accelerated after interaction with C3 and multiple activating surfaces—for example, gas bubbles, lipid surfaces, protein complexes, and biomaterial surfaces ( ).
Additional C3b will start to adhere to the target surface, which leads to the creation of C5 convertase (C3b2BbP) ( ). This triggers the final common pathway of the complement cascade.
The third pathway for complement activation is the mannose-binding lectin or mannose-binding protein, known as the MBL pathway. This pathway uses its namesake protein to activate the cascade; the binding lectin has a typical serum concentration of 1.5 μg/mL. It is produced by the liver and belongs to a group of molecules called collectins ( ; ). Other notable members of the collectin family include lung surfactant proteins A and D (SP-A, SP-D), bovine conglutinin, bovine CL-43, and ficolins ( ; ). Lectins are found in a variety of organisms, including all mammals and some birds. MBL and ficolins are described as complement-activating soluble pattern recognition molecules, meaning that they recognize pathogen-associated molecular patterns (PAMPs) on the exterior of microbes. After they bind with their specified PAMPs, they undergo a structural change so that they can then interact with a group of three associated proteins that will trigger the complement cascade. These proteins are the MBL-associated serine proteases (MASP-1, MASP-2, MASP-3) ( ).
MBL is similar in configuration to C1q from the classical pathway and will recognize specific pathogenic carbohydrates. Fortunately, it does not recognize native self-carbohydrates, for example, galactose and sialic acid ( ). MBL has been reported to interact with a wide variety of microbes, including gram-negative bacteria, acapsular gram-positive bacteria, viruses, yeasts, mycobacteria, parasites, and protozoa ( ).
After MBL has interacted with a target carbohydrate group and interfaced with MASP, the complement cascade is triggered when MASP-2 cleaves C4, creating the classical pathway C3 convertase C4b2a ( ). The serine protease MASP-1 can cleave C3 ( ), which infers that this mechanism can directly activate the alternative pathway ( ).
After creation of the classical pathway’s C3 convertase (C4b2a), the complement cascade is triggered in the same way as the classical pathway; the alternative pathway might participate as well ( ).
In addition to activating complement, MBL has a number of other functions. It is thought that it might facilitate riddance of targeted microbes by interacting with a specific receptor on phagocytes ( ; ). In addition, MBL has been thought to modulate the adaptive immune system and temper the body’s inflammatory and allergic responses ( ; ).
Pathologically, there are specific immune complexes that have been known to prompt activation of the MBL pathway. In certain patients, such as those with rheumatoid arthritis (RA), the terminal galactose on some IgG molecules may be absent (named IgG-G0 ), which allows it to interact with MBL and activate the classical pathway ( ). The MBL pathway regulation might be influenced by C1 inhibitor ( ) and α 2 -macroglobulin ( ).
The three complement pathways are triggered in different ways. However, after C3 activation, all follow a final common pathway that ends with creation of the membrane attack complex (MAC). As previously discussed, the C5 convertase is assembled in the complement pathways, which can then cleave C5 into fragments C5a and C5b. C5b is the larger molecule, which can recruit the remaining proteins of the MAC, C6, C7, C8, and C9. Their combining forms the MAC ring.
More specifically, the C5b molecule will bind to a cell membrane and then combine with C6, forming the C5b-6 complex. This will then interact with C7, which is in the fluid phase. The C5b-7 complex is amphiphilic, which allows it to insert into the cell membrane’s lipid bilayer ( ). Despite this penetration of the lipid bilayer, it is insufficient for cell lysis. Next, the C8 protein will interact with the C5b-7 complex via direct contact with C5b. With the addition of C8, the newly formed C5b-8 complex can further perforate the lipid bilayer so that a more efficient pore is created, which leads to erythrocyte lysis ( ). After the formation of C5b-C8, the final component of the MAC, C9, can adhere by interfacing with the α chain of C8, creating the fully functional MAC ( ). Notably, multiple C9 proteins will gather and polymerize to create a channel within the cell membrane. This hollow, cylindrical channel can be observed under electron microscopy ( ). Once the MAC is formed, it interrupts the crucial lipid bilayer and its hydrophilic center allows ions to move freely ( ; ). The subsequent influx of calcium ions (Ca 2+ ) shuts down mitochondrial activity, causing apoptosis ( ).
The number of C9 proteins recruited to the MAC complex varies and the number of C9 molecules will dictate the size of the pore created. As few as one or two C9 proteins are required to result in apoptosis of a nucleated cell. However, with the addition of more C9 molecules, the efficiency of cell death induced by the MAC increases.
The MAC is able to lyse gram-negative bacteria, viruses, and erythrocytes. Many nucleated cells are able to defend against a MAC-mediated attack by means of membrane-associated regulatory molecules, which inhibit formation of a MAC (see the section Regulation of Complement Activation). Metabolically active cells are able to expel the MAC from the cell surface via lipid turnover during cell membrane repair ( ). Despite these protective mechanisms, nucleated cells are still susceptible to the MAC if the number of MACs overwhelm the cell’s available defense ( ; ). The half-life of the MAC in nucleated cells is estimated at 1 to 3 minutes. In nonnucleated erythrocytes, however, the MAC can remain for up to 72 hours ( ).
The MAC can also have an activating effect on some nucleated nontarget cells, such as neutrophils and macrophages, among others. This can lead to the proliferation of neutrophils and macrophages and the creation of reactive oxygen species by them, discharge of eicosanoids from phagocytic cells, initiation of procoagulant activity in endothelial cells and platelets, induction of proinflammatory activity in endothelial cells and smooth muscle cells, production of growth factor (PDGF, bFGF-1, IGF-1), interruption of caspase-reliant apoptosis, and triggering of signal transduction pathways ( , ; ; ; ; ; ; ).
As previously mentioned, there are small, active protein by-products called anaphylatoxins that are created through cleavage of complement proteins as part of the activation cascade. Some of these protein fragments are not part of the convertases but have alternative essential functions. The protein fragments C3a, C4a, and C5a are the anaphylatoxins. Their various activities include opsonization, phagocytosis, and immunomodulation, and they assist in the promotion of inflammatory reactions.
The C3a, C4a, and C5a anaphylatoxins are about 10 kDa in size. They are unequal in terms of potency, as follows: C5a > C3a > C4a. However, these characteristics are specific to different tissues. For example, C5a seems to influence many cell types, while C3a seems to be more specific at directing eosinophils to allergic sites ( ). Activity of the anaphylatoxins can be modified into desarginine through cleavage of their carboxy-terminal arginine residues by carboxylpeptidases in the serum, reducing their activity. Several review articles further discuss specifics of anaphylatoxin structure and function ( ; ; ).
There are specified receptors on cells that anaphylatoxins will bind. The C3a receptor (C3aR) and C5aR are members of the seven-transmembrane G protein coupled receptor (GPCR) family, which activate the MAPK signaling pathway that mediates the majority of activity related to anaphylatoxins. The receptor C5L2, another member of the GPCR group, is known to bind both C5a and C5a desArg; however, its role remains unclear. For years, it was unclear what the C4a receptor was; it now appears that C4 binds to protease-activated receptor 1 (PAR1) and PAR4, both members of GPCR family. This mediates its effect on endothelial cells, increasing their permeability ( ). Further information on complement receptors and their mediated effects is discussed in the section on complement receptors.
Anaphylatoxins work to promote inflammation and generally exert their effects on smooth muscle cells, mast cells, and peripheral circulating leukocytes. The anaphylatoxins lead to neutrophil accumulation, smooth muscle contraction, increase blood vessel porousness, stimulate mucus production from goblet cells, and in nonhuman macrophages promote thromboxane generation ( ). Additionally, anaphylatoxins induce mast cell and basophil degranulation, which releases vasoactive substances such as histamine and serotonin, leading to vasodilation, thus increasing blood flow to areas of inflammation.
C5a works to activate the immune system and mediate inflammation in a variety of ways. It binds to neutrophils, increasing their adhesion and aggregation, induces an oxidative reaction, and prompts the release of lysosomal enzymes. It also promotes movement of monocytes/macrophages and neutrophils toward areas of complement activity in tissue. Generation of C5a during complement activation can vasodilate vessels in the surrounding tissue, promote aggregation of neutrophils to endothelium near the areas of inflammation, and act as a chemotactic to draw phagocytes to the area of complement activation. The C5a-induced neutrophil aggregation can be so profound that it can cause a pulmonary embolus made of the neutrophils. Lung inflammation due to immune complex formation is also thought to be mediated by C5a ( ). An experimental rat sepsis model showed that C5a, when produced in large quantities, might obstruct the bactericidal properties of neutrophils. This could contribute to the high mortality rates seen in patients suffering from sepsis ( ).
The anaphylatoxins C3a and C5a are able to provide interaction between the innate and adaptive immune systems via their ability to affect the activity of T helper 1 (Th1), Th2, Th17, and T regulatory (Treg) T cells ( ). The evaluation of anaphylatoxin interaction with the adaptive immune system relies on investigational animal models. The effect in these animal models is complicated and variable. Some of these issues include variation in the disease models used, the murine genetics, and interference by interaction with alternative receptors, such as Toll-like receptors. An asthma model demonstrated that C3a could worsen lung injury by exacerbating Th2 immunity. Alternatively, C5a repressed Th2 reaction and seemed to be protective. The theory is that the anaphylatoxins will influence dendritic cells or macrophage cytokine- and antigen-presenting cell activity, which leads to mediation of the T-cell reaction. Notably, within human myeloid cell subpopulations, there is variation in the C3aR and C5aR manifestation, which may explain the differential effects of C3a and C5a on cytokine activation and T-cell reaction.
The complement system is a crucial part of the immune system, However, without appropriate regulation, there can be disastrous damage to the host tissues. The mechanisms used to control the complement system in general work in the following ways: avoid far reaching inflammation beyond the targeted site, limit overactivation, and prevent damage to host cells during the immune response. Multiple proteins, which can be either membrane-bound or fluid-phase, work to achieve the aforementioned goals at various steps throughout the complement cascade ( Table 48.1 ).
| Protein | Molecular Weight, kDa | Target | Mechanism of Action |
|---|---|---|---|
| Fluid Phases | |||
| C1 inhibitor | 105 | C1 | Dissociates the C1 complex by binding to C1r and C1s |
| Factor H | 150 | C3b | Cofactor for C3b inactivation by factor I |
| C4-binding protein | 550 | C4 | Cofactor for C4b inactivation by factor I |
| S protein (vitronectin) | 84 | C5b-7 | Inhibits insertion of the MAC into cell membranes |
| Clusterin | 70 | C5b-7 | Inhibits insertion of the MAC into cell membranes |
| Factor J | 20 | C1, C3, B | Inhibits C1 complex formation, inhibits cleavage of C3 by Bb |
| Cell-Associated | |||
| CR1 | 190 ∗ | C3b, C4b | Dissociation of C3/C5 convertases, cofactor for C3b and C4b inactivation by factor I |
| DAF (CD55) | 70 | C3bBb, C4b2a | Dissociation of C3/C5 convertases |
| MCP (CD46) | 45–70 | C3b (C4b) | Cofactor for C3b inactivation by factor I |
| CD59 (protectin) | 18–20 | C8, C9 | Inhibition of formation of the MAC |
| HRF | 65 | C8, C9 | Inhibition of formation of the MAC |
Regulation begins early in the classical pathway at the level of C1 and is performed by C1-inhibitor (C1-Inh). C1-Inh hinders autoactivation of C1 in the fluid phase and by weak promoters of the classical pathway. However, it does not prevent the activation that occurs due to immune complexes ( ). Some evidence suggests that the C1-Inh is able to displace the C1qr 2 s 2 compound from an immunoglobulin if the binding affinity is low ( ). Not only is C1-Inh involved in the classical pathway, but it also works in the MBL pathway by inactivating MASP ( ).
Factor I (previously known as C3b/C4b inactivator ) works to regulate C4b ( ). It acts to split the C4b α chain into the fragments C4c and C4d. To achieve proteolytic cleavage, the association of C4-binding protein (C4BP) is needed. C4BP is a 570-kDa protein that is able to bind C4b in the fluid phase or in a particle bound state. It is also able to dislodge C2a from the C3 convertase C4b2a in the classical pathway ( ).
As the central convergence point of the complement pathway, regulation of the C3 molecule is crucial. Factor I will quickly inactivate unbound C3b via cleavage of three peptide bonds on the α chain and C3 (H 2 O) ( ). This cleavage results in iC3b, which is inert and unable to engage with C5 or factor B. In order to accomplish this regulation, factor I requires a cofactor, factor H, which is a 150-kDa protein. It will work with factor I to cleave C3b both in the fluid phase and membrane bound state ( ). It also acts to accelerate decay of the alternative pathway C3 convertase. After the transformation to iC3b, which is bound to the target surface, it is able to bind with complement receptor type 3 (CR3), a receptor on phagocytic cells, and can be phagocytosed.
Additional regulation takes place with the terminal complement components, which prohibit MAC insertion into the cell membrane. S protein (not to be confused with protein S), alternatively known as vitronectin , binds the C5b-7 complex, thereby inhibiting its penetration of the cell membrane ( ) and preventing C9 polymerization ( ). S protein also binds the sC5b-9 complex via C5b and C8 ( ) and inhibits complement activation via binding to the C1q receptor ( ). Even in experimental models, the complete role of S protein in controlling activation of complement is unclear. However, it has been shown in rabbit models that S protein will bind with sC5b-9 when complement is active. This compound can inhibit the C9-mediated lysis of sensitized sheep red blood cells with attached complement components 1 through 7 ( ).
Another regulator in the fluid phase is clusterin ( Sp-40 or apolipoprotein J ), which targets members of the MAC. Specifically, it inhibits the C5b-7 complex from inserting into the cell membrane ( ). It also works by targeting the MAC at C9 ( ).
In addition to fluid-phase regulators of complement, there are also cell-associated regulatory proteins that often act as complement receptors. CR1 works in this fashion by binding either C4b or C3b and then as a cofactor with factor I to mediate their cleavage. Membrane cofactor protein (MCP or CD46) is found on most cells, though not erythrocytes, and also works as a cofactor in factor I–mediated cleavage of C4b to C4c/C4d fragments and C3b to iC3b ( ; ).
Decay-accelerating factor (DAF; CD55) is another receptor that works to inhibit complement. DAF does this by increasing the rate of degeneration of C3 convertase and C5 convertase. DAF is present on all circulating cells, all endothelial cells, and many epithelial cells ( ).
Other regulators of the components of the MAC are homologous restriction factor (HRF), also known as C8-binding protein , and CD59, also known as protectin , HRF-20 , membrane inhibitor of reactive lysis , and P-18 . HRF works to bind C8, thus preventing C9 polymerization. It is present on erythrocytes, platelets, both T and B lymphocytes, neutrophils, and monocytes ( ). CD59 is expressed on all circulating cells, endothelial cells, epithelial cells, spermatozoa, and glomerular podocytes ( ). It is also found on some cells of the central nervous system ( ). It acts to inhibit MAC formation by binding C8 at the β chain and the b domain of C9 ( ).
Patients with paroxysmal nocturnal hemoglobinuria (PNH) lack DAF, HRF, and CD59 on their cells due to an abnormal glycosyl phosphatidylinositol connection ( ) (see section on hematologic disorders for further information).
Complement receptors bind the complement proteins and mediate their effects as well as having other cellular functions ( Table 48.2 ). Their presence has been described on multiple cell types.
| Receptor | Molecular Weight, kDa | Ligand | Physiologic Role |
|---|---|---|---|
| CR1 | 190 (most common isoform) | C3b, C4b, iC3b | Phagocytosis, immune complex clearance |
| CR2 | 140 | C3d, C3dg, iC3b | B-cell activation |
| CR3 | 165 (α chain) 95 (β chain) |
iC3b, C3d, C3b | Phagocytosis, cellular adhesion |
| CR4 | 150 (α chain) 95 (β chain) |
iC3b, C3b | Cellular adhesion |
| C1qRp ∗ | 126 | C1q, MBL, SP-A | Phagocytosis |
| C3aR | 48 | C3a | Chemotaxis, degranulation of serosal-type mast cells, increase in vascular permeability |
| C5aR | 43 | C5a, C5a desArg | Chemotaxis, degranulation of serosal-type mast cells, cellular adhesion, increase in vascular permeability |
Complement receptor type 1 (CR1, CD35, C3b/4b receptor) is one of the best studied complement receptors, made up of a single-chain glycoprotein. It is present on erythrocytes, mononuclear phagocytes, eosinophils, B lymphocytes, certain T lymphocytes, follicular dendritic cells, glomerular podocytes ( ), and astrocytes ( ). CR1 binds C3b, C4b, iC3b to some degree, and C1q ( ). It acts as a cofactor for factor I in its cleavage of C3b, iC3b, and C4b and accelerates the decay of C3 convertase and C5 convertase. Another critical action of CR1 is its role in phagocytosing opsonized target particles. CR1 expressed on erythrocytes works to transport tagged immune complexes to the liver and spleen, where they can be phagocytosed and thus avoid deposition into tissue ( ). There is some thought that aberrant expression of erythrocyte CR1 is at play in some chronic infections ( ).
Complement receptor 2 (CR2; CD21) is present on B cells, follicular dendritic cells, select T cells, thymocytes ( ), and astrocytes ( ). This receptor interacts with C3d and iC3b while bound to antigen to perform its major function, which is to mediate a B-cell response in the presence of antigens ( ). It also aids in factor I cleavage of iC3b attached to an antigen ( ). Interestingly, CR2 is the receptor on B cells that Epstein-Barr virus interacts with to cause infectious mononucleosis ( ).
Complement receptor 3 (CR3; Mac-1, CD11b/CD18) belongs to the β2 leukocyte integrin group of adhesion molecules ( ). It is present on a variety of cells, including, mononuclear phagocytes, granulocytes, natural killer (NK) cells ( ), and microglial cells ( ). The CR3 will bind iC3b, C3b, and C3d ( ). CR3 works to promote phagocytosis. It is also a participant in the congregation of monocytes and neutrophils to endothelial cells through contact with its ligand, intercellular adhesion molecule type 1 (ICAM-1). This action, in turn, promotes phagocytic cells to gather where endothelial cells are activated at places of tissue damage.
Complement receptor type 4 (CR4; CD11c/CD18) is a member of the same family as CR3. This receptor is present on myeloid cells, platelets, dendritic cells, NK cells, activated B cells, certain activated T cells ( ), and microglial cells ( ). It will bind both iC3b and C3b, but it binds the latter to a lesser extent ( ). CR4 remains a bit mysterious, as we do not yet understand its full function. However, it is able to bind fibrinogen and serves to increase neutrophil aggregation during inflammation ( ).
The receptor for the anaphylatoxin C5a (C5aR) is present on many cell types, including neutrophils, monocytes, basophils, eosinophils, platelets, mast cells, lung vascular smooth muscle cells, liver parenchymal cells, lung and umbilical vascular endothelial cells, bronchial and alveolar epithelial cells, astrocytes, microglial cells ( ), and human T cells ( ). The interaction of C5a to its receptor will prompt multiple proinflammatory processes, such as mast cell degranulation, chemotaxis of inflammatory cells, oxygen radical production, neutrophil and eosinophil fabrication of leukotrienes and prostaglandins, and induction of cytokines, antibodies, and acute-phase proteins ( ).
The majority (approximately 90%) of complement plasma proteins are produced in the liver. Additionally, many are acute-phase reactants, meaning that during an inflammatory event the liver will increase their production. Notable exceptions to hepatically produced complement proteins include C1q, factor D, properdin, and C7 ( ). The C1q protein is synthesized by epithelial cells, monocytes/macrophages, and fibroblasts. Adipocytes are the primary site for the synthesis of factor D. Properdin is primarily made by cells of the immune system, including monocytes, macrophages, lymphocytes, and granulocytes. C7 is synthesized in monocytes and macrophages, and, interestingly, is stored in neutrophils.
While the liver is mainly responsible for production of complement components, cells such as monocytes, macrophages, astrocytes, and cells located within synovial tissue are able to create all the proteins within the classical and alternative pathways. It is thought that this keeps inflammation localized to specific tissues.
The catalyst for complement protein production by any complement-producing cell is usually spurred on by proinflammatory proteins, such as interleukin-1α, interleukin-6, or interferon-γ. Further information about complement protein synthesis can be found elsewhere ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here