Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
“Compression of the celiac artery by the median arcuate ligament is well-documented.” Stanley and Fry published this statement in 1971 after reviewing “provocative xylose absorption tests” in ten female and five male patients over a 3.5-year period. After transecting the median arcuate ligament in these 15 patients, they concluded that intestinal ischemia was the underlying disorder in median arcuate ligament syndrome (MALS). However, 45 years later, MALS continues to be difficult to qualify, quantify, and diagnose. MALS (also known as celiac artery compression syndrome and Dunbar syndrome) is an anatomic and clinical illness resulting from extrinsic compression on the celiac axis. Clinical manifestations include postprandial and exercise-induced epigastric abdominal pain, nausea, vomiting, weight loss, and “food fear,” or fear of pain triggered by eating. Lipshutz offered the first anatomic description of the celiac artery in 1917, and Harjola performed the first surgical release in 1963. In 1967 Dunbar et al. reported a larger case series in which operative division of the MAL resulted in clinical improvement in 13 of 15 patients. Anatomically, up to 24% of the population may have MAL compression; however, less than 1% of these patients are symptomatic. Over the last several years, the theoretical etiology of MALS has shifted from a vascular disease to a neurogenic illness with compression of the surrounding celiac plexus and ganglion. Presently, patient selection remains critically important in order to obtain optimal clinical outcomes following treatment.
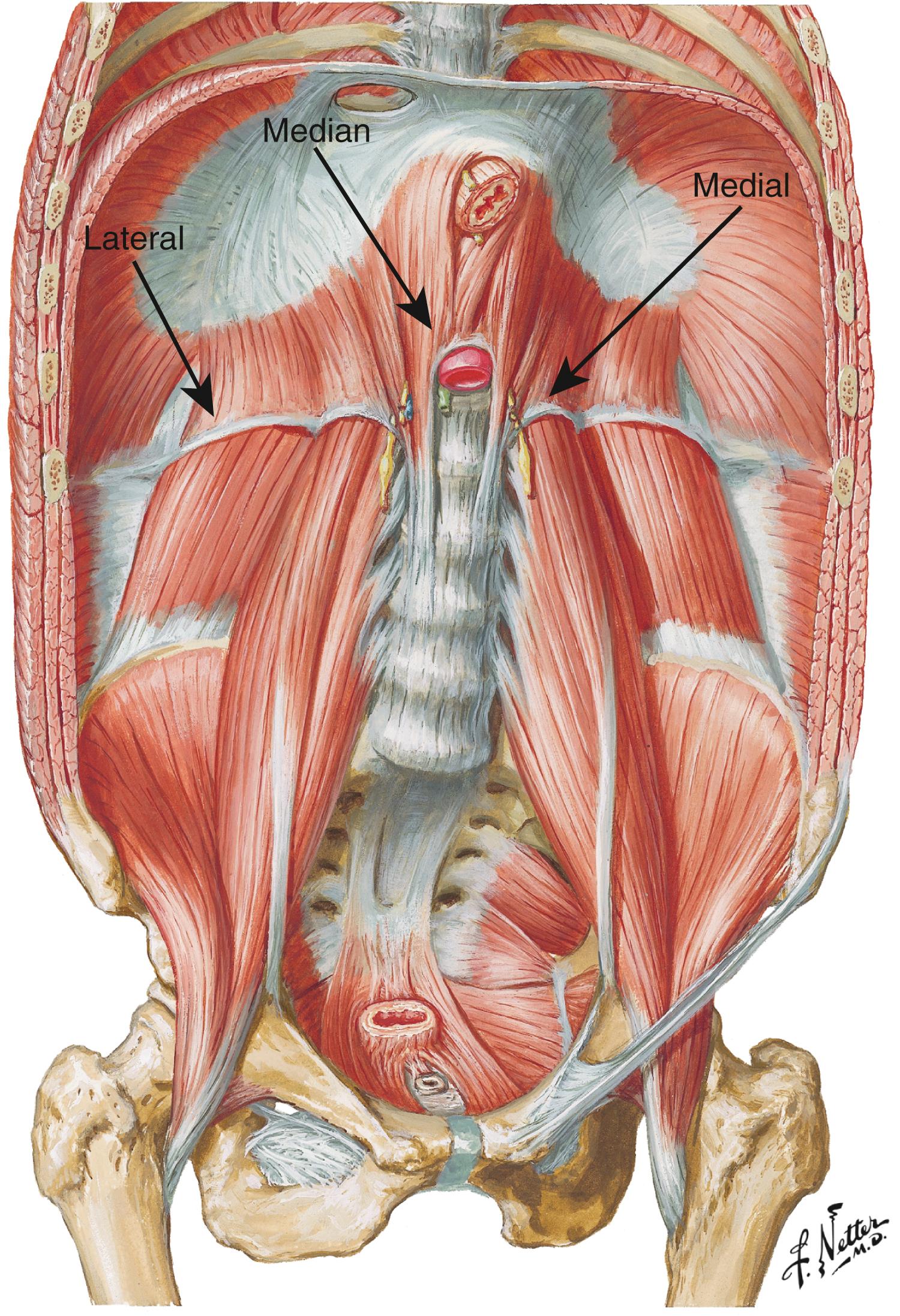
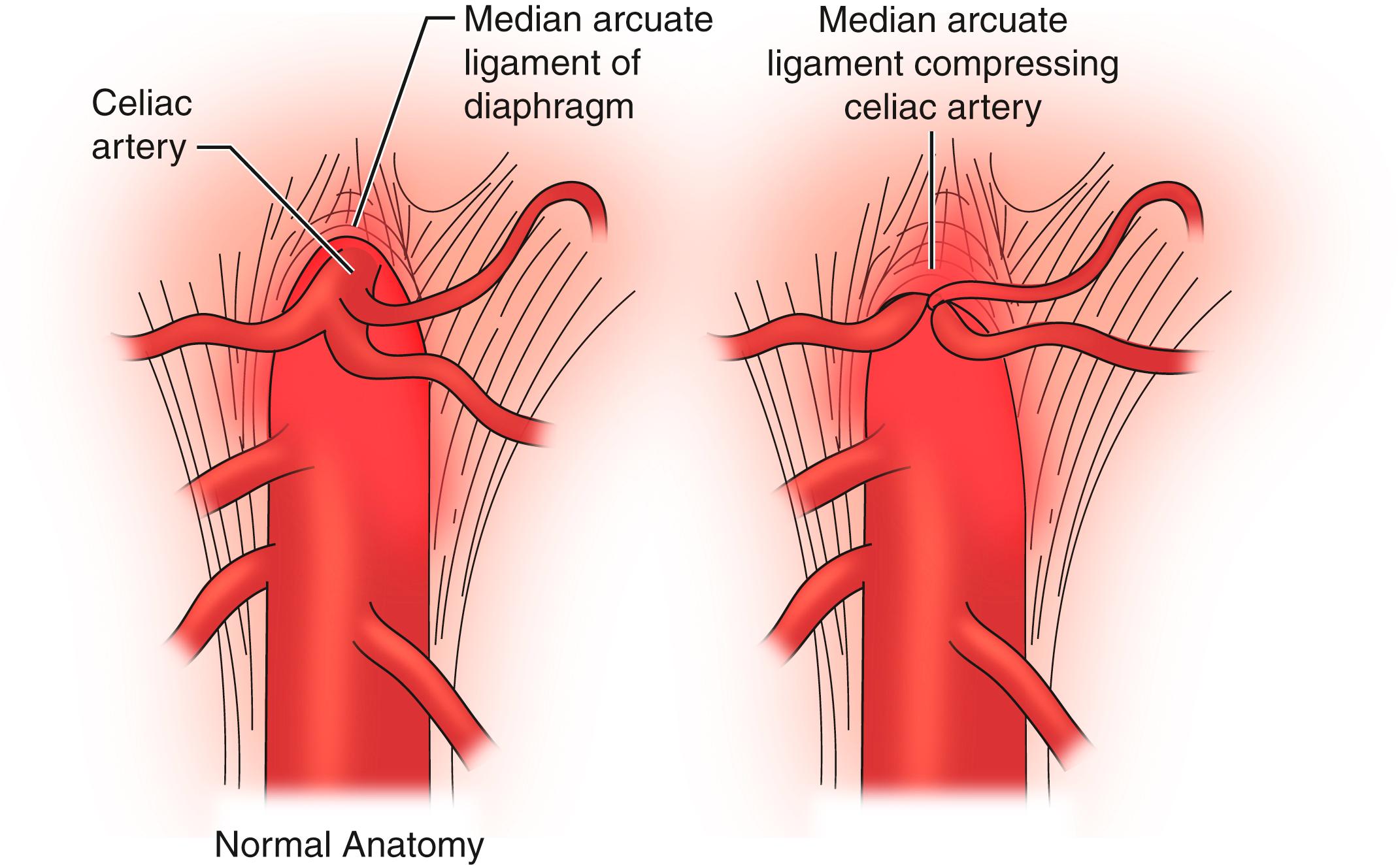
Embryologically, the diaphragm is derived from the septum transversum and descends from the neck toward the celiac axis between weeks 9 and 12 and ultimately forms the MAL. Typically the diaphragmatic crura arise from the anterior aspect of L1 to L4 and project cephalad to join the anterior longitudinal ligament of the spine overlying the celiac axis. The MAL is composed of the fibrous edge of the diaphragmatic crura that crosses the aorta at the level of the celiac artery. These fibers splay over the aorta and extend laterally toward the suspensory ligament of the fourth portion of the duodenum ( Fig. 136.1 ). In the majority of the population, the ligament does not encroach on the celiac artery and causes no compression ( Fig. 136.2 ). As noted previously, as much as 10% to 24% of the population may have MAL compression without symptoms. The celiac nerve plexus branches across the abdominal aorta between vertebral levels T11 to L1.
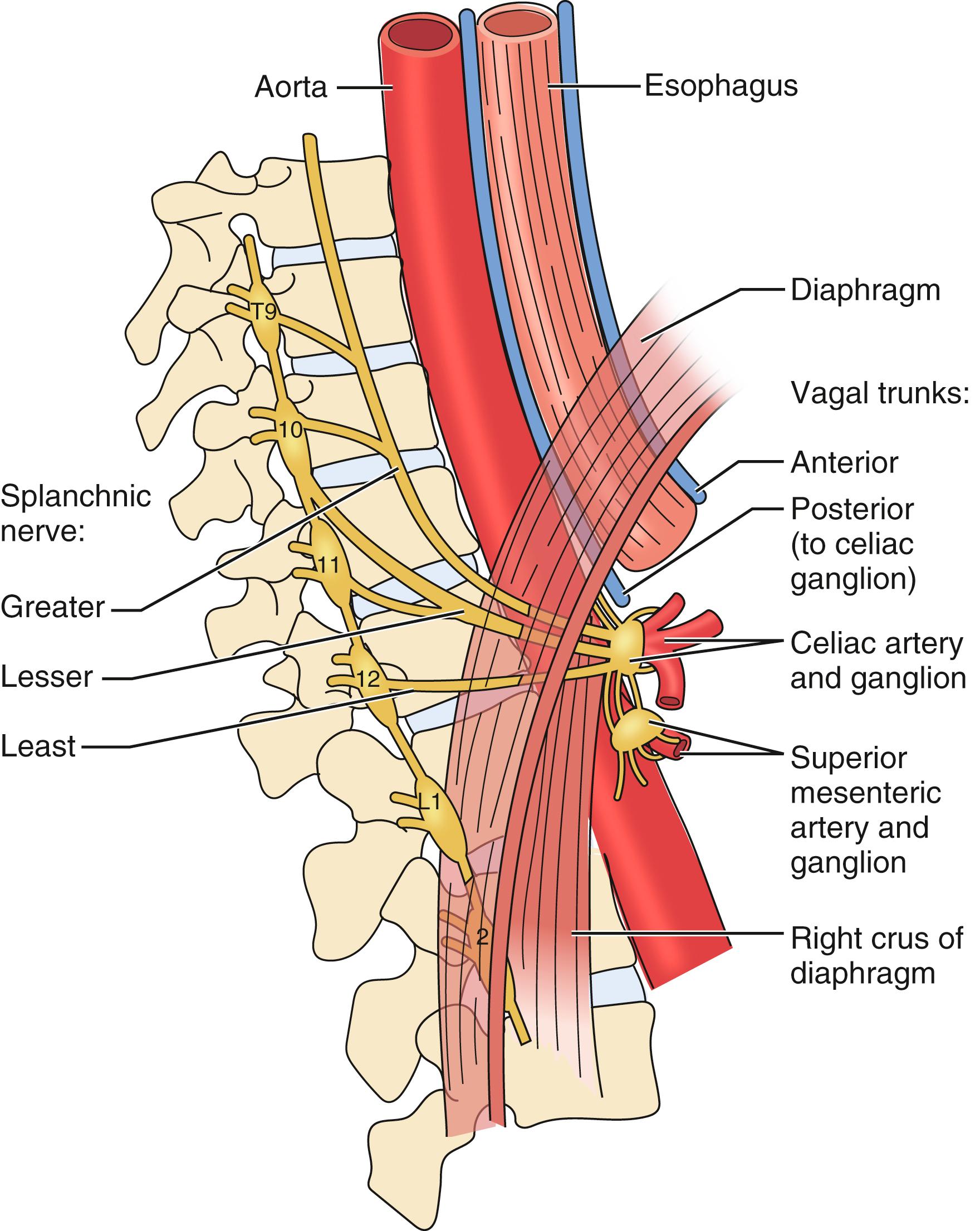
Multiple theories exist regarding the pathophysiology of the epigastric pain associated with MALS. One theory hypothesizes that increased blood demand through a compressed celiac artery leads to foregut ischemia and pain. However, collateral blood flow between the mesenteric vessels often compensates for either subtotal or intermittent occlusion of the celiac artery, especially in the patient age group presenting with MAL. Also, studies performed using gastric exercise tonometry document that mucosal oxygen delivery does not correlate with celiac artery compression or clinical symptoms. Similar findings exist with gastric perfusion studies. In addition, MAL compression is not associated with gastric necrosis or even gastric ischemia on endoscopic evaluation. Another theory states that midgut ischemia induces abdominal pain through a vascular steal syndrome. In theory, blood flow from the superior mesenteric artery may be diverted through collaterals to compensate for inefficient delivery through a stenotic celiac artery, and this mechanism results in small bowel ischemia and pain. This mechanism would be difficult to document with retrograde flow studies. Others suggest that the epigastric pain is caused by overstimulation of the celiac plexus with subsequent splanchnic vasoconstriction and ischemia ( Fig. 136.3 ). Some authors favor a neurogenic hypothesis for symptoms of MALS. Histologic analysis of the celiac plexus shows that the nerves consist of pain fibers and inhibitory motor fibers to the stomach. Thus, entrapment of the ganglion by the MAL may alter gastric myoelectrical activity and impair antral motility, and induce pain. Since the underlying pathophysiology of MALS is unknown, the diagnosis of MALS is difficult. Consequently, to enhance the benefit of patient selection and surgical intervention, the diagnosis of MALS needs to be reliable and accurate.
The goal of surgical intervention begins with accurately identifying patients with symptomatic MALS and those patients who would benefit from surgery. Initially the workup includes consultation with a vascular and general surgeon for a detailed history and physical examination. Overall, history and physical examination often prove nondiagnostic or equivocal. The history may yield previous surgical interventions, including cholecystectomy and/or diagnostic laparoscopy. The most common presenting symptom is abdominal pain, and an overwhelming majority of symptoms are classified as postprandial. Other symptoms include weight loss, bloating, nausea, vomiting, and exercise-induced pain ( Box 136.1 ). Recently, patients with autonomic neuropathy, including postural orthostatic tachycardia syndrome (POTS), have been diagnosed with MALS. Physical examination typically reveals a thin, flat, nontender abdomen. Despite the suspected underlying pathology, an abdominal bruit is usually not detected with auscultation.
Second to sixth decade of life
Female:Male 3:1
Postprandial abdominal pain
Nausea
Vomiting
Unintentional weight loss
Exercise-induced pain
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here