Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter was supported in part by the Ahlfors Center for Unbound Bilirubin Research & Development and the Kaplan-Goldstein Family Foundation. No commercial financial assistance was received in support of this chapter. We would also like to thank Angelo A. Lamola, PhD, for his contributions in the original chapter.
The pathways by which light reduces levels of circulating bilirubin and how these mechanisms decrease the levels of possible toxic byproducts of bilirubin have been subjects of intense inquiries and debates since the 1960s. The first United States national symposium on neonatal hyperbilirubinemia and phototherapy convened in 1969 by the March of Dimes Foundation reported on the effect of light on bilirubin metabolism and delineated potential clinical implications for application of phototherapy to neonatal practice. Key recommendations for the clinical use of phototherapy included a directive to “involve judgments similar to those made in deciding upon the clinical use of a new drug.” A new standard of care was then established with consideration of the advantages and risks of phototherapy, the likelihood of injury from hyperbilirubinemia, and risks associated with alternative treatment strategies. Over the intervening years, the mechanism of action of phototherapy has been extensively studied, and sufficient evidence is available to guide its use in term and late-preterm neonates. However, use of phototherapy in very or extremely preterm hyperbilirubinemic infants, although apparently effective, is confounded by preterm biology, state of maturation, concurrent disease, choice of light source, device design, and inconsistent clinical implementation.
The primary success of phototherapy has been attributed to its ability to reduce an infant’s risk for bilirubin neurotoxicity (i.e., kernicterus ) and the need for exchange transfusions. When phototherapy is instituted as an emergency measure in infants presenting with extreme hyperbilirubinemia (total serum/plasma bilirubin [TB] levels > 20 or 25 mg/dL) or early neurologic signs, care is taken to maximize the intensity of phototherapy delivered and to concurrently reduce the enterohepatic circulation of bilirubin. , , This approach, previously based only on hypothesis, has been validated by recent observations that 20% to 25% of circulating bilirubin can be converted to more water-soluble, and directly excretable, configurational photoisomers within 30 minutes of exposure to light of sufficient intensity. Phototherapy also generates structural isomers of bilirubin, called lumirubins , which are produced less efficiently but excreted more rapidly. Both types of photoproducts are believed to be less likely than bilirubin to cross the blood-brain barrier (BBB), and some investigators have proposed that this may confer neuroprotection even without enhancing excretion.
Phototherapy for neonatal hyperbilirubinemia is relatively simple and efficacious if properly applied. Disciplined efforts are now aimed at standardizing prescribing practices. Furthermore, a much better definition of the phototherapy action spectrum has emerged, taking into account improvements in light sources, variables affecting efficacy (such as dosimetry and hematocrit levels), and potential undesirable effects (such as heating by absorption of light wavelengths that are relatively useless therapeutically). The advent of blue light-emitting diodes (LEDs) with their narrow bandwidths has significantly contributed to development of affordable and optimized phototherapy devices.
In this chapter, we review bilirubin photochemistry, photobiology, and photomedicine to delineate how phototherapy should be viewed as a drug that interacts with bilirubin molecules ( Table 92.1 ). A review of selected terms related to photobiology products can be found in Box 92.1 . In the spirit of primum non nocere , there is an obligation for clinicians to optimize therapy and deliver the safest and most effective care.
| Checklist | Recommendation | Implementation |
|---|---|---|
| Light source (nm) | Emission spectrum in 460–490 nm blue-green light region | Know the spectral output of the light source |
| Light irradiance (μW/cm 2 /nm) | Irradiance: ≥30 μW/cm 2 /nm within the 460–490-nm wavelength band | Measure irradiance over entire light footprint area to ensure uniformity |
| Body surface area (cm 2 ) | Expose maximal skin area (35%–80%) | Reduce blocking of light |
| Timeliness of implementation | Urgent or crash-cart intervention for excessive hyperbilirubinemia | May perform other procedures while infant is under phototherapy |
| Continuity of therapy | May briefly interrupt for feeding, parental bonding, and nursing care | After confirmation of adequate TB decrease |
| Efficacy of intervention | Periodically measure rate of response in bilirubin load reduction | Degree of TB concentration decrease |
| Duration of therapy | Discontinue at desired bilirubin threshold; be aware of possible rebound increase | Serial TB measurements based on rate of decrease |
Biliprotein: This is a molecular complex containing stoichiometric proportions of bile pigment and protein. The term is limited to molecules in the bile pigment.
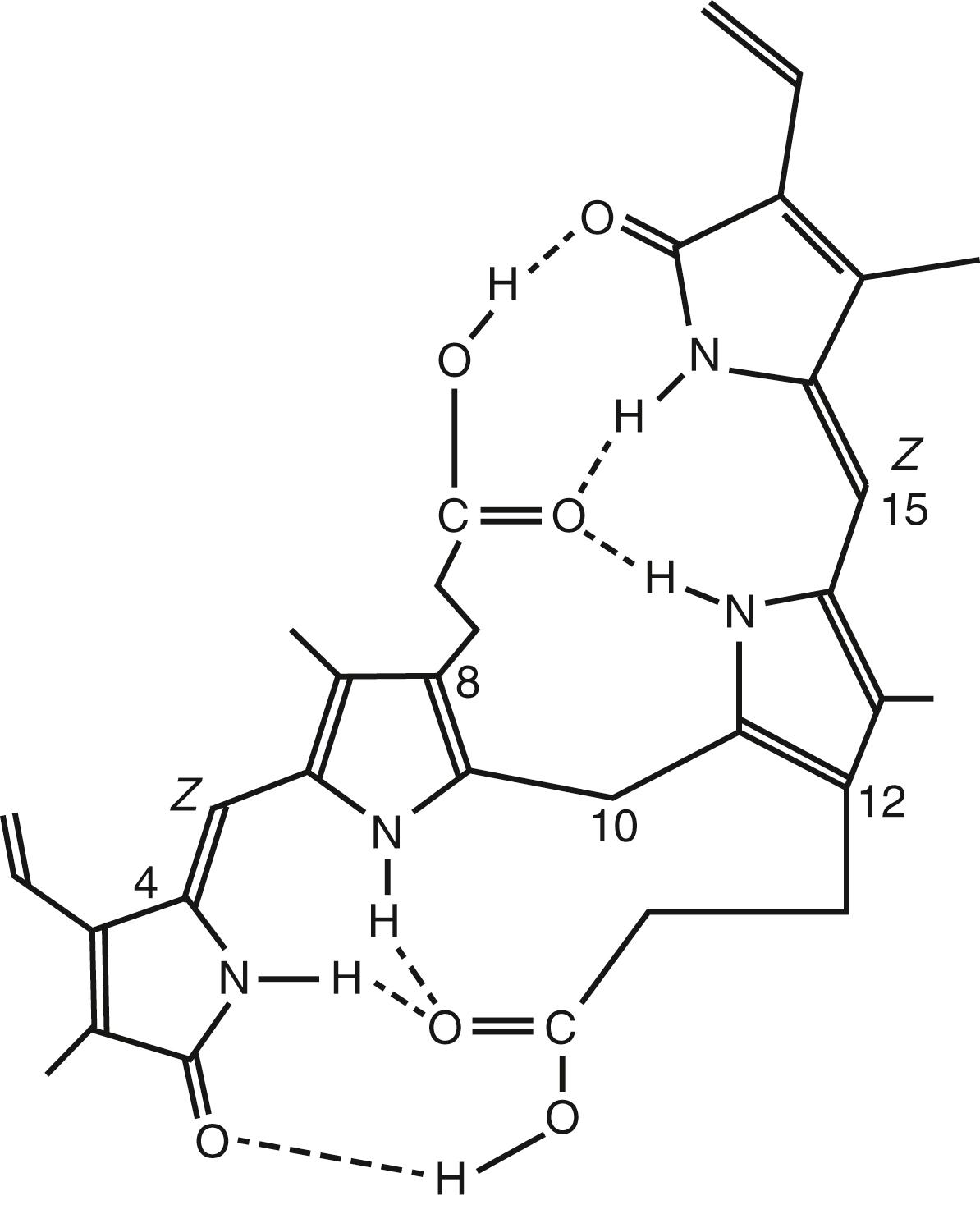
Bilirubin: This term refers specifically, by convention, to the naturally produced 4Z,15Z -IXa isomer, unless indicated otherwise.
Chiral: A structure that is not superimposable on its mirror image is said to be chiral.
Configurational isomers: These are molecules that have the same sequence of atoms and bonds but different fixed three-dimensional arrangements of these atoms. They can only be interconverted by breaking and remaking chemical bonds between adjacent atoms.
Conformation isomers: These are molecules that can be interconverted by rotations about single bonds between atoms and without making or breaking covalent bonds between atoms. In general, conformational isomers interconvert very rapidly at room temperature and cannot be separated.
E/Z bilirubin: A nomenclature system for unambiguously designating the arrangement of atoms around double bonds in molecules is derived from the German words entgegen (opposite) and zusammen (together). Asymmetric substituted double bonds that are not contained within a ring system can have two possible configurations, which are designated as E and Z. Pairs of E/Z configurational isomers are also called geometric isomers.
Photo-bilirubins: A nonspecific term that has been used to designate several products obtained by irradiating bilirubin with light. Because the structures of most bilirubin photoproducts have been elucidated, the term is now redundant and may be abandoned except for use as a collective term to describe photoisomers derived from bilirubin.
Photoisomerization: This reflects conversion of a bilirubin molecule to an isomeric molecule by irradiation with light.
Of the three naturally occurring biologic pigments (the red of hemoglobin, the yellow of bilirubin, and the green of chlorophyll), bilirubin ( Fig. 92.1 ) is unique in that it retains its photosensitivity. Ancient and traditional knowledge of this yellow compound and social practices to use natural sunlight to reduce hyperbilirubinemia in newborns intersected with scientific inquiry in 1956 to 1958 at the Rochford General Hospital, Essex, United Kingdom, where the photoreactivity of bilirubin was originally observed. The scientific community was first introduced to this phenomenon at a meeting on June 1957. The “device-biodesign innovation team” included Dr. RJ Cremer (pediatrician in training), Dr. PW Perryman (biochemist), DH. Richards (laboratory technician), and B Holbrook (device engineer). They provided “evidence for the reduction of circulating bilirubin levels in some cases of neonatal jaundice by exposing these infants to sunlight.” Details were published in their landmark paper in The Lancet , which described their “cradle illumination machine.” It consisted “of a hemi-cylindrical stainless-steel reflector suspended on a movable gantry and adjustable for height. Eight 24-inch 40W blue fluorescent tubes (General Electric Corporation) at 2-inch separation were arranged around the curve of the reflector.” The equipment was designed for use with a bassinet that was wheeled beneath the lights, and a switch was provided to allow illumination to be halved, if desired. Light in the region of 420 to 480 nm, filtered of any dangerous ultraviolet (UV) or x-ray components, was delivered at a very high intensity. Although The Lancet recognized this as a contribution of importance to merit publication, it received only limited attention both in Europe and North America. The breakthrough scientific concepts, novel prototype device, and change in clinical practice, however, subsequently made their way to Italy, Brazil, and other Latin American nations. A decade after the original scientific publication, Dr. JF Lucey opined “no adverse effects had been noted” with the use of phototherapy devices in Latin America. However, his own studies led to serious debates about the effectiveness, timeliness, and safety of using phototherapy. Dr. AK. Brown then conducted the pivotal National Institutes of Health (NIH)-sponsored clinical study of the effectiveness of phototherapy to prevent exchange transfusions in preterm infants. ,
Catabolism of heme from red blood cells (RBCs) occurs in the reticuloendothelial system, where heme oxygenase (HO) degrades heme to biliverdin, which is then rapidly reduced to the lipid-soluble unconjugated bilirubin. Almost all of the bilirubin in blood is reversibly bound to its transport protein, albumin, in a form that can be distributed to a variety of tissues. The bilirubin-binding capacity (BBC) of albumin controls a dynamic relationship between an infant’s levels of bound and unbound (“free”) bilirubin (UB) and his/her ability to tolerate increasing bilirubin loads. The ability of albumin to bind bilirubin is influenced by a variety of molecular, biologic, and metabolic factors, including the rate of bilirubin production, an infant’s gestational age (GA), and the presence of circulating competitive antagonists. UB, in dynamic equilibrium with albumin-bound bilirubin, can cross membranes and enter cells. The cellular uptake of bilirubin is considered a reversible, passive diffusion process, such that bilirubin can be “pulled out of cells” by increasing the extracellular BBC.
The normal lipid- to water-soluble conversion of unconjugated bilirubin is mediated through a process of conjugation occurring in the liver. The uridine diphosphoglucuronosyltransferase (UGT) family of microsomal enzymes mediates active glucuronidation. Once conjugated, the now water-soluble bilirubin is excreted into urine or bile. Phototherapy-induced conversion of bilirubin to more water-soluble and colorless products bypasses this hepatobiliary excretion process.
Direct neurologic measures that quantify bilirubin toxicity have remained elusive. Precise assessment of neurotoxicity must address multiple domains of sensory processing. Table 92.2 lists current and prospective biomarkers for identifying infants most at risk for neurotoxicity. In clinical practice, the ability to use phototherapy more effectively and to reduce the need for exchange transfusion warrants development of an evidence-based risk assessment paradigm to replace the current consensus-based TB thresholds modulated by GA, clinical signs of hemolysis, and the bilirubin-albumin molar ratio (BAMR). ,
| Biomarkers | Specifications | Clinical Use |
|---|---|---|
| Total serum/plasma bilirubin | Consensus threshold values | Adjusted for maturity |
| Rate of bilirubin rise | Increased; at any age | ≥0.2 mg/dL/h |
| Bilirubin production rate | Exhaled carbon monoxide (ETCOc) | >3.5 ppm |
| Bilirubin production rate | Carboxyhemoglobin (COHbc) | >2.5% |
| Unbound bilirubin | ≥10 nmol/L | Phototherapy threshold |
| Unbound bilirubin | ≥18 nmol/L | Crash-cart phototherapy threshold or exchange |
| Utilized bilirubin-binding capacity | ≥45% | Phototherapy threshold |
| Utilized bilirubin-binding capacity | ≥65% | Crash-cart phototherapy threshold or exchange |
Light absorption by bilirubin in the vasculature and extravascular space in the skin transforms the native toxic, nonpolar Z,Z -bilirubin into more readily excretable polar photoisomers: the configurational isomers Z,E - and E,Z -bilirubin and the structural isomers Z - and E -lumirubin. The matching of the absorption spectrum of a bilirubin-albumin solution in vitro with a source of blue light with a peak emission of approximately 460 nm is now considered the global standard of treatment for hyperbilirubinemia.
The perception of light as a continuous energy stream obscures the reality that it comprises discrete packets (quanta) of energy called photons . The energy (E) carried by a photon (quantum) is inversely proportional to the wavelength (λ) of the light as follows:
where ℏ is Planck’s constant, and c is the velocity of light. From this equation, a photon at 400-nm wavelength (visibly blue) contains approximately 25% more energy than one at 500-nm wavelength (visibly green)—that is, the same light exposure measured in energy units (such as μW/cm 2 ) delivers approximately 25% more photons at 500 than would be the case for light at 400 nm. Photochemical reactions require absorption of single photons by individual molecules. The absorption spectrum is a plot of the probability of light absorption as a function of the wavelength of the light. Fig. 92.2 shows the absorption spectra of hemoglobin and bilirubin. Bilirubin appears yellow-orange in white light because it absorbs the blue light portion of the visual spectrum (as well as the UV portion that we cannot see). Fig. 92.2 also shows that hemoglobin strongly absorbs light throughout most of the region of the bilirubin spectrum. The spectrum in this illustration is actually that of bilirubin bound to albumin, which is the form of virtually all the bilirubin in blood.
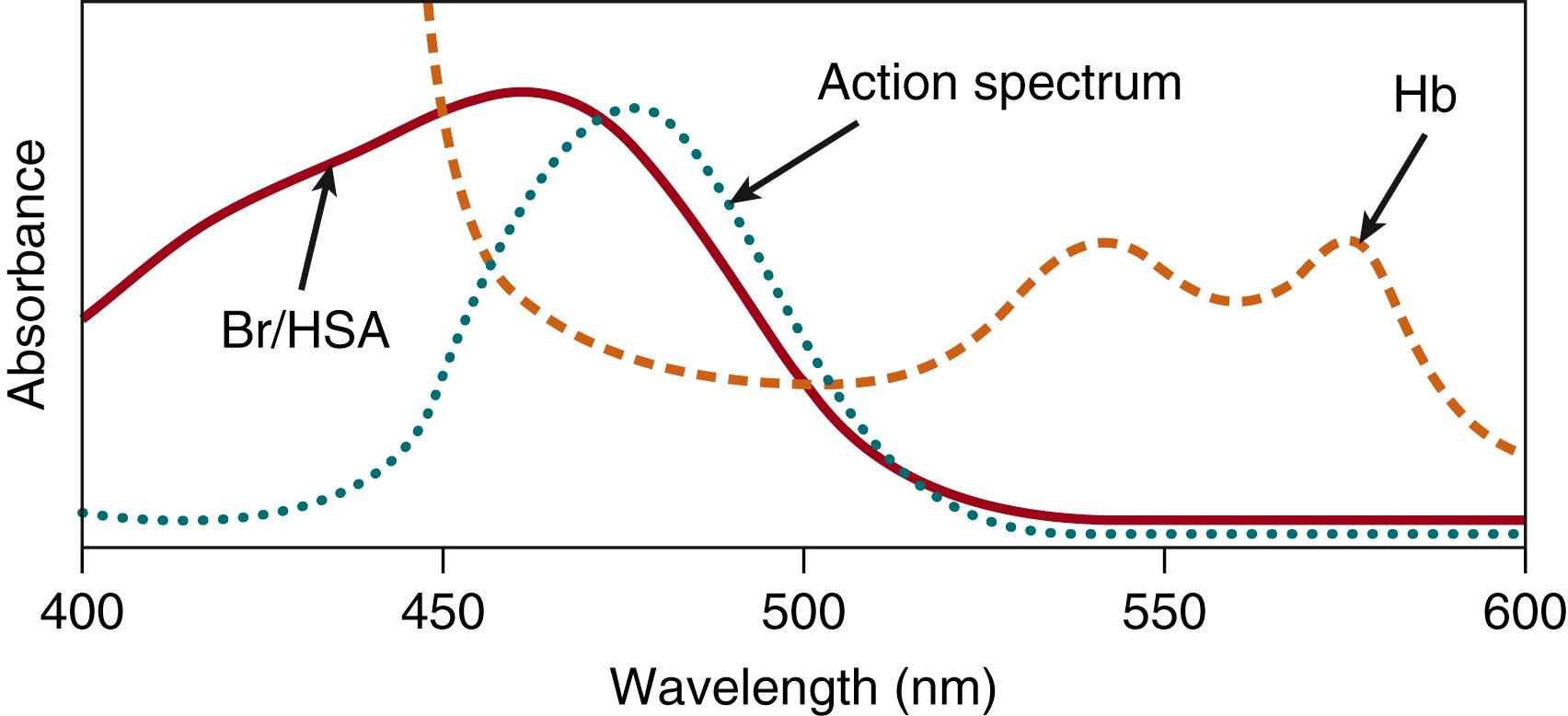
Photons are analogous to the molecules of a drug. Therefore, the wavelength (color) of light (drug) designed to interact with the molecular target (bilirubin) can be predicted by determining its absorption by the molecular target. Additional specificity of the wavelength range may be dictated by the avoidance of untoward side effects. For example, bilirubin absorbs UV light, as do almost all biologic molecules, such as proteins and nucleic acids. UV light absorption by the latter can lead to photochemical alterations that can be deleterious. However, those without prosthetic groups do not absorb blue light; therefore, it is possible to have blue light absorbed by bilirubin without affecting proteins and nucleic acids. The number of therapeutic photons absorbed by the molecular target is analogous to the dose of a drug. The intensity or irradiance (photons per unit time) of the light is analogous to the drug dose. One way absorbed light generally differs from molecular drugs is in the deposition of heat. When a photon is absorbed by a molecule of bilirubin, the energy of the photon is transferred to the molecule and transformed into heat that is quickly transferred to the surrounding environment. These principles of molecular photochemistry are well described.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here