Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mechanical circulatory support (MCS) for the failing heart has become a mainstay of the modern management of patients with both acute and chronic heart failure refractory to pharmacologic and other usual interventions.
Outcomes with MCS have improved so dramatically that the main focus of this arena has now shifted away from simple survival and toward mitigation of risk and minimization of adverse events.
Data taken from experience gained with the first generation of pulsatile devices may no longer be applicable in the current era of nonpulsatile support, but the valuable lessons learned continue to help shape management and clinical decision making.
In addition to the traditional indications for MCS (eg, short-term bridge to recovery and long-term bridge to transplantation), MCS is currently employed for a variety of both short- and long-term modern indications.
Patient status at the time of implementation of rescue MCS is a key factor determining outcome. Deterioration from delayed implementation is associated with worse outcome.
The timing of implantation of a durable left ventricular assist device (LVAD) (eg, as a bridge to transplantation and/or as destination therapy) and perioperative optimization of the patient's nutritional status are key factors determining outcome.
Nonpulsatile support devices have supplanted the first generation of pulsatile ventricular assist devices worldwide, and outcomes have improved dramatically with the technology now available.
Extracorporeal membrane oxygenation is being incorporated more and more often into modern extracorporeal life support algorithms.
The implantable total artificial heart has undergone a resurgence of interest as a bridge to transplantation for patients with biventricular failure and in other scenarios where an LVAD alone would not be ideal.
Mechanical circulatory support (MCS) for the failing heart has become a mainstay of current management of patients with both acute and chronic heart failure refractory to pharmacologic and other usual interventions. In fact, the successes realized to date have been so significant that the main focus of this arena has now shifted away from simple survival and toward mitigation of risk and minimization of adverse events. Undeniably, continued advances in device technology have made this possible, but when coupled with analyses of the enlarging patient management experience, we now have better understandings regarding optimal patient selection and timing of intervention, the expectation of significant improvement in multiorgan function during ventricular assist device (VAD) support, and the ways in which preexisting and demographic risk factors may result in complications.
Although some of the data taken from the experience with the first generation of pulsatile devices may no longer be applicable in the modern era of nonpulsatile support, the valuable lessons learned help shape management and clinical decision making. All of these factors have now resulted in more widespread acceptance of VADs by physicians and patients as a management strategy, as well as an earlier use of VADs in the course of a patient's cardiac deterioration. As such, in addition to the traditional indications for MCS (eg, short-term bridge to recovery and long-term bridge to transplantation), MCS is currently employed for a variety of both short- and long-term indications, including rescue of patients from acute low cardiac output situations (bridge to immediate survival), prevention of further myocardial damage following an ischemic event, prevention of deterioration in multisystem organ function, as a temporizing measure to buy time for recovery, as a bridge to the next step of management, as a bridge to improved candidacy (for transplantation) and, increasingly, as a final management strategy for end-stage heart failure (destination therapy).
Equally important to the advances in MCS technology has been the formal sharing of outcomes data from centers nationwide through the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), a North American registry database sponsored by the National Heart, Lung, and Blood Institute; the US Food and Drug Administration (FDA); and the Centers for Medicare and Medicaid Services. INTERMACS was established in 2005 for adult patients receiving long-term MCS device therapy to treat advanced heart failure. A similar European-based database called EuroMACS exists in Europe. Additional databases collect pediatric MCS data (PEDIMACS) and data about adult heart failure patients with higher (less sick) INTERMACS levels (discussed later) still being medically managed (MEDAMACS).
INTERMACS collects clinical data about patients implanted with durable MCS devices at 1 week, 1 month, 3 months, 6 months, and every 6 months thereafter. Major outcomes after implant (eg, death, explant, rehospitalization, and adverse events) are updated frequently and also as part of the defined follow-up intervals. Additional end points include patients' level of function and quality of life, and the reported improvements in both of these areas have been compelling. These data have proven invaluable to appropriate risk stratification and patient selection, and as new devices are introduced, documentation of functional outcomes beyond simple survival assist in differentiating the value of MCS devices.
The sixth INTERMACS annual report, released in 2014, summarizes the enrollment and outcomes of more than 12,300 patients implanted with left ventricular assist devices (LVADs) between 2006 and 2013. This latest INTERMACS report reveals the dynamic and expanding landscape of modern MCS:
Patient accrual now exceeds 2000 VAD implants per year in the United States alone, and the number of implanting centers in the United States has grown to 158.
At 1 year, overall survival with a durable MCS device now approaches 80%. This is a marked improvement over the 52% 1-year survival rate demonstrated with the pulsatile HeartMate VE in the REMATCH trial reported in 2001 and is a major advance when compared to the medically managed patients in that trial that demonstrated only a 25% 1-year survival rate.
Overall survival rate at 2 years with a durable MCS device now approaches 70%, 3-year survival rate now approaches 60%, and 4-year survival rate now approaches 50%. Survival rate after destination therapy is now higher than 75% at 1 year and higher than 50% at 3 years.
Survival has improved dramatically in recent years, but this has also been influenced by the earlier implantation in the course of a patient's cardiac deterioration. Classification in this regard is denoted by INTERMACS level, a scale of clinical condition ranging from 1 to 7. An INTERMACS 7 patient is simply in the advanced stages of heart failure, with the clinical condition of the patient worsening as the INTERMACS profile number gets lower. An INTERMACS 4 has symptoms at rest, an INTERMACS 3 is hemodynamically stable but inotrope-dependent, an INTERMACS 2 is clinically deteriorating with signs of end-organ dysfunction despite the use of inotropes, and an INTERMACS 1 is in cardiogenic shock.
Based on the collective outcome data in the INTERMACS registry, guidelines for device implantation have developed. For early elective implantation of a durable LVAD, at numerically higher INTERMACS levels (5–7), the risks of adverse events may outweigh the benefits. Conversely, waiting until the patient is at too low an INTERMACS level (1 or 2) with multisystem organ failure is associated with a low probability of ultimate rescue and poor survival. Consequently, at least in the United States, elective LVAD patients are being implanted with durable LVADs when at an INTERMACS level 3 (and in some cases 4), because this seems to be the best timing to balance the risks and benefits and to achieve the best outcomes.
Until 2009, MCS was used most often as a bridge to transplantation. Since 2010, use of destination therapy has grown exponentially, once the HeartMate II received approval as a destination therapy device. INTERMACS data show that destination therapy is now the most common utilization of MCS in the United States, accounting for 41.6% of all LVAD implants in the time period 2011–2013 (compared with 14.7% in 2006–2007). Bridge to candidacy (for transplantation) is now the second most common indication for VADs. The percentage of patients listed for transplantation at the time of VAD implantation has decreased to 21.7% in 2011–2013, compared to 42.4% in 2006–2007. Bridge to recovery and bridge to next decision with short-term VADs and/or extracorporeal membrane oxygenation (ECMO) currently constitutes only a very small percentage of the usage of this technology, compared with the long-term indications.
Cardiogenic shock may be defined as the inability of the heart to deliver sufficient blood flow to meet the metabolic requirements of the body, despite the presence of adequate intravascular volume. Generally, cardiogenic shock entails sustained hypotension (systolic blood pressure [SBP] <90 mm Hg or 30 mm Hg below baseline), low cardiac output with high central filling pressures (eg, cardiac index <2.2 L/min/m 2 with pulmonary capillary wedge pressure (PCWP) >12 mm Hg) and signs of diminished tissue perfusion.
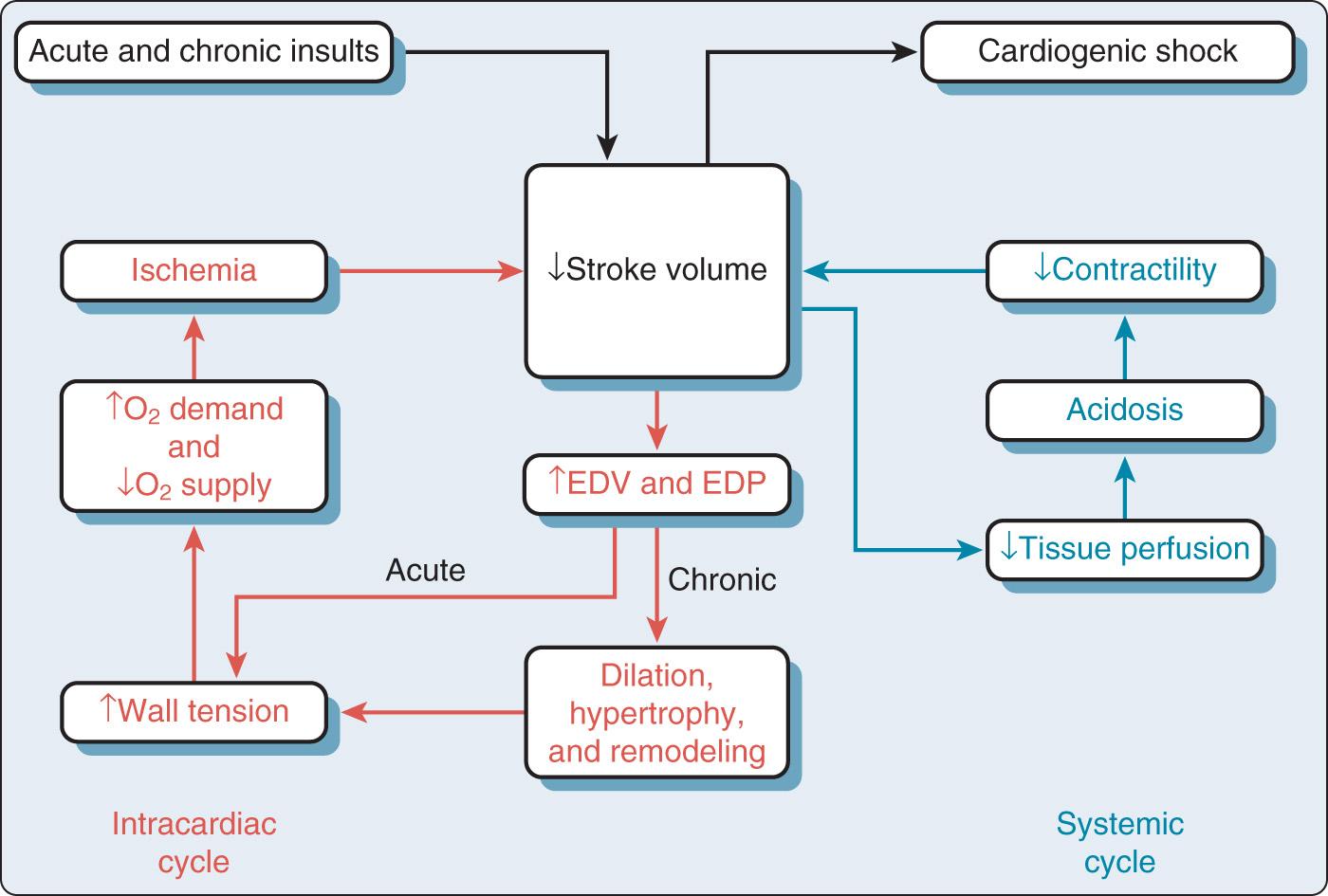
What distinguishes cardiogenic shock from the other forms of shock is the mechanical impairment of pump function. Once a patient develops mechanical pump failure and the intracardiac volumes and pressures begin to rise, the vicious cycles ( Fig. 22.1 ) can result in an imbalance of myocardial oxygen supply and demand, worsening ischemia and resulting in further decreases in ventricular function. Cardiogenic shock may ultimately result if the cycle is not broken.
Manipulations and optimization of preload, afterload, heart rate, and contractility are generally the first-line treatments for acute heart failure. Facilitating recovery requires maintaining adequate myocardial oxygen supply, with the lowest feasible myocardial oxygen demand.
Pharmacologic therapies can potentially improve hemodynamics and stabilize the patient with mild or perhaps moderate cardiac failure. In severe failure, however, pharmacologic management with inotropes and vasopressors comes at the cost of increased myocardial oxygen demand and decreased perfusion to the peripheral and splanchnic circulations during attempts to attain acceptable central hemodynamics. For the myocardium, β-adrenergic stimulation may improve contractility of areas that are well perfused, but it will greatly increase myocardial oxygen demand, feeding into and fueling the vicious cycle.
Vasoconstriction may improve coronary and systemic perfusion pressures but, depending on which vasoconstrictor is used, α-adrenergic stimulation will increase both systemic and pulmonary vascular resistances, making it harder for failing ventricles to eject. This is especially problematic when there is right ventricular (RV) failure, because this will increase the workload of the already struggling right ventricle. Furthermore, intentional vasoconstriction often leaves the peripheral and splanchnic beds underperfused.
Afterload reduction with vasodilators is a common strategy to assist the failing heart because the physiologic principle of ventriculoarterial coupling holds that, regardless of the poor intrinsic systolic mechanics of the failing ventricle, its overall function as a pump can be improved by decreasing the afterload against which it must pump. However, decreased afterload in the setting of developing cardiogenic shock results in hypotension and poor tissue perfusion that predisposes the patient to multisystem organ failure and a poor outcome.
This is where mechanical circulatory assistance can play an important role, effectively breaking the cycle and improving the balance between myocardial supply and demand as well as systemic perfusion. By decompressing the failing ventricle, the increased wall tension that is adversely affecting the supply-to-demand ratio is addressed, which potentially sets the stage for myocardial recovery. Concurrently, effective perfusion is resumed to the heart and the rest of the body, which can stave off multisystem organ failure.
Thus, by using a mechanical device to take over the pumping function of the failing ventricle, the ravages of cardiogenic shock can often be addressed with the one intervention, albeit an extremely invasive one, with potential advantages and disadvantages. Thus, the implementation of mechanical assistance is often approached in a stepwise fashion.
The first step that specifically targets the problem is implementation of intraaortic balloon pump (IABP) counterpulsation. Despite the fact that it was introduced in 1968, the IABP still remains a very commonly used VAD (especially in the United States) because counterpulsation with a properly timed IABP simultaneously increases myocardial oxygen supply and decreases oxygen demand, and is often an effective treatment for left ventricular (LV) failure.
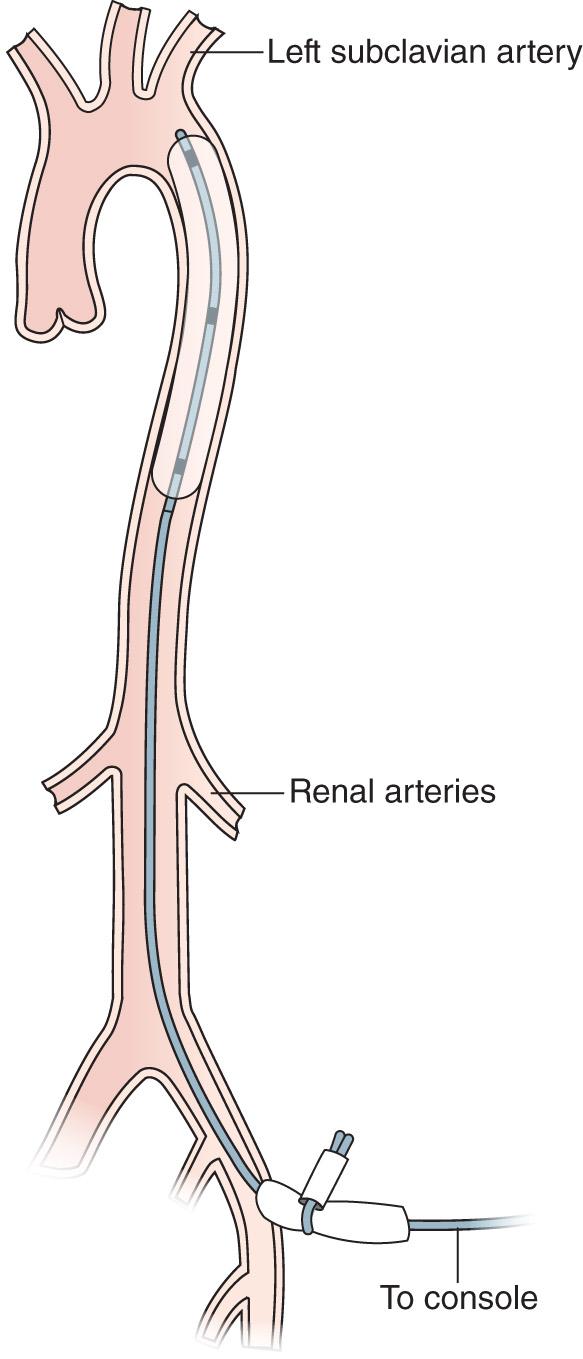
Fig. 22.2 demonstrates a deployed IABP. The device has been inserted percutaneously into the femoral artery and then advanced retrograde up the aorta to its correct position that is just distal to the left subclavian artery. Balloon inflation during diastole occludes the aorta and displaces arterial blood, abruptly increasing the aortic root pressure. This increases coronary perfusion pressure, which increases myocardial oxygen supply (assuming the patient has an adequate level of saturated hemoglobin). Abrupt deflation just before the next systolic ejection decreases the pressure in the aorta in a sudden fashion, facilitating forward ejection from the heart by decreasing impedance to opening of the aortic valve. This results in increased stroke volume and decreased myocardial work and therefore less oxygen demand on the struggling left ventricle. It has been reported that a properly timed, optimally functioning balloon pump can increase cardiac output by 20% or perhaps 30% and decrease afterload by as much as 15%. Of the two, it is generally believed that it is the decrease in oxygen demand that most benefits the failing ventricle supported by this device. In the setting of acute myocardial stunning (eg, as a result of an acute myocardial infarction [AMI]), such a decrease in oxygen demand can help to set the stage for myocardial recovery. In the setting of an acute deterioration of a chronically failing ventricle, the IABP may be used to stabilize hemodynamics as a bridge to intervention. Additional reported benefits of IABP counterpulsation include reduction in systemic acidosis and improvements in cerebral and renal microcirculatory perfusion. However, although a balloon pump is well known to improve cardiac function and overall hemodynamics, as mentioned earlier, it augments forward cardiac output by only 25% to 30% at maximum, and it will not augment anything if there is a complete absence of LV output. As a sole intervention, the IABP cannot be expected to rescue a patient from catastrophic myocardial failure.
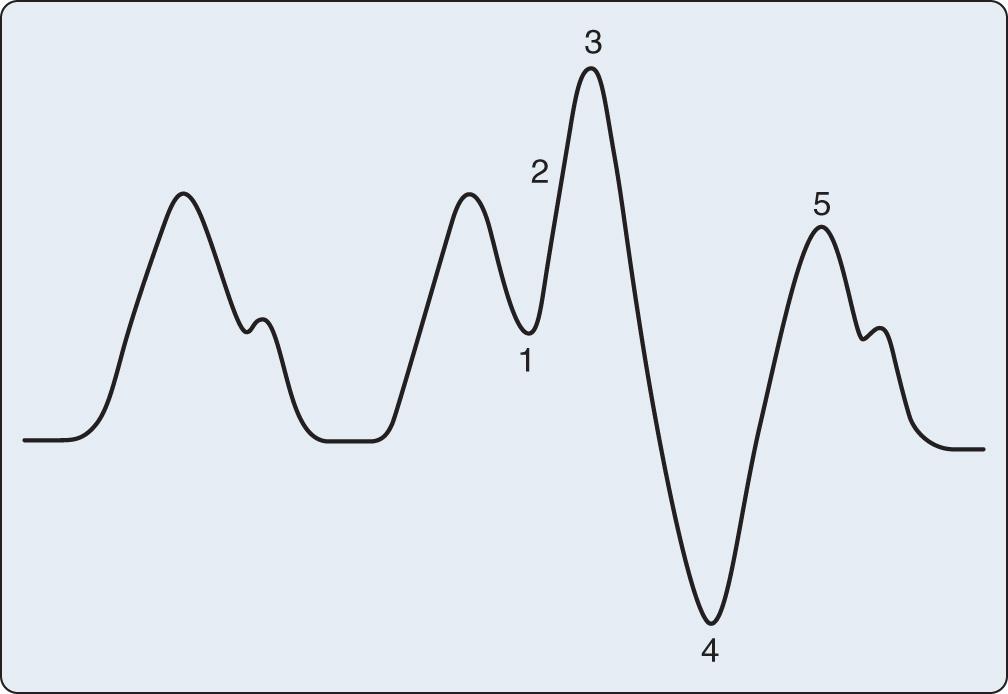
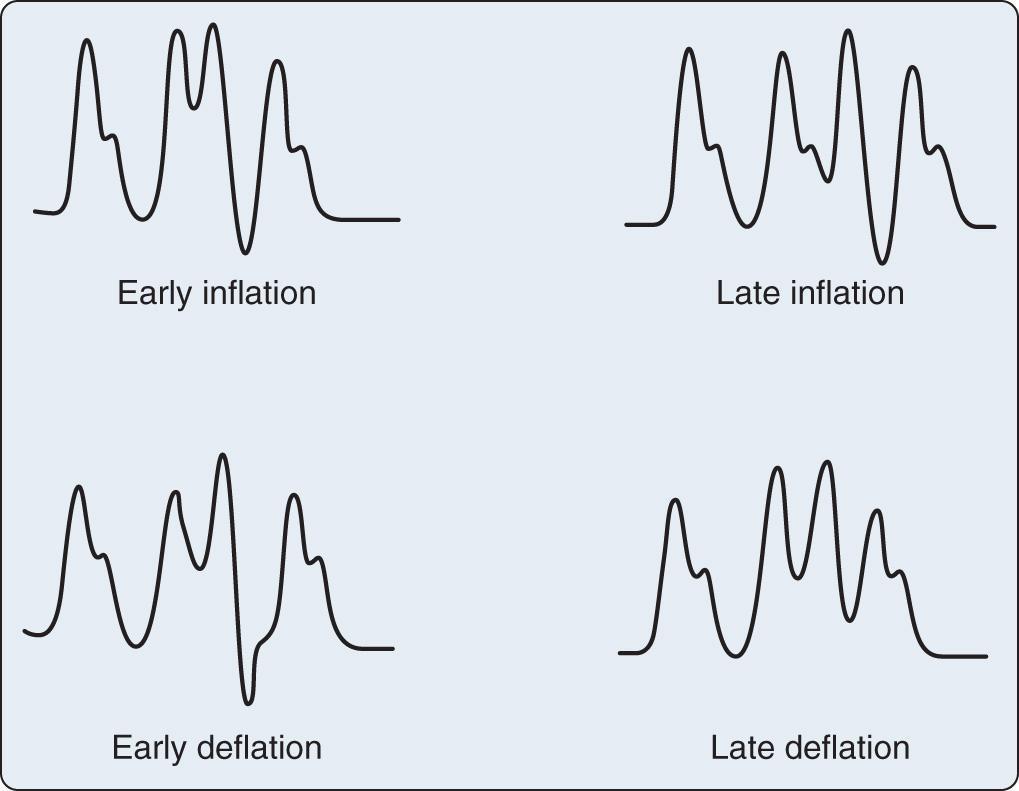
Appropriate timing of balloon inflation and deflation is key to realizing the hemodynamic benefits of the device. The usual trigger for balloon inflation is the R wave of the patient's electrocardiogram (ECG); however, an arterial pressure tracing and pacing spikes can also be used. Regardless of the trigger used, as illustrated in Fig. 22.3 , inflation should always coincide with the dicrotic notch of the arterial tracing and should continue throughout diastole. Deflation should always occur just at end-diastole, immediately before the next systolic ejection. Inflation and deflation at any other point in the cardiac cycle must be manually corrected by adjustments in balloon timing. Fig. 22.4 shows and discusses potential timing errors. Helium is used as the inflation gas in the IABP because of its low viscosity and inert nature. Depending on the level of assistance required, the balloon can be triggered with each cardiac cycle (so-called 1 : 1 assistance), every other cycle (1 : 2), every third cycle (1 : 3), and so forth. Ratios of 1 : 2 or 1 : 3 are ideal for optimizing the timing of inflation and deflation.
Contraindications to the use of the IABP include clinically significant aortic insufficiency, aortic aneurysms, and significant friable atherosclerotic plaques in the aorta. However, the widespread availability of echocardiography to assess patients with cardiac problems, as well as the nearly routine use of transesophageal echocardiography (TEE) during IABP placement in the operative setting, can detect significant atherosclerotic disease in the arch and descending aorta and may help identify patients at high risk. While an ascending aortic dissection still contraindicates IABP use, a descending aortic dissection may no longer constitute an absolute contraindication to IABP use, because, in this era of echocardiography, TEE can be used to ensure the device comes to rest in the true lumen of the aorta.
Indications for the IABP have not changed, but routine IABP usage has recently become somewhat controversial, especially in Europe. It is estimated that 5% to 10% of patients will develop cardiogenic shock following an AMI, and early survival rates for these patients have always been reportedly of the order of 5% to 21%. However, 75% of such patients who were unresponsive to pharmacologic interventions were well known to exhibit hemodynamic improvement with IABP therapy alone, and early survival rates in these patients were reported to approach 93% when treated with IABP counterpulsation. Although decades of nonrandomized studies and clinical observational trials reported such benefit of IABP use, until recently, limited data were available from randomized trials regarding the outcomes of patients with AMI cardiogenic shock in whom IABP counterpulsation was employed.
In the era of thrombolysis as a primary management of AMI, the IABP enjoyed a class I recommendation in international guidelines. However, in current international guidelines ( Box 22.1 ), in the era of percutaneous coronary interventions (PCIs), the recommendation for routine IABP use in the setting of AMI cardiogenic shock has now been downgraded from class I to class IIa in the 2013 American Heart Association (AHA) guidelines and to a class IIb recommendation in European guidelines, on the basis of registry data and a small number of retrospective meta-analyses and randomized trials that failed to demonstrate a mortality benefit from use of the device. However, a number of serious concerns and criticisms (eg, regarding patient selection and timing of intervention) have been raised about the methodologies and protocols used in these trials (and thus in trials analyzed in the meta-analyses), and their negative conclusions have been questioned at the international level because several modern-era trials and analyses have demonstrated outcome benefits from IABP use in the AMI cardiogenic shock population.
Emergency revascularization with either PCI or CABG is recommended in suitable patients with cardiogenic shock due to pump failure after STEMI irrespective of the time delay from MI onset. (Level of evidence: B)
In the absence of contraindications, fibrinolytic therapy should be administered to patients with STEMI and cardiogenic shock who are unsuitable candidates for either PCI or CABG. (Level of evidence: B)
The use of IABP counterpulsation can be useful for patients with cardiogenic shock after STEMI who do not quickly stabilize with pharmacologic therapy. (Level of evidence: B)
Alternative LVADs for circulatory support may be considered in patients with refractory cardiogenic shock. (Level of evidence: C)
ACCF , American College of Cardiology Foundation; AHA , American Heart Association; CABG, coronary artery bypass graft; IABP, intraaortic balloon pump; LVADs, left-ventricular assist devices; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; VAD, ventricular assist device.
A summary of available published data at the time of this writing regarding the routine use of the IABP to treat patients with AMI cardiogenic shock is as follows:
There are no strong data to support the routine use of the IABP in the management of AMI with or without cardiogenic shock, certainly when the device is deployed after PCI.
Conversely, no strong data support the avoidance of the use of an IABP in a timely manner in appropriately selected patients who might benefit from the hemodynamic optimization it can provide. Overall, minimal harm has been demonstrated from its use, specifically regarding the incidence of stroke, bleeding, peripheral ischemic complications, and sepsis.
There are data suggesting that the routine inclusion of an IABP before high-risk PCI decreases the number of procedural complications and the need for rescue.
There are data suggesting that long-term mortality is improved by timely inclusion of an IABP when placed before PCI to ameliorate myocardial ischemia, decompress the ischemic left ventricle, and assist forward flow.
Clearly, the IABP remains useful for stabilizing and improving the hemodynamics of selected patients with low cardiac output. It cannot, however, substantially augment forward cardiac output in patients with severe LV failure. This is where more formal MCS comes into play. Despite the absence of strong data and only a class IIb recommendation in current American College of Cardiology (ACC)/AHA guidelines regarding MCS for acute situations (see Box 22.1 ), the immediate survival of acute cardiogenic shock of any origin will be minimal if nothing is done and is disappointingly low (<20%) if medical management alone is instituted.
When the patient with a failing ventricle has failed to improve substantially following all the usual attempts to optimize and maximize, including an IABP, signs that the patient likely needs formal MCS include the following:
Hypotension (mean arterial pressure [MAP] <60 mm Hg or SBP <90 mm Hg)
Cardiac index <2 liters per minute [LPM]/m 2
PCWP or right atrial pressure (RAP) >20 mm Hg
Systemic vascular resistance (SVR) >2000 dyne-sec/cm
Oliguria, low mixed venous oxygen saturation, and rising lactate.
It cannot be overemphasized that fixing numbers will not inevitably improve outcome. Even if somewhat acceptable central hemodynamics can be created pharmacologically, it is very important to consider evidence of poor organ and peripheral perfusion, such as oliguria, low mixed venous oxygen saturation, and rising serum lactate as indications of the need to support the circulation in a more formal way.
Moreover, it is critically important that the failure of the usual maneuvers to adequately stabilize the patient be promptly recognized, because experience of the past few decades has shown that the timing of implementation of MCS is the most important factor in patient outcome.
One cannot wait to initiate support until there is profound cardiogenic shock with deterioration in major organ function. Some recovery may be possible with restoration of adequate perfusion, but it is very difficult to predict, and study after study has shown that patient status at the time of implantation is the primary determinant of outcome. The longer one waits, the worse the outcome.
To provide formal MCS, the heart and great vessels must be cannulated and connected to a pump. Fig. 22.5 shows the classic cannulation strategies in the heart and great vessels that, until recently, were the only options, regardless of which manufacturer's device was selected to provide the support.
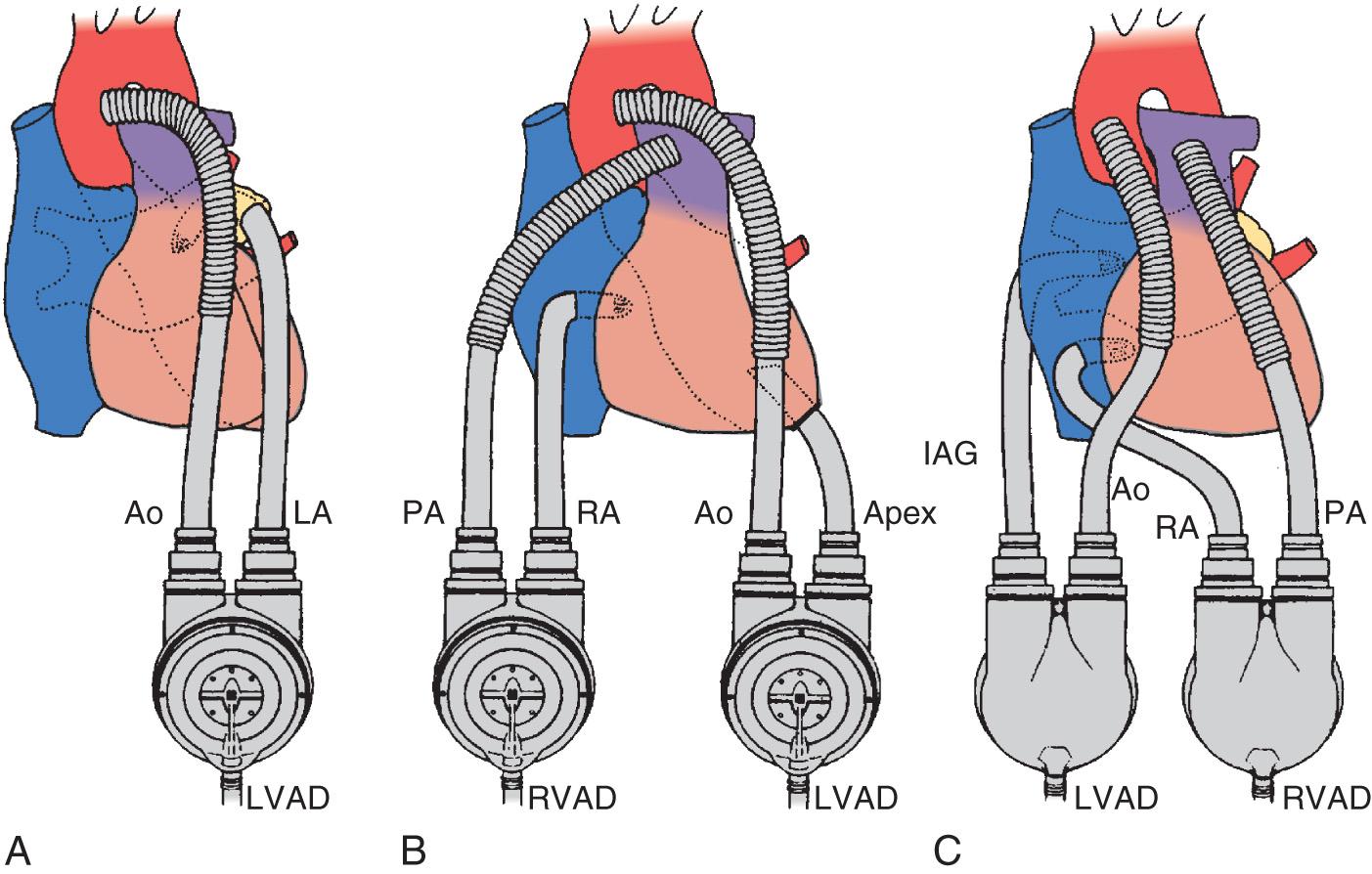
While diversion of blood to the pump provides the stroke volume that is ejected, it also facilitates decompression of the failing ventricle, which is critical, because decreased ventricular wall tension dramatically reduces myocardial oxygen demand, interrupting the cycle of worsening ventricular failure. It should also be noted that currently available VADs do not provide any oxygenation or removal of waste from the blood but simply act as pumps that can promote perfusion of the arterial circulation downstream from the failing ventricle. It is possible with some devices, however, to introduce an in-line membrane oxygenator and extracorporeal carbon dioxide (CO 2 ) removal system for patients with concomitant respiratory failure.
Once the decision to provide MCS has been made, an appropriate device is selected. A number of different devices may be available, and the device selected for a given patient depends primarily on the following factors:
The anticipated duration of support required (different devices have different intended durations of use rooted in their engineering, and there are also considerations of FDA approval of the various devices).
Whether univentricular or biventricular support is required (some devices are intended to support just the left ventricle, although some can be configured to support either, or both simultaneously).
The degree of pulmonary dysfunction such that ECMO is required.
The urgency of the situation (some devices can be deployed rapidly, perhaps even at the bedside, whereas others require transfer to the operating room for sternotomy and cardiopulmonary bypass [CPB]).
The availability of the device.
As patient management experience in the field has grown and the outcomes have improved with more advanced devices, there has been an interesting shift away from the question, who needs a VAD? to the more pertinent question, who probably should not receive one? The only absolute contraindications to even temporary VAD use are prognostic factors that would preclude survival even with the restoration of perfusion to the vital organs and peripheral tissues. Thus, the potential for recovery is the paramount consideration. On many occasions, however, the myocardial insult is at least initially the primary problem, and more relative contraindications to VAD support will need to be considered.
Box 22.2 lists a number of commonly encountered considerations and relative contraindications to VAD support that include a variety of anatomic issues and other patient factors that create management issues, make VAD placement or use difficult, make complications more likely, or make meaningful recovery unlikely. Although the advent of modern devices and management strategies has rendered some of these relative contraindications essentially moot, all must be considered and addressed.
The patient will not survive regardless of the restoration of adequate systemic perfusion
Patient is not a transplant candidate (unless destination therapy or bridging to improved candidacy is the intention and a durable LVAD is being implanted)
In situ prosthetic valves
Clinically significant aortic insufficiency
Clinically significant tricuspid insufficiency
Mitral or tricuspid stenosis
Congenital heart disease
Intracardiac shunts
Previous cardiac surgery
Poor nutritional status
Extremes of body surface area
Advanced systemic disease (severe COPD, malignancy, ESLD, ESRD, sepsis, progressive neurologic disorder, etc.)
COPD, Chronic obstructive pulmonary disease; ESLD, end-stage liver disease; ESRD, end-stage renal disease; LVAD, left ventricular assist device.
INTERMACS data reveal that short-term MCS support constitutes a relative minority of the usage of this technology, but the use of a VAD as a bridge to recovery remains critical to the survival of patients with acute, refractory, severe cardiac failure. However, the traditional conception of short-term use of a VAD only as a bridge to recovery has now been expanded to include concepts such as a bridge to immediate survival, bridge to next decision, bridge to a bridge, and bridge to surgery (sometimes at another center). It is common for MCS to be implemented to allow for patient transport to a transplant center.
Thus, as listed in Box 22.3 , common scenarios in which temporary VAD insertion may be indicated include ventricular failure due to stunned myocardium following open heart surgery, AMI, following a failed heart transplantation, cardiogenic shock due to acute myocarditis, stress-induced cardiomyopathy, following a cardiac catheterization laboratory complication, and in the setting of RV failure in the patient already supported by an LVAD.
Stunned myocardium following open heart surgery
Acute myocardial infarction
Following a failed heart transplantation
Cardiogenic shock due to acute myocarditis
Stress-induced cardiomyopathy
Following a cardiac catheterization lab misadventure
In the setting of right ventricular failure in the patient already supported by left ventricular assist device
Before 1992, when the Abiomed BVS 5000 became clinically available, standard centrifugal pumps were used to provide either univentricular or biventricular short-term mechanical circulatory assistance. Currently, this type of very basic device would be used for pediatric applications (with small-caliber cannulas to limit flow) or for ECMO; clinicians are beginning to incorporate ECMO more frequently into resuscitative efforts as a bridge to next decision. Such a strategy has been termed extracorporeal life support , in which a patient with refractory cardiogenic shock with uncertain outcome is placed on ECMO for a few days. In this fashion, a less expensive centrifugal device is used to determine if there is reasonable likelihood of survival, before committing the patient to a more formal (and much more expensive) VAD. As experience grows, ECMO utilization is likely to increase in these circumstances, concurrent with the availability of more advanced devices that are replacing standard centrifugal pump head technology.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here