Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We would like to acknowledge the work of Patrick Duff, MD, whose previous chapter on Maternal and Fetal Infections served as the template for this chapter and has been edited accordingly.
Infectious diseases are among the most common problems encountered by obstetricians. Pregnancy is a period of immune reshaping, with alterations in the maternal immune system aimed at protecting the fetus from rejection potentially rendering greater maternal susceptibility to infection. In addition, mucosal and epithelial changes occurring during pregnancy may render women more susceptible to acquisition of certain infections. Viral infections can be an important cause of morbidity to the maternal-fetal dyad. Some infections such as influenza, measles, or yellow fever pose a primary risk of morbidity to mothers. Other viral infections such as those caused by herpes simplex virus, parvovirus B19, cytomegalovirus, rubella, or Zika virus are a cause of severe complications to the fetus and newborn infant, many of which are responsible for significant teratogenicity or other fetal/infant adverse events. Other viruses can pose serious threats to maternal-fetal health, as in the case of chikungunya virus, herpes zoster virus, human immunodeficiency virus, Ebola, and, more recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This chapter reviews the most common viral infections occurring during pregnancy.
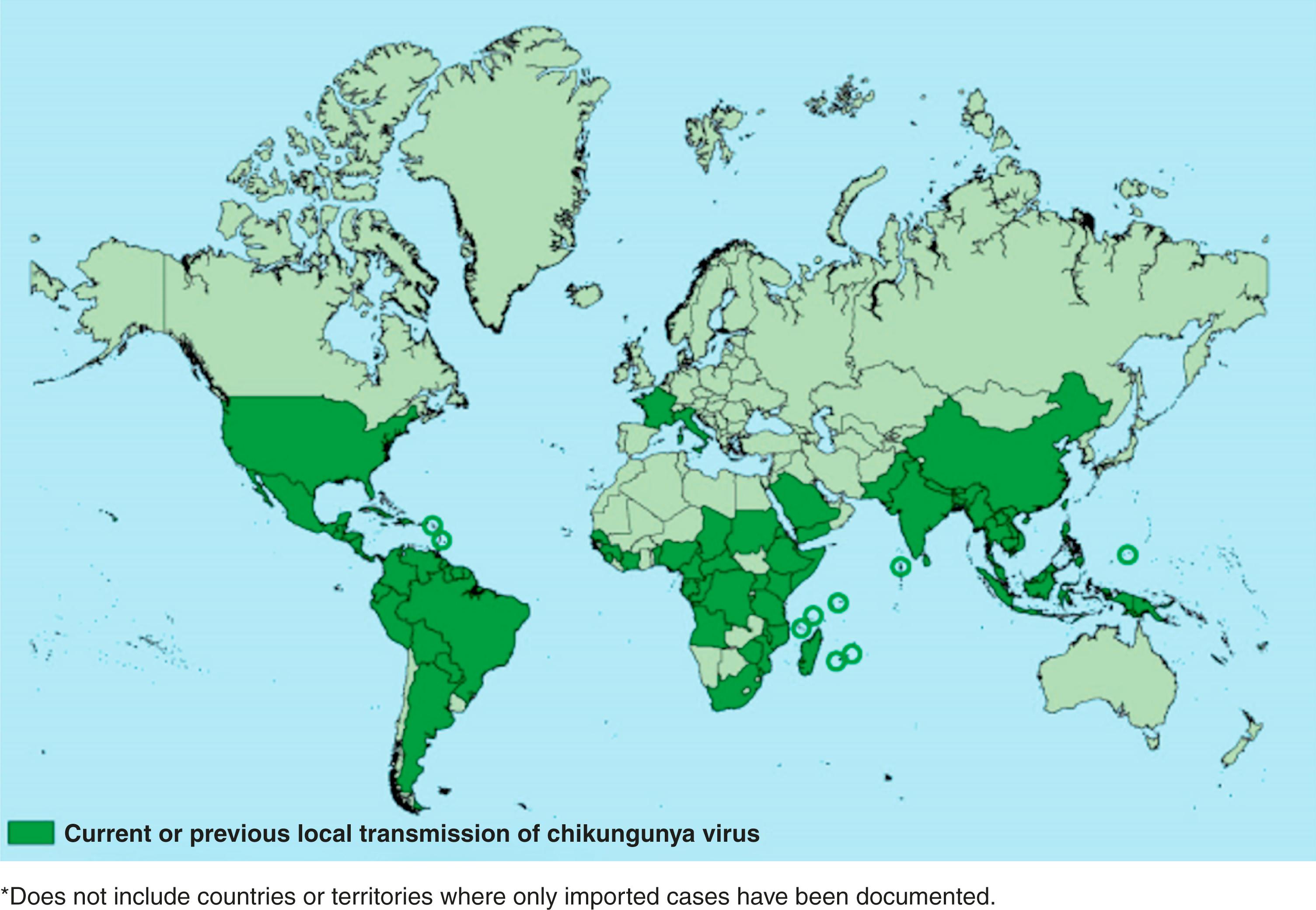
Chikungunya is a term in the Makonde language, a central Bantu language spoken in southeast Tanzania and northern Mozambique. It comes from the verb kungunyala , which means “that which bends up,” and is thought to reflect the contorted posture of people with severe joint pain due to this arboviral illness. The disease was first described in 1955, following a 1952 outbreak on the Makonde Plateau. Since its discovery, multiple outbreaks of this disease have occurred in Africa, South Asia, the Pacific Islands, and, more recently, in the Americas ( Fig. 49.1 ). In the continent where it was first described, chikungunya followed mainly a sylvatic cycle. Since 2005, however, after many decades of inactivity, it reemerged, causing large outbreaks globally. The major outbreak was on the Indian Ocean Island of Réunion of East Africa, where it infected approximately one-third of the island population of 770,000. Much of the information about chikungunya in pregnancy and repercussions to the neonate is known from this large outbreak. In 2006, the virus caused a large outbreak in India, with over 1.25 million suspected cases. It was introduced in the Americas between 2013 and 2014 and has been a leading cause of outbreaks since then. Globally, chikungunya virus is responsible for about 3 million cases a year.
Chikungunya virus is a single-stranded RNA virus in the genus Alphavirus in the Togaviridae family. It is vector-borne, which makes it an arbovirus, but it is genetically very distinct from other common arboviruses such as Zika virus, dengue viruses, Japanese encephalitis virus, and yellow fever virus, all of which are flaviviruses. Therefore serologic diagnostic tests for chikungunya virus do not cross-react with these other common arboviruses. The increased severity of outbreaks since 2005 may be due to a mutation in the genetic sequence of the viral E1 protein, the E1-A226V variant, which allows the virus to multiply more easily in mosquito cells. This might have expanded the capacity of the virus to infect additional mosquito hosts beyond its usual vector Aedes and further enable global dissemination.
In contrast to dengue and Zika viruses, most people infected with chikungunya virus have symptomatic illness (72%–97% of individuals). The disease most often is characterized by acute onset of high fever (typically >39°C [102°F]) and polyarthralgia. Other symptoms may include headache, myalgia, arthritis, conjunctivitis, nausea, vomiting, or maculopapular rash. Fever typically lasts for several days to a week and can be biphasic. Rash usually occurs after onset of fever and typically involves the trunk and extremities, but the palms, soles, and face may be affected. Joint symptoms are often severe and debilitating, usually are bilateral and symmetric, and occur most commonly in the hands and feet but can affect more proximal joints. Clinical laboratory findings may include lymphopenia, thrombocytopenia, increased creatinine, and hepatic transaminases.
Acute symptoms typically resolve within 7 to 10 days, but rare complications include uveitis, retinitis, myocarditis, hepatitis, nephritis, bullous skin lesions, hemorrhage, meningoencephalitis, myelitis, Guillain-Barré syndrome, and cranial nerve palsies. Some patients have persistent debilitating joint pain following acute illness that may persist for months to years. Others report relapse of rheumatologic symptoms in the months following acute illness. Mortality from this condition is rare, although the virus has been associated with fetal death and severe neonatal complications. The disease does not appear to be more severe in pregnant women than in the general population but is associated with poor pregnancy outcomes.
The major vector of transmission is Aedes mosquitoes, and upon inoculation the virus replicates in the human skin and blood, although it has been detected in muscles, joints, lymph nodes, spleen, and brain. As with other arboviruses, vertical transmission of chikungunya virus can occur. During the outbreak in Réunion Island the first reports of vertical transmission surfaced, and the risk to pregnancy and fetal survival became more evident. In mothers with high viremia at the time of birth, maternal-to-child transmission can be as high as 49%. In newborns, infection with chikungunya can result in multiorgan involvement, with the presence of fever, rash, irritability, and meningoencephalitis, as well as reports of microcephaly and neurodevelopmental delay. , , Neonatal deaths have also been reported, and the virus has been associated with fetal loss between 12 to 15 weeks of pregnancy. The viral genome has been detected in the amniotic fluid, placenta, and fetal brain by reverse transcriptase polymerase chain reaction (RT-PCR). Placental studies in aborted fetuses have shown inflammatory infiltrate in the decidua and chorionic villi, areas of calcification, edema and deposition of fibrinoid material, and ultrastructural changes, such as mitochondria with fewer cristae and ruptured membranes, endoplasmic reticulum with dilated cisterns, dispersed chromatin in the nuclei, and the presence of apoptotic bodies. The virus has been identified in placentas through immunohistochemistry and electron microscopy.
In a meta-analysis of 42 studies selected from a pool of 227 reports, for a total of 266 chikungunya-affected neonates, the vertical transmission rate was 50% (although this was primarily in women viremic at birth on Réunion Island), preterm delivery was reported in 45% of pregnancies, fetal distress in 20%, and fetal loss in 2% of cases. Among infants with perinatally acquired chikungunya, the frequency of encephalopathy/encephalitis was 69%. Most infected newborns were healthy at birth but developed a sepsis-like picture within the first week of life.
Preliminary diagnosis is based on the patient’s clinical features, locations and dates of travel, and activities. In endemic areas, clinical working definitions have been established to differentiate chikungunya, dengue, and Zika infections in the absence of widespread availability of laboratory testing. Laboratory diagnosis generally is accomplished by testing serum to detect virus, viral nucleic acid, or virus-specific immunoglobulin M (IgM) and neutralizing antibodies. During the first week after onset of symptoms, infection often can be diagnosed by performing RT-PCR on serum. Chikungunya virus–specific IgM and neutralizing antibodies normally develop toward the end of the first week of illness. A plaque-reduction neutralization test (PRNT) can be performed to measure virus-specific neutralizing antibodies and to discriminate between cross-reacting antibodies with similar viruses (i.e., Mayaro and O’nyong-nyong viruses). The IgM antibodies usually persist for 30 to 90 days, but longer persistence has been documented. Thus a positive IgM test result on serum occasionally may reflect past infection. Immunohistochemical staining can detect specific viral antigen in fixed tissue. Routine molecular and serologic testing for chikungunya virus is performed at state health departments and the US Centers for Disease Control and Prevention (CDC). Immunohistochemical staining and PRNT are performed at the CDC and other reference laboratories.
There is no antiviral treatment available for chikungunya. The primary treatment is supportive care and includes rest, fluids, analgesics, and antipyretics. In areas where dengue is endemic, acetaminophen is the preferred treatment for fever and joint pain until a dengue diagnosis is ruled out, to reduce the risk of hemorrhagic complications. Patients with persistent joint pain may benefit from the use of nonsteroidal antiinflammatory drugs, corticosteroids, and physical therapy. For infants, neonatal critical care support is generally necessary.
There is no available vaccine. Prevention relies on avoidance of vector exposure, use of repellents, wearing long pants and long sleeves while outdoors, use of air-conditioned dwellings, and limit of outdoor activities. Screening of blood products and organs for donation is necessary in endemic areas.
Dengue is an arboviral infection caused by one of four flaviviruses, dengue viruses 1–4. These viruses cause symptomatic disease in about 25% of patients and asymptomatic disease in 75% of infections. The virus is transmitted by an infected mosquito of the Aedes species, most commonly Aedes aegypti , but several other types of Aedes mosquitoes can transmit dengue, including Aedes albopictus , which is more prevalent in temperate climates. These mosquitoes also transmit other arboviral diseases, including other flaviviruses such as Zika virus or yellow fever, or distinct viruses such as chikungunya. Infection with one dengue virus produces lifelong immunity against that virus but does not confer complete protection against the other dengue viral types. After 1 to 3 years, the individual who was infected with one viral strain loses cross-protection against other strains and becomes susceptible to the other three strains. Infection with a different strain in the presence of ineffective cross-protection tends to predispose individuals to more severe forms of dengue upon repeat infection, a phenomenon termed antibody-dependent enhancement .
Dengue is an immense public health problem in tropical and subtropical regions of the planet, with 50 to 100 million dengue cases occurring annually in over 100 countries, with 40% of the global population living in endemic areas. Dengue is endemic in Puerto Rico and the Virgin Islands, with periodic outbreaks also occurring in American Samoa. Dengue outbreaks have been reported in Florida, Texas, and Hawaii in the last decade. Dengue is also an important cause of febrile illness in returning travelers from the Caribbean, South and Central America, and South Asia. Although dengue occurs in children and adults alike, it is most likely to cause severe disease in young infants, pregnant women, and patients with chronic illnesses and immunosuppression.
Aedes aegypti mosquitoes are originally from the African continent but over centuries established themselves in the American continent accompanying human migration. Humans are the main host for dengue viruses and the main source of viral delivery for Aedes mosquitoes. The female mosquito is the one who feeds on blood meals and is a daytime feeder. Mosquito eggs are extremely resilient and can become viable following contact with water after extended periods of time in dry weather. Therefore warm climates, storms, and pooled/stagnant water are excellent mosquito breeding grounds. A sylvatic nonhuman primate dengue virus transmission cycle exists in parts of Africa and Southeast Asia, but humans are very rarely affected. Dengue virus incubation period in the mosquito is generally 8 to 12 days (extrinsic incubation), during which time the virus reproduces in the midgut of the mosquito and then migrates to the salivary glands, where it is inoculated into humans. Mosquitoes remain infected with dengue throughout their 30-day life span.
Infected individuals have a 3- to 14-day incubation period (intrinsic incubation) and are viremic for generally a week. If bitten by a second mosquito, they are capable of perpetuating the dengue cycle. Regardless of the presence of symptoms, infected individuals can transmit dengue virus to mosquitoes 1 to 2 days before symptom onset and for the 7 days of ensuing viremia. While viremic, individuals can also transmit infection through blood products, transplantation of organs, or accidental exposure to blood products. Dengue has also been shown to be transmitted in utero, at the time of labor and delivery, and through breastfeeding.
Most dengue infections are asymptomatic, while symptomatic illness has a wide range of manifestations. The severe forms of dengue, such as dengue hemorrhagic fever or dengue shock syndrome, occur in about 5% of cases, depending on the dynamics and timing of prior epidemics where other strains of dengue viruses circulated. Other complications of dengue that are uncommon include myocarditis, pancreatitis, hepatitis, and neuroinvasive illness. Dengue generally has an acute onset with high fever accompanied by severe myalgias and joint and/or bone pain (severe enough for dengue to be known as breakbone fever), headache, retro-orbital pain, facial redness, erythematous oropharynx, maculopapular rash, as well as leukopenia and thrombocytopenia with petechiae. This febrile phase of dengue lasts from 2 to 7 days. Once the fever subsides, some patients experience a period of increased vascular permeability with an increasing hematocrit due to hemoconcentration. The time when plasma leakage occurs is the critical phase and lasts about 24 to 48 hours. This is followed by a convalescent phase where there is gradual improvement of the hemodynamic instability. Patients who progress to a complicated dengue course usually present with persistent vomiting, severe abdominal pain, bleeding gums, and dyspnea during the end of the febrile phase. These patients can present with early signs of shock and rapidly declining platelets. If the vascular permeability is pronounced, patients may develop pleural effusions, ascites, hypovolemic shock, and bleeding.
There are multiple publications in the medical literature highlighting a higher risk of adverse pregnancy and infant outcomes when women have dengue virus infection during pregnancy. In a registry population-based study evaluating almost 17 million live births in northeastern Brazil, where birth and dengue registration records were linked from 2006 to 2012 to identify women who had dengue during pregnancy, dengue hemorrhagic fever was associated with preterm birth (odds ratio [OR] = 2.4; 95% confidence interval [CI], 1.3–4.4) and low birth weight (OR = 2.1; 95% CI, 1.1–4.0) but not small for gestational age (OR = 2.1; 95% CI, 0.4–12.2). The magnitude of the effect was higher in the acute disease period. Another analysis from the same group of investigators using the same methodology demonstrated that dengue in pregnancy increases the risk of maternal death by 3-fold (95% CI, 1.5–5.8), while dengue hemorrhagic fever increases the risk of maternal death by 450-fold (95% CI, 186.9–1088.4) when compared to mortality of pregnant women without dengue. The increase in risk occurs mostly during acute dengue (OR = 71.5; 95% CI, 32.8–155.8), compared with no dengue cases. Using the same cohort, investigators also reported a very high frequency of congenital brain malformations in mothers who had dengue during pregnancy prior to the introduction of Zika in the country. In an analysis of over 16 million live births using the Brazilian single health care system database (Datasus), investigators reported that dengue virus infection during pregnancy increased the risk for any neurologic congenital anomaly in the infant by roughly 50% and for other congenital malformations of the brain fourfold. However, these results were not statistically significant (confidence intervals included 1.0). In this study, neurologic congenital abnormalities occurred in 13,634 infants or 0.8% of the cohort. Authors alluded to the possibility that flaviviruses other than Zika virus may be associated with central nervous system (CNS) malformations, but future prospective studies should further investigate this finding. In a study of 82 patients with dengue delivering at nine Mexican public hospitals along the Gulf of Mexico in 2013, among the 13 patients with severe dengue complications, fetal distress, emergency surgical deliveries, prematurity and low birth weight, obstetrical hemorrhage, and maternal death (5 patients) were highly prevalent. Nevertheless, not all studies demonstrate such degrees of maternal and infant adverse outcomes. In a study from Malaysia from 10 years earlier, evaluation of 2531 paired maternal-cord blood specimens at delivery identified a dengue-specific positive IgM in 63 women (2.5%; 95% CI, 1.9–3.2%) with one positive infant result in 64 infants tested (1.6%; 95% CI, 0.0–9.5%). All RT-PCR tests were negative, and rates of preterm birth, mode of delivery, postpartum hemorrhage, low birth weight, and neonatal outcomes were not different. However, it is possible that in this older study fewer women had severe dengue illness. A meta-analysis encompassing 6071 pregnant women, 292 of whom had dengue during pregnancy, found associations between dengue and miscarriage (OR = 3.51; 95% CI, 1.15–10.77), preterm birth (OR = 1.71; 95% CI, 1.06–2.76), and low birth weight (OR = 1.41; 95% CI, 0.90–2.21).
During the viremic phase, RT-PCR can identify the dengue virus. Other methods of diagnosis include antigen tests, which identify the dengue virus nonstructural protein-1 (NS-1), or detection of IgM antibodies to dengue, which can be present within a few days of disease onset, by enzyme immunoassay (EIA). Serologic assays, however, can have false-positive results due to cross-reactivity with other flaviviruses, particularly Zika virus. Dengue IgG remains positive for life and is not helpful for diagnosis of new acute dengue infections. It also cross-reacts with several other flaviviral antibodies, including Zika, West Nile, Japanese encephalitis, or yellow fever. Serologic distinction between dengue and Zika virus infections often requires performance of PRNT assays, which are not routinely performed in commercial labs. Reference testing for dengue virus is available through the CDC dengue branch.
There is no specific antiviral treatment for dengue, and treatment is supportive. Hydration and avoidance of the use of aspirin and nonsteroidal antiinflammatory drugs are recommended to minimize risk of bleeding. Early recognition of shock and intensive supportive therapy can reduce risk of death 10-fold. Fluid volume replacement and hemodynamic stability are key to management of severe disease. Early signs of shock should be monitored, as well as occult bleeding. Plasma leak can lead to end-organ damage. The possibility of fluid overload should also be monitored.
A recombinant live attenuated tetravalent dengue vaccine with a three-dose schedule administered at 0, 6, and 12 months has been approved for use in 11 countries (i.e., Brazil, Costa Rica, El Salvador, Guatemala, Indonesia, Mexico, Paraguay, the Philippines, Peru, Singapore, and Thailand) with the caveat that they should be given to individuals who previously had dengue. A number of other vaccine candidates are in clinical trials to evaluate immunogenicity, safety, and efficacy, as are innovative approaches to vector control.
People traveling to areas with endemic dengue are at risk of infection and should use precaution against mosquito bites. Air-conditioned accommodations are preferable and those with screened windows and doors. As Aedes mosquitoes bite mainly during the daytime, bed nets are indicated for those who sleep during the daytime, such as children. Travelers should wear clothing that fully covers arms and legs, especially during early morning and late afternoon. Adults, including pregnant women, should use mosquito repellents containing up to 50% N,N-diethyl-meta-toluamide (DEET) on themselves or on their clothing. For individuals with laboratory-confirmed prior dengue infection, immunization with a tetravalent dengue vaccine is an option. Dengue is a notifiable disease in the United States, and suspected cases should be reported to local or state health departments.
West Nile virus (WNV) is a flavivirus originally isolated in a woman in the West Nile region of Uganda in 1937. WNV is endemic in Africa, Asia, and the Middle East and was first identified in North America in 1999 when it caused its first outbreak. Most cases of WNV are asymptomatic, with symptoms present in 20% to 40% of patients. The fact that asymptomatic infections tend not to be reported may explain the high ratio of neuroinvasiveness identified in CDC-reported cases. Neuroinvasive potential, especially in cases of encephalitis, is associated with advancing age. Immune deficiency states, diabetes, hypertension, alcohol abuse, renal disease, and male gender are additional risk factors.
WNV is an RNA virus and a member of the Japanese encephalitis virus antigenic complex. It is maintained in nature through biologic transmission involving bird reservoirs and mosquitoes, with humans being an incidental host via a bite of an infected mosquito. WNV is transmitted to birds through the bite of infected Culex species of mosquitoes, the main vectors in the United States being Culex pipiens , Culex tarsalis , and Culex quinquefasciatus . Mosquitoes become infected themselves by biting infected birds. Predator birds (such as hawks and owls) or scavengers (such as crows) may become infected after eating infected sick or dead birds. Humans and other mammals, such as horses, for example, become infected through the bite of infected mosquitoes, but they do not develop enough high-level viremia to pass the virus on to biting mosquitoes. For this reason, they are considered “dead end” hosts. West Nile cases in humans tend to occur in the late summer months, and there is no documented animal-to-human (except mosquitoes) or human-to-human transmission, except through rare cases of mother-to-child transmission or transfusion of blood products. The incubation period is 2 to 14 days.
Although most pregnant women with WNV infection deliver healthy infants without repercussions, there is evidence of transplacental transmission, and there are a few reported cases of infants born with CNS malformations (chorioretinitis and severe brain damage) after maternal WNV infection in the second trimester and reports of neonates born to mothers with recent WNV infection who developed encephalitis soon after birth. Following the Zika virus epidemic, animal studies of teratogenicity in different arboviruses found evidence that both WNV and Powassan virus (a tick-borne flavivirus) caused fetal demise and CNS malformations in mice. Larger-scale studies of WNV-infected pregnant women are needed to determine the true frequency of problems associated with infection during pregnancy.
Most human WNV infections are subclinical—only one in five develop a mild febrile illness, with symptoms lasting 3 to 6 days, termed West Nile (WN) fever, which is very similar in clinical presentation to other arboviral conditions such as dengue or Zika. Rash in WN fever occurs in 25% to 50% of patients who have symptoms and is similar to the maculopapular rash seen in Zika, often being pruriginous. Some studies suggest that the presence of a rash is inversely proportional to the risk of neuroinvasive disease and death. Neuroinvasive disease is diagnosed in less than 1% of adult cases. Pregnant women with symptoms may present with fever, headache, fatigue, a truncal skin rash, lymphadenopathy, and eye pain.
The diagnosis of WNV is based on clinical symptoms and serology. WNV serum IgM and IgG and cerebrospinal fluid (CSF) IgM are used for laboratory confirmation; cross-reaction with antibodies to other flaviviruses has been reported. PCR testing is limited secondary to transient and low-level viremia. Amniotic fluid, chorionic villi, fetal serum, or products of conception can be tested for evidence of WNV, though the sensitivity and specificity are not known. Serologic studies for WNV following an outbreak in Colorado found evidence of IgG antibodies in the cord blood of 4% of infants, none of whom were symptomatic.
There is no known effective antiviral treatment, and management is supportive.
Patients with neuroinvasive disease often have long-term sequelae including fatigue, memory loss, difficulty walking, muscle weakness, and depression. Fetal infection has been reported, complicating pregnancy as outlined above. Transmission through breast milk has been reported, though it is thought to be rare.
The primary strategy for preventing exposure in pregnancy is the use of mosquito repellent containing DEET, avoidance of outdoor activities around stagnant water, and use of protective clothing. A vaccine is not currently available.
Yellow fever is an acute viral hemorrhagic disease transmitted by infected mosquitoes. The “yellow” in the name refers to the jaundice that affects some patients. Symptoms of yellow fever include fever, headache, jaundice, muscle pain, nausea, vomiting, and fatigue. A small proportion of patients who contract the virus develop severe symptoms, and approximately half of those die within 7 to 10 days. Yellow fever virus (YFV) is a flavivirus that is transmitted to humans by the Aedes and Haemagogus species of mosquito. Most infections are asymptomatic, but symptomatic infection causes fever, headache, myalgia, photophobia, back pain, vomiting, restlessness, and anorexia. Approximately 15% of patients experience severe disease with jaundice and, in some cases, hemorrhagic symptoms and multiorgan complications. In severe disease with hepatorenal dysfunction, the case fatality rate ranges from 20% to 50%.
YFV is endemic in tropical areas of Africa and Central and South America. Large epidemics of yellow fever occur when infected people introduce the virus into heavily populated areas with high mosquito density and where most people have little or no immunity, due to lack of vaccination. In these conditions, infected mosquitoes of the Aedes aegypti species transmit the virus from person to person (urban epidemic). Viral transmission by non- Aedes species of mosquitoes such as Haemagogus sp. is more typical of sylvatic epidemics. These have intermediate hosts such as nonhuman primates. The reason for this is that different mosquito species live in different habitats; some breed around houses (domestic), others in the wild, and some in both habitats (semidomestic). Therefore there are three types of transmission cycles:
Sylvatic (or jungle) yellow fever: In tropical rainforests, nonhuman primates, which are the primary reservoir of yellow fever, are bitten by wild mosquitoes of the Aedes and Haemagogus species, which pass the virus on to other monkeys. Occasionally humans working or traveling in the forest are bitten by infected mosquitoes and develop yellow fever.
Intermediate yellow fever: In this type of transmission, semidomestic mosquitoes (breed in the wild and around households) infect both nonhuman primates and people. Increased contact between people and infected mosquitoes leads to increased transmission, with many distinct communities in a geographic area developing simultaneous outbreaks.
Urban yellow fever: Large-scale epidemics occur when infected people introduce the virus into heavily populated areas with high density of Aedes aegypti mosquitoes and where most people have little or no immunity, due to lack of vaccination or prior exposure to yellow fever. In these conditions, infected mosquitoes transmit the virus from person to person.
There are 47 countries in Africa and Central and South America where yellow fever is endemic; these countries are at risk for yellow fever outbreaks. Occasionally travelers who visit endemic countries bring the disease to unaffected countries. To prevent disease importation, many countries require proof of vaccination against yellow fever before they issue a visa, particularly if travelers come from, or have visited, yellow fever endemic areas. In past centuries, yellow fever was transported to North America and Europe, causing large outbreaks that disrupted economies and development and in some cases decimated populations.
Viral incubation is approximately 3 to 6 days. Many people do not experience symptoms; however, if present, the most common findings are fever, muscle pain with prominent backache, headache, anorexia, nausea, or vomiting. In most cases, symptoms disappear within 3 to 4 days. A small percentage of patients enter a second, more toxic phase within 24 hours of improvement of initial symptoms. High fever returns and several body systems are affected, usually the liver and the kidneys. In this phase, people are likely to develop jaundice, dark urine, and abdominal pain with vomiting. Bleeding can occur from the mouth, nose, eyes, or stomach. Half of patients who enter the toxic phase die within a week to 10 days.
Yellow fever may be difficult to diagnose, particularly in its early stages. More severe cases can be mistaken for severe malaria, leptospirosis, fulminant forms of viral hepatitis, other hemorrhagic fevers, infection with other flaviviruses (hemorrhagic dengue), and certain forms of poisoning. PCR testing in blood and urine can detect the virus in early stages of the disease if performed in the correct window for viremia and viruria. In later stages, serologic testing using EIA or PRNT, which help differentiate YFV from other flaviviruses, is warranted.
Early supportive treatment for patients requiring hospitalization improves survival rates. There is currently no specific antiviral drug approved for yellow fever, but specific treatment of dehydration, electrolyte imbalances, fever, and liver and kidney failure improves outcomes. Associated bacterial infections can be treated with antibiotics. During the 2017 yellow fever outbreak in Brazil, sofosbuvir, a drug normally used to treat hepatitis C (also a flavivirus), was used in clinical studies for treatment of patients with severe disease.
Most of the literature concerning yellow fever during pregnancy centers around immunization of pregnant women. Symptomatic yellow fever may evolve into an illness that carries significant morbidity and mortality risk, and pregnant women are at risk for adverse outcomes, including maternal-fetal demise due to complications of yellow fever. Nevertheless, there are no studies demonstrating that infected pregnant women are at higher risk of adverse outcomes than the general population. Few studies on yellow fever during pregnancy are available, but vertical transmission has been described.
Yellow fever is prevented by an extremely effective vaccine, which is safe and affordable. A single dose of yellow fever vaccine (YFV) is sufficient to grant sustained immunity and lifelong protection. A booster dose of the vaccine is not needed. The vaccine provides effective immunity within 10 days for 80%–100% of people vaccinated and within 30 days for more than 99% of people vaccinated. In fact, yellow fever vaccination has been available for more than 60 years, but because it is a live virus vaccine, there are concerns about its use in pregnancy. Per the CDC’s Advisory Committee on Immunization Practices (ACIP) guidelines, while most live vaccines are contraindicated in pregnancy, yellow fever vaccination should be considered with caution and recommended if risks of yellow fever exposure outweigh the theoretical risks of vaccination. Medical waivers are available for yellow fever vaccine if the risks outweigh the benefits and travel to an area that requires vaccination is necessary.
Pregnant women are usually excluded from yellow fever vaccination campaigns but can be inadvertently vaccinated early in pregnancy. The official guideline is for pregnant women to avoid or postpone travel to an area where there is risk of yellow fever if possible. A small multicenter European study of 74 women following yellow fever vaccination showed no increase in risk of miscarriage or fetal anomaly, while a Nigerian study also reported no increased risk of adverse pregnancy outcomes in vaccinated mothers. A Brazilian study of 441 women showed good immunogenicity of the vaccine with a seroconversion rate of 98.2% and no increase in adverse pregnancy outcomes, and a more recent study described the safety of yellow fever immunization in a population of pregnant women serving in the military.
Yellow fever vaccine should be avoided in breastfeeding women if possible. Three cases of yellow fever vaccine–associated acute neurotropic disease were reported in exclusively breastfed infants whose mothers were vaccinated but not the infants. In Brazil, yellow fever viral RNA was detected in a CSF specimen of an infant who was not immunized, with the nucleotide sequence of the amplified PCR product being identical to the yellow fever vaccine virus. A second similar case was also reported in Brazil. In Canada, an infant who had been breastfed by a recently vaccinated mother had evidence of acute neurotropic disease, with a sample of CSF being positive for yellow fever antigen. The YFV was also isolated in breastmilk. Given these anecdotal reports, if nursing mothers cannot avoid or postpone travel to areas endemic for yellow fever and vaccination is recommended, these women should be cautioned about the potential risk of transmission to the infant following vaccination. There are no data on whether ceasing breastfeeding for a period post-vaccination will lessen the risk of transmission. Viremia occurs between 4 and 10 days after primary vaccination, so avoidance of breastfeeding for a period after vaccination may be useful. The Brazilian Ministry of Health recommends delaying vaccination of breastfeeding mothers until the infant is 6 months of age or older, but more data are needed to guide recommendations for temporary suspension of breastfeeding after yellow fever immunization.
One large-scale initiative to prevent yellow fever outbreaks across the globe is the Eliminate Yellow Fever Epidemics (EYE) Strategy launched in 2017. With more than 50 partners involved, the EYE partnership supports 40 at-risk countries in Africa and the Americas to prevent, detect, and respond to yellow fever suspected cases and outbreaks. The partnership aims to protect at-risk populations, prevent international spread, and contain outbreaks rapidly. By 2026, it is expected that more than 1 billion people will be protected against the disease through this initiative.
Zika virus (ZIKV) was once known as an obscure flavivirus transmitted through Aedes genus mosquitoes in sub-Saharan Africa. It was first identified as an arbovirus which infected macaques in the Zika Forest of Uganda in the late 1940s. In 2007, it was responsible for a small outbreak in Micronesia, and in 2013–14 it triggered a larger epidemic in French Polynesia. It was not until the virus reached Brazil and triggered a large-scale epidemic in 2015–16 that its potential for teratogenicity was recognized, after thousands of infants were born with CNS malformations, including microcephaly. The catastrophic repercussions of the Zika epidemic and the global health panic that ensued triggered the World Health Organization (WHO) to declare it a public health emergency in 2016.
Following this epidemic, ZIKV cases declined globally, although the virus became endemic in Central and South America, the Caribbean, and southern Asia. Outbreaks occurred in 2018 in India and Angola, and a locally acquired ZIKV case was identified in France in 2019. Global warming and population mobility have increased the environmental span of Aedes sp. mosquitoes, and the threat of new outbreaks lingers, particularly since arboviral outbreaks are cyclical. In addition, ZIKV, unlike most arboviral infections, can be transmitted via sexual contact, so women who are pregnant can be infected by their partners without necessarily traveling to endemic areas.
The similarity of ZIKV’s genomic structure to that of dengue viruses 1–4 has important diagnostic implications. Other related flaviviruses include yellow fever, Japanese encephalitis, and WNVs (all arboviruses), as well as hepatitis C virus. Over time, ZIKV evolved from the African lineage to the Asian lineage, which shares approximately 90% homology with the African lineage, acquiring potentially higher teratogenic potential. The recent ZIKV epidemic was caused by the Asian strain.
ZIKV infections are generally asymptomatic, with about 20% of patients developing mild symptoms resembling rubella within 7 to 10 days of exposure. These generally consist of a maculopapular pruritic rash, arthralgia, and conjunctival erythema. Fever is usually absent and, if present, low grade. Clinical findings have been used for the development of ZIKV case definitions in areas where multiple arboviral infections co-circulate, as laboratory diagnosis for differentiation can be difficult. The presence of rash, pruritus, and conjunctival hyperemia, as well as absence of fever, petechiae, and anorexia, is often used as a clinical case definition for Zika infection to differentiate it from dengue and chikungunya. ZIKV infection is typically self-limited, with symptoms resolving within one week. Most people with acute infection recover without complications, including pregnant women and children without congenital ZIKV infection. However, during the 2015–16 epidemic, there were case reports of rare unusual findings following acute ZIKV infection, including neurologic complications such as Guillain-Barré syndrome, acute sensory polyneuropathy, as well as myelitis, including acute disseminated encephalomyelitis, meningoencephalitis, encephalopathy, and neuropsychiatric changes. Other reported unusual complications include temporary hearing impairment, uveitis, prostatitis, myocarditis, and even death. Pregnant women do not have a different pattern of illness than nonpregnant individuals. Nevertheless, the absence of clinical symptoms of ZIKV during pregnancy does not indicate absence of clinical repercussions to the fetus; asymptomatic infected women were found to have infants with microcephaly and other abnormalities. Maternal ZIKV serum virus load during acute infection, severity and frequency of symptoms, and prior dengue immunity have not been shown to be associated with infant outcomes.
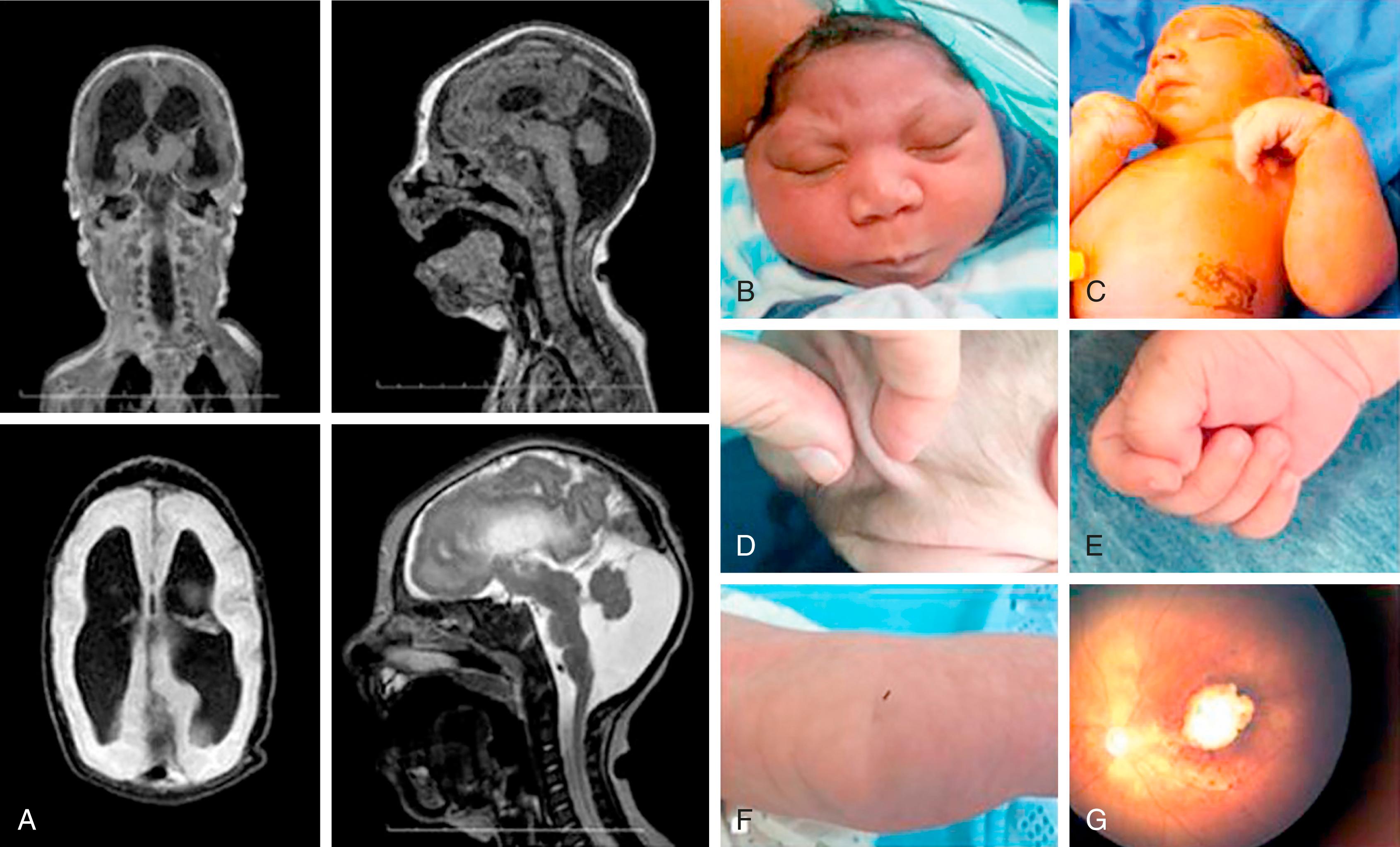
Following the ZIKV epidemic in Brazil, the teratogenic potential of ZIKV became quite evident, with CNS findings being the most frequent manifestations. Although the CDC coined the term congenital Zika syndrome (CZS), which refers to infants most severely affected by in utero ZIKV infection, studies have demonstrated that there is a spectrum of clinical manifestations attendant in utero exposure ranging from no clinical findings to severe microcephaly. , CZS is defined as a constellation of clinical findings at birth ( Fig. 49.2 ) that include (1) severe microcephaly (>3 SD below the mean for gestational age and gender); (2) brain abnormalities (subcortical calcifications, ventriculomegaly, cortical thinning, gyral pattern anomalies, hypoplasia of the cerebellum, or corpus callosum anomalies); (3) ocular findings; (4) congenital contractures, also known as arthrogryposis; and (5) neurologic impairment. Microcephaly rates in the few prospective studies conducted during the epidemic have ranged from 3% to 7% among infants with in utero ZIKV exposure. The most common CNS abnormalities include cerebral calcifications, cortical developmental malformations (lissencephaly, pachygyria, agyria), ventriculomegaly due to brain atrophy, posterior fossa alterations including brainstem or cerebellar hypoplasia, corpus callosum abnormalities, enlarged extra-axial CSF spaces, and enlarged cisterna magna. Ophthalmologic and sensorineural hearing loss have been reported in 7% and 12%, respectively, of infants followed since the time of maternal acute infection. , Hearing deficits and eye abnormalities are more frequently identified in children with additional CNS findings; however, they can also be isolated findings. Eye manifestations of in utero infection include focal pigment mottling of the macular epithelium, optic nerve hypoplasia, chorioretinal atrophy; unusual manifestations include colobomas and microphthalmia. , Affected children with abnormal eye exams generally have abnormal visual function in early infancy. Eye abnormalities following in utero infection do not tend to progress.
Interestingly, 10% of children with confirmed maternal in utero ZIKV exposure were found to have congenital heart defects. Studies evaluating longer-term outcomes of infants with in utero ZIKV exposure have shown that approximately 14.5% will have severe neurodevelopmental problems and sensorineural abnormalities by 3 years of age. , While not all children with abnormalities at birth have later neurodevelopmental repercussions, infants exposed to ZIKV in utero and found to be normal at birth might have abnormal developmental outcomes years later. , Prospective studies demonstrated that 31.5% of in utero–exposed children have below-average neurodevelopment or abnormal eye or hearing findings, with 29% of children scoring below average in Bayley IIII neurodevelopmental assessments in the third year of life. Secondary microcephaly, microcephaly that develops after birth, and a higher rate of autism spectrum disorder have been identified in children exposed to ZIKV in utero, underscoring the need for long term follow-up.
Individuals with ZIKV infection have a short window of viremia, usually no longer than 14 days. Following primary infection, the virus infects CD14+ blood monocytes, an ideal target as these cells can be used as a “Trojan horse” to infiltrate immune-sheltered tissues such as the placenta, testes, and brain. During the period of acute maternal viremia, ZIKV can cross the placenta, infecting placental macrophages. The virus is exceedingly neurotropic and has been shown to disrupt neural progenitor cell development, leading to the occurrence of microcephaly in animal models. Worse fetal outcomes are associated with maternal infection earlier in pregnancy, with CNS malformations most common following first- and second-trimester infections. Epidemiologic studies suggest a link with spontaneous abortions, and prospective series have found high rates of fetal growth restriction. Late fetal demise has been shown to occur as well, due to placental vascular involvement leading to a focal necrotic vasculitis and placental failure. For this reason, adverse outcomes following ZIKV infection have been noted in all stages of pregnancy. The virus has also been found to induce CNS calcifications and bone fusion, with craniosynostosis being another feature of congenital ZIKV infection. , Because the virus can persist for extended periods of time in the semen, pregnant women could potentially be at risk for infection weeks to months following their partner’s travel to an endemic area.
The diagnosis of ZIKV infection during pregnancy can be difficult. ZIKV identification in blood and urine by PCR has a narrow diagnostic window, generally not exceeding 14 days from the time of acute infection in adults and children postnatally infected. Since most individuals have asymptomatic infection and most who do have symptoms have only mild findings, it is relatively easy to miss the diagnosis of acute ZIKV infection, particularly in nonepidemic settings where awareness is diminished. Serologic diagnosis is fraught because of the cross-reactivity of ZIKV serologic assays with preexisting dengue virus (DENV) antibodies. There is significant cross-reactivity between ZIKV NS1 and DENV NS1 antibodies, as well as cross-reactivity with E (envelope) protein–based antibodies. For confirmatory serologic diagnosis of ZIKV infection, PRNT assays are optimally performed; however, these assays are cumbersome and generally unavailable except through health departments and the CDC. They are also time-consuming, so immediate results are not feasible. Zika IgM can be used for diagnostic purposes in some settings, such as travelers to endemic areas. However, IgM responses to ZIKV are short-lived, making retrospective diagnosis of ZIKV even more difficult.
Prenatal ultrasonography to detect fetal abnormalities consistent with CZS is recommended for all pregnant women with laboratory evidence of ZIKV infection. Pregnant women with possible exposure but without laboratory evidence of infection during pregnancy should undergo ultrasound examination as recommended for routine prenatal care. Ultrasound is for this reason the major modality used to screen for CZS; nevertheless, its sensitivity, specificity, and positive and negative predictive values do not appear to be as high as expected. Findings such as microcephaly are generally not identifiable until the mid- to late second trimester of pregnancy. In addition, a prenatal diagnosis of microcephaly is presumptive and should be confirmed during postnatal follow-up. Fetal growth restriction is another important finding. In a prospective study of 92 mother-infant pairs in Brazil with PCR-confirmed maternal diagnosis of ZIKV infection in pregnancy, abnormal fetal ultrasound findings had 49% sensitivity and 68% specificity for association with adverse neonatal outcomes including perinatal death, abnormal neonatal exam at birth, or postnatal CNS imaging. The sensitivity was even lower at 22% when only ZIKV-associated abnormal prenatal ultrasound results were considered (i.e., microcephaly, cerebral calcifications, ventriculomegaly, fetal growth restriction), although the specificity increased to 98% in this setting. As severe abnormalities are noted in only 15% of cases of in utero ZIKV exposure, it is not surprising that ultrasound has low sensitivity.
A positive ZIKV PCR of the amniotic fluid implies fetal infection. Nevertheless, the timing of performance of the amniocentesis should also be taken into consideration. There should be enough time following maternal infection for the virus to cross the placenta and infect the fetus and, in addition, fetal kidneys should be developed sufficiently for the virus to be excreted in the urine (generally 18 to 21 weeks of gestation). A negative PCR result in amniotic fluid does not rule out Zika infection. In cases of high suspicion, testing of the placenta and the infant postnatally is critical. Placental inflammation and cell death are not universally seen, and placental involvement can often be very focal. Neonatal PCR testing, placental findings, and infant outcomes can be discordant between co-twins with antenatal ZIKV exposure. Delayed villous maturation, stromal fibrosis, Hofbauer cell hyperplasia, basal villitis, and lymphocytic deciduitis were commonly discordant between ZIKV-exposed twin pairs, with localized foci of pathology.
There is no specific treatment for ZIKV infection, and there is currently no vaccine available for prevention. Studies have shown that US Food and Drug Administration (FDA)-approved drugs such as azithromycin or chloroquine carry therapeutic benefits in animal models of infection; no human studies have yet been reported. CDC guidelines recommend that men with recent ZIKV infection or exposure should wait at least 3 months from symptom onset/exposure before attempting conception with their partner, and women with recent infection or exposure should wait at least 2 months from symptom onset/exposure before attempting to conceive.
There are no data to suggest that prior ZIKV infection is deleterious to future pregnancies when conception occurs several months after acute ZIKV infection, nor are there data to suggest that women who had infants with CZS are at risk of adverse outcomes in future pregnancies. There are no contraindications to breastfeeding in women with ZIKV exposure. Postnatal infection of children with ZIKV does not appear to cause long-term sequelae. Children born to women with ZIKV infection during pregnancy should be followed prospectively, as long-term neurodevelopmental manifestations can be identified as late as 3 years of age and potentially later. Children with confirmed or suspected antenatal ZIKV exposure should have eye examinations and hearing assessments regardless of symptoms.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), joins the list of highly pathogenic betacoronaviruses, including SARS-CoV-1 and Middle Eastern respiratory syndrome coronavirus (MERS-CoV). The large, single-stranded RNA virus codes for the spike protein ( Fig. 49.3 ), a surface protein responsible for attachment and fusion with the angiotensin-converting enzyme 2 (ACE2), which is the site of entry in host cells. During the SARS-CoV-1 epidemic in Hong Kong in 2003, the case fatality rate in pregnancy was estimated to be as high as 25%, raising significant concerns about the implications of SARS-CoV-2 infection in pregnancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here