Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors wish to acknowledge Kiang Hiong Tay, who contributed to this chapter in the previous edition.
Peripartum hemorrhage (PPH) is reported as the most common maternal morbidity in developed countries and a major cause of death worldwide. Globally, postpartum hemorrhage may account for 25% of delivery-associated deaths. The incidence may be up to 20% of pregnancies beyond 18 weeks’ gestation. In the United States, PPH may occur in 1% to 5% of deliveries.
Figures vary widely depending on the precise definition of PPH. Generally postpartum hemorrhage is defined as a blood loss of 500 mL during a vaginal delivery or more than 1000 mL blood loss with a cesarean delivery. Clinicians may add the specification of a 10% drop in hemoglobin, although the laboratory findings may lag significantly behind the patient’s true blood loss status. More than 99% of PPH will occur within 24 hours of delivery.
The use of embolization to control pelvic hemorrhage was first described in 1972. As a treatment for pelvic fractures, the technique was successful and later applied to the treatment of PPH as described by Brown et al. in 1979. With several refinements over the years, this procedure has emerged as a safe and highly effective option for treating PPH.
The etiologies for PPH can be summarized in the 4Ts mnemonic: tone, trauma, tissue, and thrombin.
Uterine atony is the most common cause of PPH, accounting for 70% to 80% of cases. This occurs when there is decreased contraction of the myometrium. The postgravid uterus is floppy or flaccid and unable to provide adequate compression for hemostasis. Risk factors for atony include multiple gestation, fetal macrosomia, and prolonged labor.
Possibly related to uterine atony, uterine inversion is a rare condition that can cause PPH. The incidence is around 0.05%. The myometrium of the inverted uterus is unable to contract and retract, resulting in severe blood loss. The mechanism for development of uterine inversion is unclear but may be related to combined factors of uterine atony, fundal placenta, increased fundal pressure, and undue cord traction.
Birth trauma leading to lacerations and hematomas may be the cause of significant PPH, with an incidence of about 20% in these patients. Injuries to the vagina, cervix, and uterus leads to hemorrhage, given the highly increased vascularity that has developed during pregnancy. Risk factors that increase the trauma of delivery include fetal macrosomia, any instrumentation (e.g., forceps, vacuum), vaginal birth after cesarean section, or episiotomy.
A small subset of patients with birth trauma may sustain substantial injury to the uterus classified as uterine rupture. The incidence is 0.6% in patients who undergo vaginal birth after cesarean section. The risk for uterine rupture increases with classical cesarean incisions, short interval between pregnancy, and a history of multiple cesarean deliveries, especially in women with no previous vaginal delivery. The use of pharmaceutical induction and/or augmentation of labor can increase the rate of uterine rupture as well.
On average the placental delivery occurs within 10 minutes after the fetus. The diagnosis of retained placenta is defined as the absence of placental expulsion by 30 minutes. In about 10% of patients with PPH, retained placental tissue is the primary etiology. The operator may attempt several manipulations to retrieve the retained tissue and/or treat the ensuing blood loss. However, in a fraction of these patients, the tissue plane between the uterine wall and the placenta cannot be identified or separated, and the diagnosis of abnormal placentation or invasive placenta should be considered. Risk factors for retained placenta include any events that may have damaged the endometrial lining before becoming pregnant, multilobed placenta, prior uterine surgery, or cesarean section delivery.
The presence of invasive placenta can be life-threatening. In the United States, the overall incidence is 1 in 533 deliveries, a rate that appears to be increasing and likely secondary to the increased rate of cesarean section or other uterine instrumentation. There are three different types of invasive placenta with reference to the depth of invasion: (1) placenta accreta , which adheres to the myometrium, (2) placenta increta , which invades the myometrium, and (3) placenta percreta , which penetrates the myometrium to or through the serosa.
Coagulation disorders may result in PPH and may be the causal etiology in 1% of patients. Preexisting disease may be discovered before delivery and allow for preparation and appropriate prophylaxis. Examples include idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, factor X deficiency, familial hypofibrinogenemia, and von Willebrand disease. Disseminated intravascular coagulation can occur in the setting of sepsis, placental abruption, and amniotic fluid embolism.
In most obstetric patients, the diagnosis of PPH is a clinical one defined by the amount of blood loss and the hemodynamic status of the patient. No preprocedure imaging is usually required to make the clinical diagnosis.
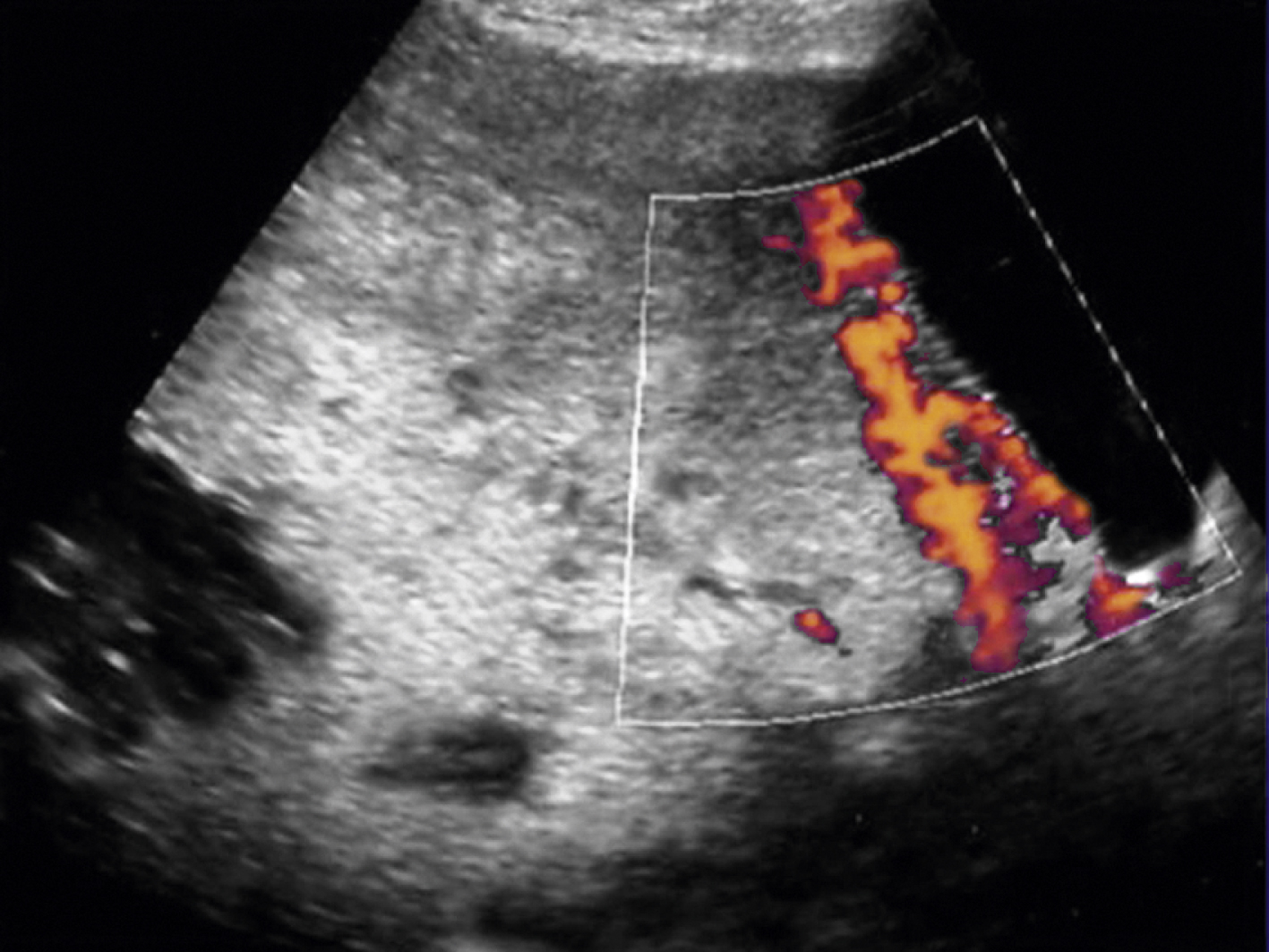
For patients with suspected PPH, ultrasound may show hematoma, retained placental fragments, or possibly abnormal placental invasion. Color flow Doppler images are especially helpful to identify the vascularity of any products of conception and demonstrate the abnormal lacunae of an invasive placenta ( Fig. 44.1 ).
Computed tomography scan with intravenous contrast findings are nonspecific. Magnetic resonance imaging is usually not possible in the acutely hemorrhaging patient.
In the smaller subset of patients with invasive placentation, preprocedure imaging is essential in preparation for delivery and the consideration for endovascular therapy. Ultrasound is the primary diagnostic tool, and most patients should present for ultrasound examination at 18–20 weeks’ gestation. Ultrasound findings for placenta accreta include placenta previa, placental lacunae with turbulent flow, irregular bladder with extensive associated vascularity, loss of the retroplacental clear space, myometrial thickness <1 mm or loss of visualization of the myometrium, and gap in the retroplacental blood flow.
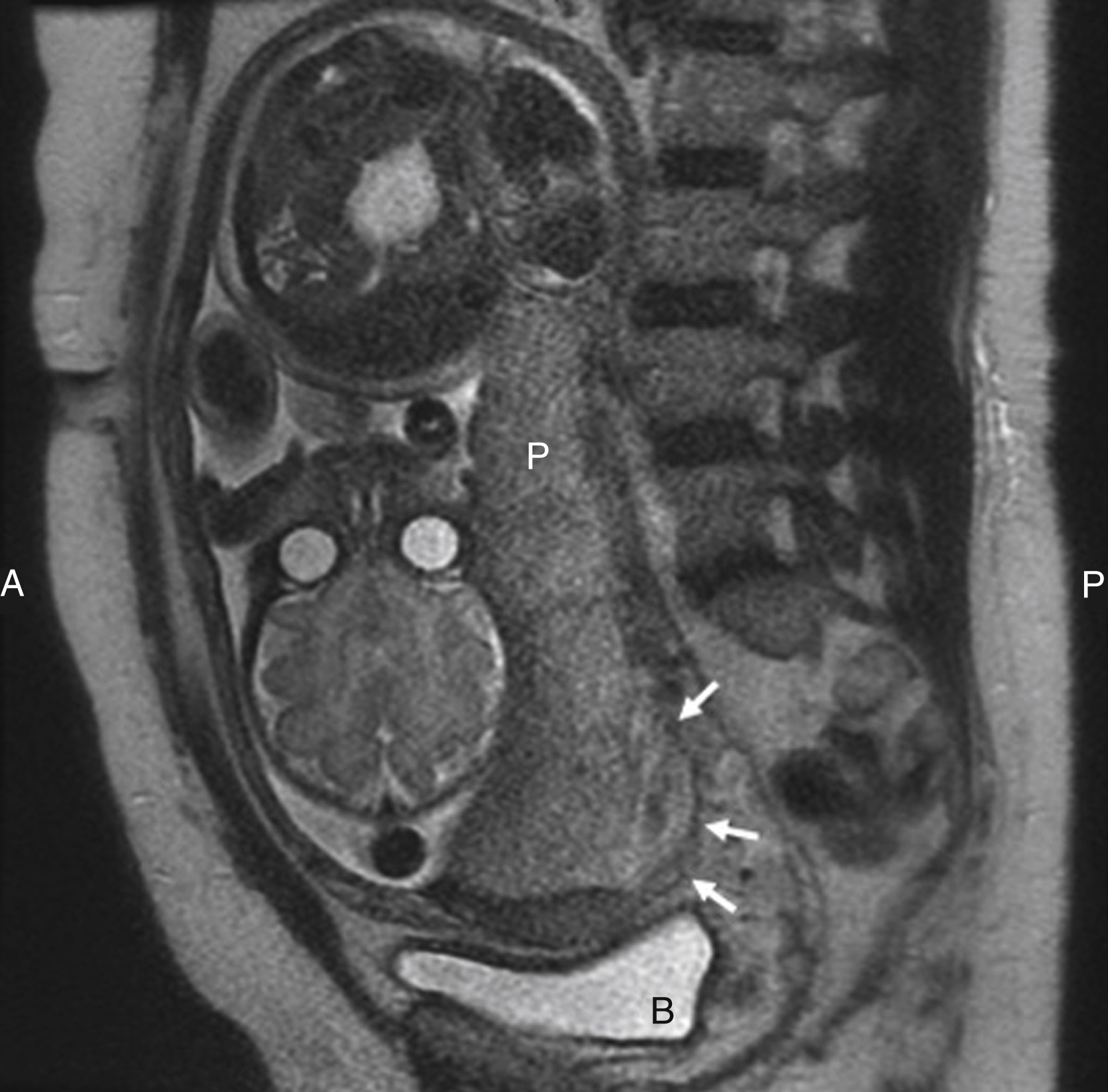
Magnetic resonance imaging can be helpful for the evaluation of invasive placentation to provide information on the depth of invasion and more clearly depict a posterior placenta. The magnetic resonance findings for placenta accreta include placenta previa, uterine bulging, heterogeneous signal intensity within the placenta, dark intraplacental bands on T2-weighted images, focal interruptions in the myometrial wall, bladder tenting, and direct visualization of pelvic structures by placental tissue ( Fig. 44.2 ).
When hemorrhage persists despite resuscitative efforts, vasopressors, and/or surgical intervention, then pelvic arterial embolization is indicated (see Additional or Adjuvant Therapies). The decision to proceed with endovascular therapy is usually multidisciplinary, with input from the obstetric surgeon and the interventional radiologist as well as the intensivist and anesthesiologist.
Few absolute contraindications to endovascular therapy exist in the face of persistent life-threatening PPH. If a patient has a history of severe anaphylaxis to iodinated contrast, endovascular therapy may not be possible unless alternative contrast media are used (e.g., carbon dioxide, gadolinium). However, if the contrast allergy is relatively mild, pretreatment with diphenhydramine and steroids may be sufficient to avoid a reaction from contrast exposure.
Preexisting renal insufficiency is a relative contraindication to embolization therapy. Because the patient may be in extremis, the risk for renal failure must be weighed against the morbidity and mortality of continued PPH.
The desire to maintain fertility may be a relative contraindication to pelvic arterial embolization. The pelvis has a rich and varied arterial supply, optimal for collateral arterial supply and support of the uterus after embolization. However, the risk for temporary or even permanent menopause postembolization exists, given the common occurrence of anastomoses between the ovarian and uterine arteries. Additionally there may be increased risk for subsequent pregnancy complications such as spontaneous abortion, preterm delivery, and abnormal placentation after uterine artery embolization. Although future fertility is an important consideration for all patients who undergo pelvic embolization, in the face of ongoing hemorrhage this may not be the clinical priority.
Several embolic agents are available. Most operators will choose absorbable gelatin sponge or Gelfoam (Pharmacia & Upjohn/Pfizer, New York, NY). A gelatin slurry may be mixed relatively quickly with small cut cubes of gelatin sponge and dilute contrast homogenized through a three-way stopcock. Gelatin sponge particles of varying sizes are usually the materials of choice because they are safe, inexpensive, and easy to use. In addition, they have the potential for recanalization. Some operators will choose microcoils for embolization in PPH, particularly if there is a pseudoaneurysm present, hemorrhage is refractory to gelatin sponge embolization, and/or definitive devascularization is necessary. Caution should be taken in the use of coils because their presence proximally in an artery may preclude reentry should new hemorrhage develop.
Particle embolics such as polyvinyl alcohol and tris-acryl gelatin microspheres (Embosphere [BioSphere Medical, South Jordan, UT] and Embozene microspheres [Celonova BioSciences, Peachtree City, GA]) are another choice in the treatment of PPH. The gelatin microspheres are approved by the U.S. Food and Drug Administration and used frequently for uterine artery and bland tumor embolization; microspheres are considered permanent embolic agents. However, particles are more costly than gelatin sponges, and there is some controversy over the increased likelihood for ischemia or necrosis.
N -butyl cyanoacrylate glue (Trufill n-BCA Liquid Embolic System, DePuy Synthes, Raynham, MA) has also been described as an effective embolic for pelvic hemorrhage. The delivery of glue is more technically challenging, but it is a viable option if the use of other embolic materials is unsuccessful.
Most interventional radiologists will use a 4F or 5F vascular sheath for access. A nonselective flush catheter may be used initially (e.g., pigtail catheter). Many selective catheters are available, and the choice is operator dependent. Examples of commonly used selective catheters include a Cobra-shaped catheter, a nontapered angled catheter (JB1), and the Roberts uterine curve catheter (RUC [Cook Medical, Bloomington, IN]). A microcatheter may be used when necessary; it should have a sufficient inner diameter to accommodate the embolic agent of choice. Examples include the 2.4F to 3F Renegade HI-FLO Microcatheter (Boston Scientific, Natick, MA), with a 0.027-inch inner diameter, and the 2.4F to 2.8F Progreat microcatheter (Terumo Medical, Somerset, NJ), with a 0.027-inch diameter.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here