Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Traumatic head and neck vascular injuries such as dissections, transections, pseudoaneurysms, arteriovenous fistulae, and large artery occlusions are relatively uncommon but can result in potentially devastating stroke, severe blood loss, and even exsanguination and death. Rapid, early diagnostic imaging followed by effective management of neurovascular injuries can help improve patient outcome by focusing attention on prompt treatment of these lesions.
Advances in multidetector computed tomography (CT) technology ( Fig. 66.1 ) have greatly facilitated rapid noninvasive screening of trauma patients with suspected neurovascular injury. Although the use of CT angiography (CTA) for definitive diagnosis remains somewhat controversial, it can provide sufficient information to “fast-track” a patient with significant vascular injury to definitive surgical or endovascular intervention. Alternatively, CTA may help determine whether a patient with minor injury is better served by delaying angiographic evaluation or treatment in favor of managing concomitant visceral or orthopedic injuries. Angiographic and endovascular resources can thus be directed where they are most appropriate and most needed.
Obvious signs of trauma, including facial fracture; penetrating wounds; epistaxis; hemorrhage from the mouth, orbit, or ears; periorbital ecchymosis, or Battle’s sign; expanding cervical hematoma or a pulsatile mass, cervical bruising or abrasions; or cervical bruit in a young patient
Fractures in proximity to the internal carotid or vertebral arteries, such as basilar skull fractures, cervical spine fracture-dislocations, or fractures extending into the foramen transversarium
History of significant blunt force injury to the head and neck, especially with a mechanism of injury involving cervical hyperextension-rotation, hyperflexion, or direct blow to the neck
New focal neurologic deficit or stroke of unexplained cause on head CT in a patient with history of trauma; focal neurologic deficit in the setting of questionable or minimal trauma, especially if the patient was normal at admission
Pain in the neck, face, scalp, or head in the setting of questionable or minor trauma
Relative contraindications are as follows.
Presence of dangerous collaterals or routes of blood flow to the orbit and brain; if these are present and cannot be protected, endovascular therapy may pose a significant risk for stroke.
Reopening of an acute traumatic vascular occlusion should be done with extreme caution because of the risk of thromboembolic stroke, and should be performed only when essential for cerebral perfusion. In many instances, particularly in patients with an intact circle of Willis and good intracranial collaterals, intentional occlusion of severely injured vessels or coil embolization to secure a traumatic vascular occlusion should be considered among the treatment options.
Some patients may be better served by medical or surgical therapy.
Triage issues may dictate the timing or extent of treatment.
Angiography tray
Rotating hemostatic valves
Continuous flush system using heparinized saline (2000–4000 units/L) for sheaths, catheters, and microcatheters ( Fig. e66.1 )
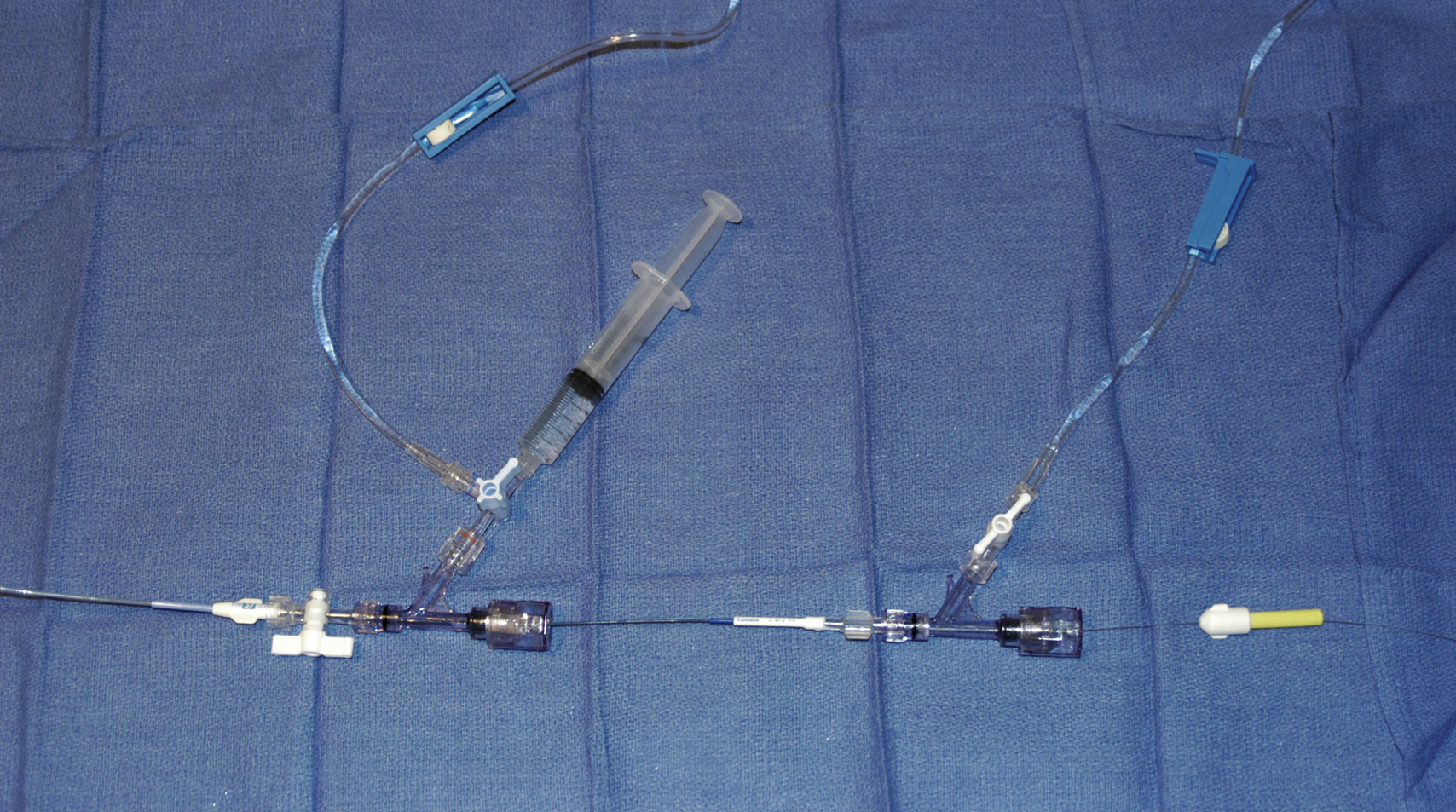
6F (or larger) access sheath
5F to 6F guide catheter
6F (or larger) introducer guide sheath, 90 to 100 cm long, for stent delivery
End-hole microcatheter appropriate for use with chosen embolic agents, and 0.014-inch microwire; exchange-length microwire may be needed for some stent delivery systems or tortuous anatomy
Particulate embolic agents for epistaxis and facial hemorrhage
Liquid embolic agents ( N -butyl cyanoacrylate, Onyx) have been used for treatment of life-threatening facial hemorrhage.
Fibered 0.018-inch push coils and coil pusher for distal arterial injuries
Fibered 0.035-inch push coils and Benson 0.035-inch wire for proximal arterial injuries
Detachable 0.020-inch, 0.018-inch, 0.014-inch, and 0.012-inch coils for occlusion of pseudoaneurysm sacs
Stent(s) suitable for delivery into the extracranial internal carotid or vertebral arteries for treatment of cervical arterial dissection or pseudoaneurysm
Covered stent(s) suitable for delivery into the extracranial internal carotid artery for treatment of cervical arterial lacerations and transections, including arteriovenous and cavernous carotid fistulae
The cervical, facial, and cranial regions are supplied by a rich vascular tree, including the external carotid artery (ECA), internal carotid artery (ICA), vertebral artery (VA), thyrocervical trunk, and costocervical trunk. Branching patterns can be variable, and numerous potential pathways of collateral flow exist between the extracranial and intracranial arteries. Thus, careful vigilance for the presence of these dangerous collaterals is essential in preventing inadvertent embolization of the retina, brain, or cranial nerves.
If time and the patient’s condition permit, CTA to evaluate the extent of bony injury and identify potential sites of arterial injury often facilitates delivery of definitive treatment, and is well worth the additional “cost” in examination time, contrast administration, and patient radiation exposure.
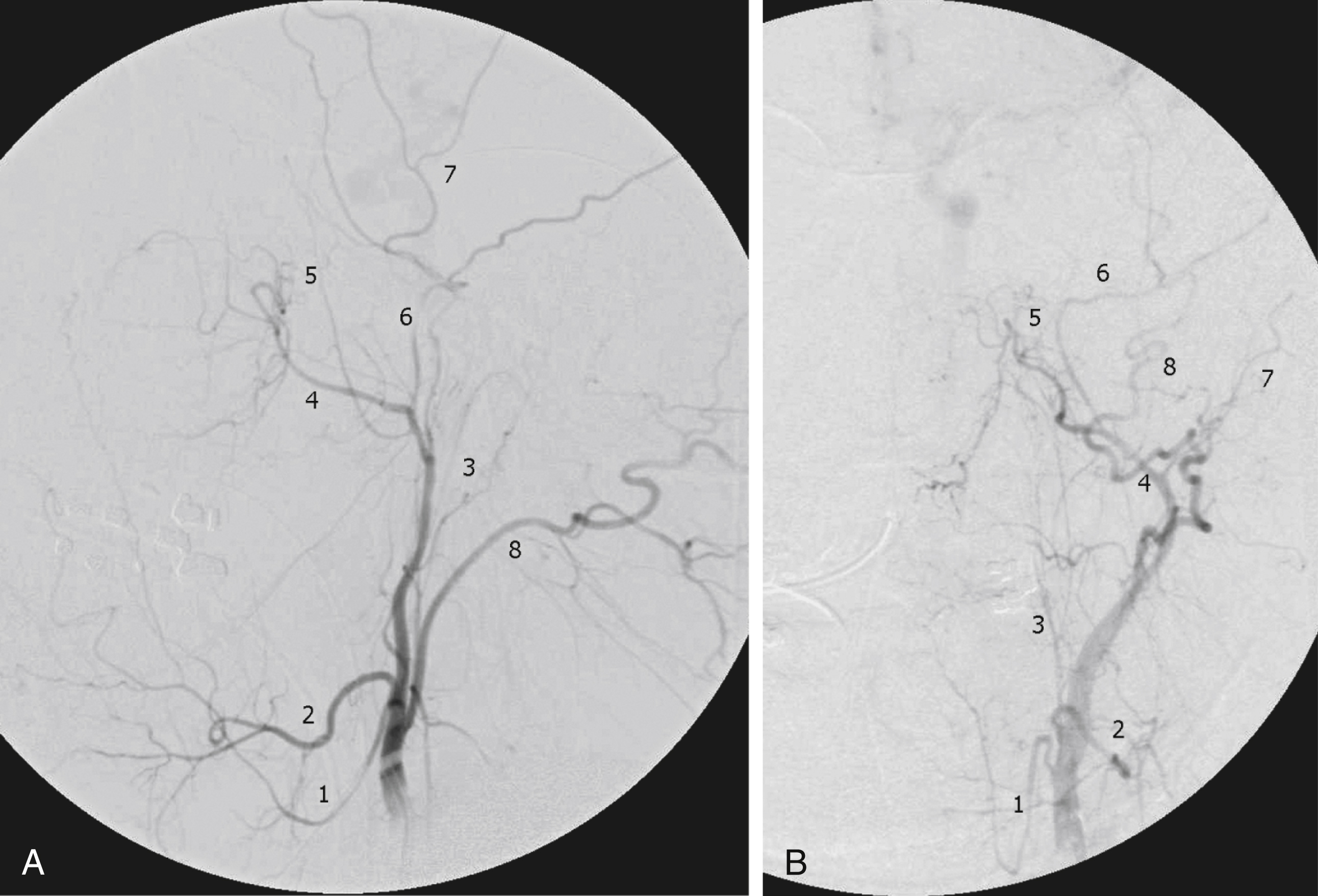
In epistaxis and facial smash injuries, the most common culprits amenable to embolization are branches of the facial and internal maxillary arteries. The facial artery arises as a proximal branch of the ECA (either directly or as a common trunk with the lingual artery), ascends superficial to the mandible, then courses medially in the superficial soft tissues of the face, giving off branches to the lips, mandible, cheeks, and nasal cavity. The internal maxillary artery (IMA) arises as one of the terminal branches of the ECA at the level of the ramus of the mandible. It passes forward almost horizontally, medial to the mandibular ramus, giving off branches to the soft tissues (transverse facial artery), meninges (middle and accessory meningeal arteries), and, as it subsequently courses either deep or superficial to the lateral pterygoid muscle, to the muscles of mastication (masseter, temporalis, mylohyoid, and pterygoids). The most distal part of the IMA passes through the pterygomaxillary fissure and into the pterygopalatine fossa; this distal segment gives rise to numerous branches that can be injured in facial fractures, particularly LeFort fractures, including the anterior and posterior superior alveolar arteries, descending palatine artery, infraorbital artery, sphenopalatine artery, and branches to the nasal cavity ( Fig. 66.2 ).
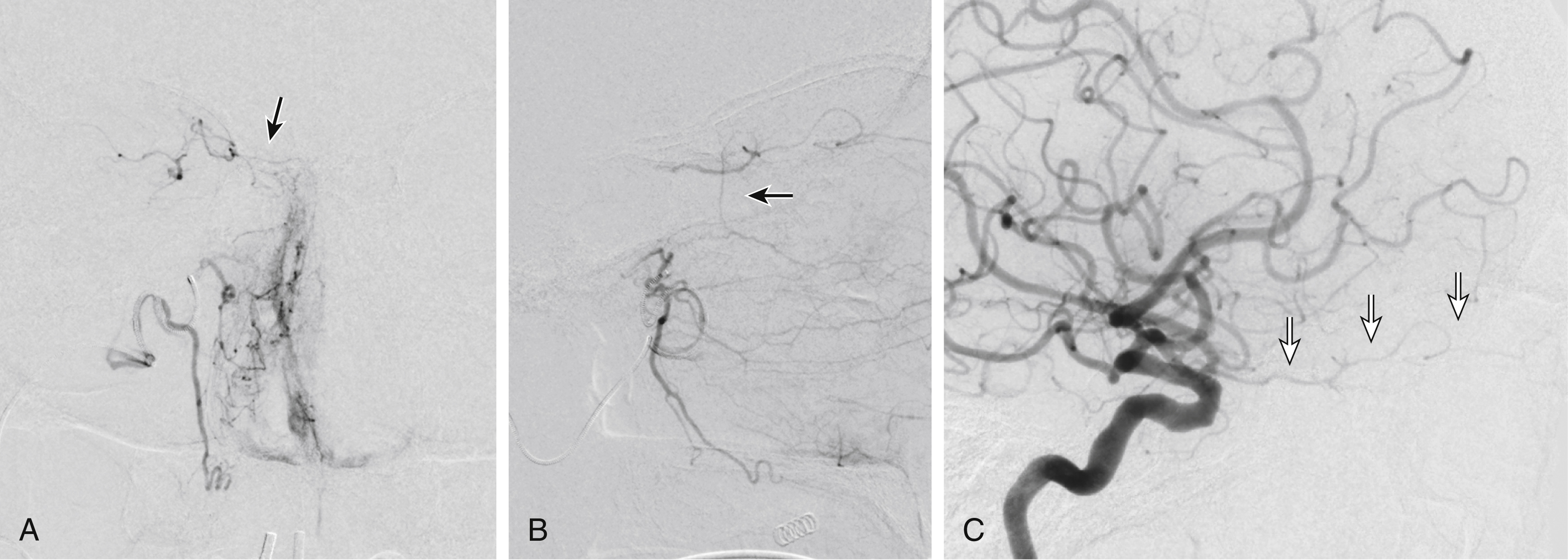
Facial and nasal cavity bleeding can also occur as a consequence of injury to intracranial branches of the ICA. Persistent nasal bleeding may arise from the posterior and anterior ethmoidal arteries , which arise from the ophthalmic artery (OphA) distal to the takeoff of the central retinal artery. The posterior ethmoidal artery courses medially, passing between the medial rectus and superior oblique muscles, and enters the nasal cavity via the posterior ethmoidal canal to supply posterior nasal air cells and the upper nasal septum. The anterior ethmoidal artery also courses medially, passing through the anterior ethmoidal canal, and supplies the anterior and middle ethmoidal air cells, frontal sinus, and anterosuperior aspect of the lateral nasal wall. These ethmoidal branches are generally not amenable to safe direct embolization, and a failure to recognize them before ECA branch embolization can result in unilateral vision loss or stroke ( Fig. 66.3 ).
In patients with epistaxis or facial injury, it is generally prudent to secure the airway by intubating the patient. The common femoral artery is entered using Seldinger technique, and a 6F or larger sheath inserted. A 5F or 6F guide catheter is then passed over a suitable 0.035- or 0.038-inch guidewire into the aortic arch. The large internal diameter of many available guide catheters is adequate to obtain a good road map by injecting around a coaxially placed microcatheter.
There are two general approaches to angiographic protocol: (1) diagnostic angiography of all vessels that are potentially a source of bleeding, followed by embolization of relevant arteries, or (2) angiography and embolization of one vessel, then moving on to another. The second approach is recommended only when the source of bleeding is clear on the basis of antecedent clinical or imaging findings. In either case, careful evaluation of the injured vessel and its runoff for possible communication with cerebral collaterals must be performed before intervention.
The common carotid artery (CCA) is selected, with care to keep the catheter and wire below any potential site of injury. Digital subtraction angiography (DSA) over the neck, skull base, and head is then performed to evaluate for ICA dissection, pseudoaneurysm, or extravasation arising from the cervical ICA or ECA. Treatment of cervical ICA dissections, pseudoaneurysms, and lacerations is addressed in a separate section later.
Large ECA branch lacerations causing profuse hemorrhage are best treated with particulate embolization followed by coil occlusion. Although the ECA main trunk and proximal portions of ECA branches can be occluded using 0.035-inch fibered push coils (delivered via a 5F catheter) or by 0.018-inch fibered push coils (delivered via microcatheter) alone, the offending ECA branch may continue to fill via retrograde collaterals from the opposite side. Selective road map imaging of the ECA, followed by passage of a microwire and microcatheter across (if possible) or closely proximal to the site of extravasation, are performed. Subsequent DSA with microcatheter injection is then done to evaluate for dangerous collaterals. If none are present, small branches that could serve as potential channels for collateral supply are then occluded with polyvinyl alcohol (PVA) particles larger than 250 μm, followed by occlusion of the lacerated branch with 0.018-inch fibered push coils, using either the microwire or a coil pusher for delivery.
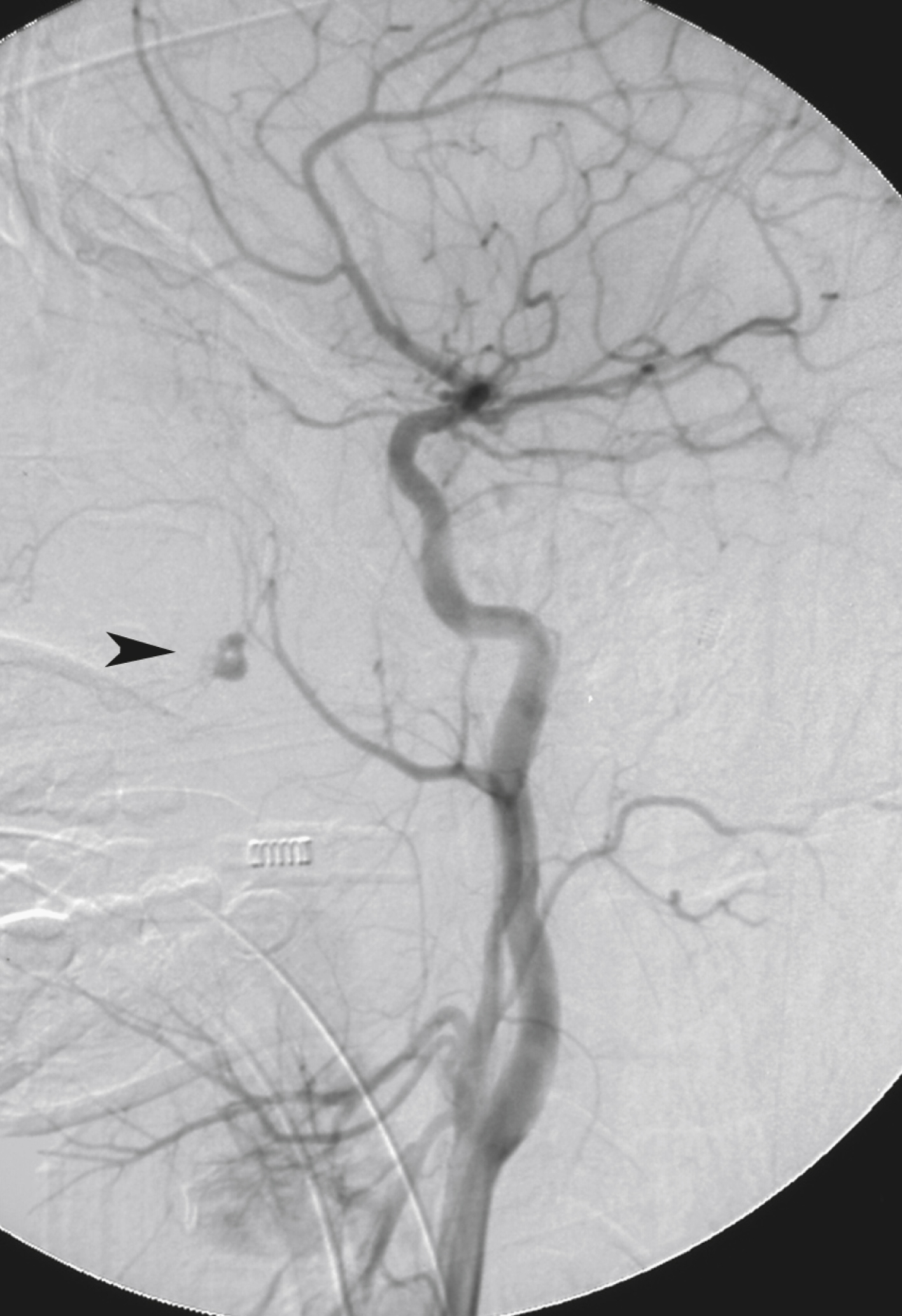
In many if not most cases of epistaxis or bleeding due to facial fractures, however, no obvious source of bleeding is evident on the initial CCA angiogram. Often the source of hemorrhage is seen only on selective or superselective angiography. Selective ICA angiography should be performed to rule out laceration of this vessel as a direct source of bleeding into the sphenoid sinus, evaluate ethmoidal branches of the OphA as either direct or collateral sources of hemorrhage, and look for other potential dangerous collaterals. Selective ECA angiography should then be performed to evaluate for arterial truncations (and thus potential lacerations), extravasation, pseudoaneurysms, or regions of unusual vascular blush ( Fig. 66.4 ). The ECA angiogram and any selective branch angiograms should be carefully evaluated for the presence of any branches projecting over the orbit and any collateral branches filling the ICA or OphA before performing embolization (see Fig. 66.3 ).
Therapeutic small-artery occlusion in the setting of epistaxis or trauma is most commonly performed using various preparations of PVA particles, typically 250 to 355 μm in size, suspended in a 1:1 or 2:1 mixture of contrast material to saline. This choice of particulate size effectively controls bleeding but allows small mucosal arteries to remain intact. Particles larger than 250 μm also help avoid inadvertent embolization of perineural vessels and some potentially dangerous collaterals. The concentration of PVA in the suspension should be sufficiently dilute to avoid clumping or clogging of the microcatheter.
Great care should be taken to prevent proximal reflux of particulate material into potential anastomotic branches and the ICA. It is also important to avoid a wedged catheter position, because the resultant increased injection pressure can sometimes open up previously unseen dangerous collateral branches.
If obvious sources of bleeding are seen on the diagnostic angiograms, these vessels are embolized first. If the site of bleeding is occult but clearly unilateral, the ipsilateral IMA and facial arteries may be embolized empirically. When the source of difficult-to-control bleeding is not evident, branches of the IMA are often to blame. In all cases, however, a maximum of three out of the four arteries providing most of the supply to the midline midface (right IMA, left IMA, right facial, left facial) should be embolized.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here