Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Injury management has been an important assignment of the practicing surgeon. Throughout the history of medical care, the treatment of trauma necessitates a mastery of diverse skills spanning all areas of anatomy and physiology. Because of the great disease burden due to injury sustained in conflict, care for the trauma patient has been advanced most profoundly during wartime. Box 17.1 lists some major contributions to trauma care that were developed during major U.S. wars. Common themes that have evolved over time include improvements in wound management, resuscitation, and systems of care. Military-based programming and funding continue to formalize this research in the development of care provided in austere and civilian environments. Likewise, advancements in civilian care have had a reciprocal effect within the military. Civilian training of military providers and clinical research on hemostasis and damage control techniques have saved the lives of countless service personnel across the globe.
Wound contraction during healing
Primary and secondary healing
Description of granulation tissue and epithelialization
Exhaustive therapy (bleeding, diarrhea, vomiting, salivation, sweating)
Centralization of medical care
Establishment of first medical school
Primary amputation (vs. secondary)
Use of topical antiseptic agents
Whole blood transfusion
Development of specialty hospitals (eye/ear, orthopedics, hernia)
Extremity traction splinting
Laparotomy for penetrating abdominal trauma
Wound debridement and delayed closure
Early use of plasma and crystalloid
First blood bank
Guillotine amputation and delayed primary closure
Exteriorization of colon injuries
Mobile surgical teams
Organ dysfunction after injury described
Vascular surgery for limb salvage
Hypovolemic shock recognition
Mobile Army Surgical Hospital (MASH) units
Aeromedical transfer (helicopter)
Sulfamylon for burn care
Recognition of acute respiratory distress syndrome (Da Nang lung)
Damage control resuscitation
Highly efficient trauma systems
Re-emergence of tourniquet use
Traumatology has matured into a distinct surgical field with a unique infrastructure over the last century. After the formation of the American College of Surgeons in 1913, the leadership of the organization appointed a committee to report on the management of fractures. Created in 1922 and chaired by Charles L. Scudder, the Committee on Fractures evolved in 1949 to become the Committee on Trauma (COT), as the need for formal oversight became evident. Beginning with the publication of Early Care of the Injured , the COT has been instrumental in advancing trauma care throughout the world via initiatives such as the Advanced Trauma Life Support (ATLS) course, verification of trauma centers, and the development of trauma systems to improve access to care. One of the ways in which the COT has been highly effective is through creation of state-level divisions. Activities of the state committees frequently include (1) trauma system development with the creation of triage documents, maximizing the use of local prehospital and hospital resources, (2) injury prevention initiatives, (3) maintenance of statewide trauma registries, and (4) advancement of performance improvement efforts. To standardize the way in which trauma centers define appropriate structure, process, and outcome, the COT first created in 1976 the Resources for the Optimal Care of the Injured Patient reference manual, now freely and electronically accessible in its sixth edition on the American College of Surgeons’ website with an associated update for 2019. The COT has also developed the National Trauma Data Bank (NTDB), which is the largest database of trauma ever assembled, currently including more than 7 million patients from 747 trauma centers. Data from the NTDB are included throughout this chapter to provide the reader with up-to-date information on specific injuries.
Beyond the COT, several other professional organizations have been developed with the primary goal of promoting the improvement of trauma care. The American Association for the Surgery of Trauma (AAST) originated in 1938 and is the oldest and largest of all trauma professional organizations. The AAST conducts an annual scientific conference in September that recently has become the Annual Meeting of the AAST and Clinical Congress of Acute Care Surgery. The maturation of this meeting reflects the inclusion of emergency general surgery as a component of acute care surgery into the scientific proceedings. The AAST has also been the lead organization in the development of the acute care surgery training paradigm, which now includes advanced education in trauma, emergency general surgery, and surgical critical care. Since program inception in 2008, there are currently 21 centers providing training in acute care surgery in accordance with a standardized curriculum. In addition to the AAST, the Eastern Association for the Surgery of Trauma (EAST) and the Western Trauma Association (WTA) comprise partnering academic organizations that promote the exchange of scientific knowledge in trauma care. Both groups contain active multi-institutional trial committees and have focused on the development of practice management guidelines, available electronically on their respective websites. Furthermore, the American Trauma Society, founded in 1968, has been an instrumental part of injury prevention and trauma systems development by advocating for the injured patient and promoting trauma-related legislation. Finally, the Orthopedic Trauma Association, American Association of Neurological Surgeons, and Society of Trauma Nurses represent three organizations whose members are part of the multidisciplinary team dedicated to improving the care of the injured patient.
At the most basic level, the primary goal of a trauma system is to get the right patient to the right place at the right time . Outcomes in trauma are highly dependent on the geography of injury, and regions that respond best have developed an organized approach to providing all the key elements to maximize meaningful recovery, called a trauma system . The ideal trauma system includes the entire care continuum, beginning with prevention and encompassing prehospital care, acute hospital services, postinjury rehabilitation, and research.
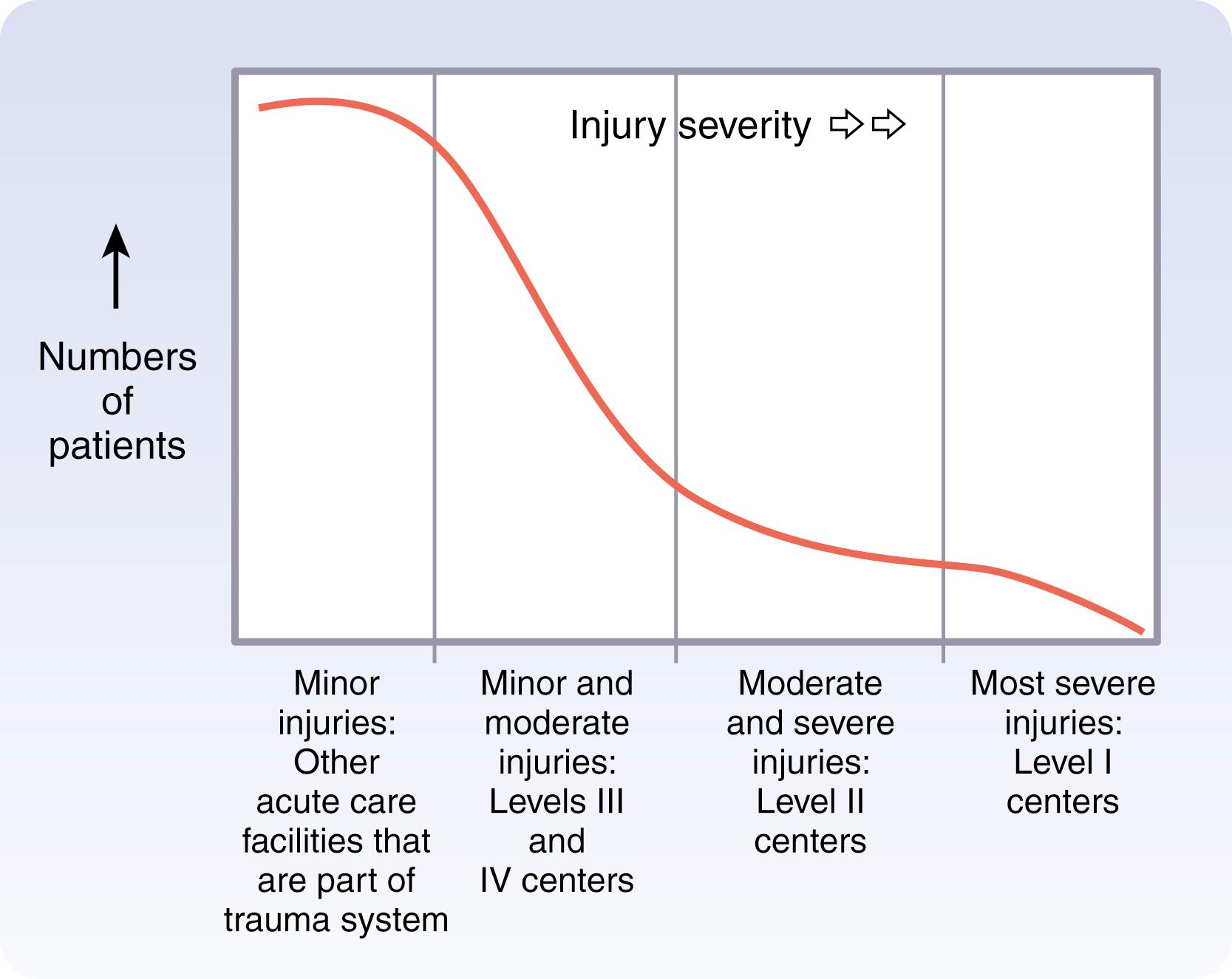
As the American healthcare system developed, trauma care was initially centered on the large, academic hospital. All patients were transported to the major trauma center, regardless of the degree of injury. Although this “exclusive” trauma system was beneficial to the severely injured, it resulted in the movement of a significant number of minimally injured patients and failed to capitalize on local resources. Data emerged, revealing similar outcomes and improved measures of efficiency in minimally injured patients managed outside of Level 1 trauma centers.
The solution was the development of a trauma system that includes all hospitals to address the needs of injured patients, regardless of designation ( Fig. 17.1 ). Inclusive trauma systems identify roles for facilities as a continuum, from critical access hospitals to the large Level I and Level II trauma centers. Guided by triage protocols, injured patients are transported to facilities that are appropriate to the severity of the injuries. Although this may require transfer of patients from smaller hospitals to trauma centers, most can receive appropriate treatment within the local network. Box 17.2 lists the common components of an inclusive trauma system that must be coordinated to maximize the effectiveness of care. The benefits of this approach include a reduction in wastefulness of medical resources and allowance of appropriate care within the community.
Injury prevention efforts
Prehospital care
Triage
Communication
Transportation
Acute care facilities
Trauma center designation and verification
Postacute care and rehabilitation
Performance improvement
Education and outreach
Legislation
The genesis of trauma systems in the United States followed the publication of “Accidental Death and Disability: The Neglected Disease of Modern Society,” a landmark report by the National Academy of Sciences in 1966. Congressional legislation (National Highway Safety Act of 1966) was subsequently passed to allocate funding for care of the injured following motor vehicle accidents. Maryland, Illinois, and Florida capitalized on this initiative, first implementing state trauma infrastructures approximately 40 years ago with demonstrable reductions in mortality. A follow-up report, “Injury in America: A Continuing Public Health Problem,” was published in 1985 and revealed trauma to be an ongoing issue at the national level. The National Center for Injury Prevention and Control was subsequently installed into the Centers for Disease Control and Prevention (CDC), and Congress legislated the Trauma Care Systems Planning and Development Act of 1990, which formally addressed the need and funding of new or revised state trauma systems. Further advancement occurred in 1992 when the Health Resources and Services Administration released the “Model Trauma Care System Plan,” intending to provide each state with a template for systems development. Revised in 2006 and renamed the “Model Trauma System Planning and Evaluation,” this work applied a public health disease-based approach to trauma and identified three critical functions: (1) epidemiological assessment, (2) policy implementation for public protection, and (3) high-quality well-regulated care provision.
Evidence for the mortality benefit of trauma system care is provided by two seminal publications. In 2006, the National Study on Costs and Outcomes of Trauma (NSCOT) was performed to evaluate variations in the care provided between trauma centers and nontrauma center hospitals. Supported by the National Center for Injury Prevention and Control of the CDC, NSCOT represents one of the largest epidemiological studies ever to evaluate the care of the injured patient. Including more than 5000 patients from 69 hospitals, NSCOT established that patient outcomes are improved when care is provided at a trauma center versus a nontrauma center. After correction for injury severity, care at a trauma center was associated with a 20% in-hospital mortality reduction and a 25% reduction in 1-year mortality. At the system level, Nathens and colleagues demonstrated the value of a coordinated response to injury after studying 400,000 patients during a 17-year period. The study spanned a length of time (1979–1995) during which trauma systems were established and optimized. After accounting for all possible contributors to improved outcomes, the development of a trauma system resulted in an 8% reduction in mortality during a 15-year period.
Despite the clear progress that has been made in national trauma care over prior decades, there is no “one size fits all” approach and systems implementation must be tailored to locations ranging from rural to urban geographies. In 2015, the COT developed the Needs-Based Assessment of Trauma Systems (NBATS) tool to assist with designation or creation of new trauma centers within a region. Criteria for the tool include point values assigned to six categories within a trauma service area: (1) population, (2) median transport time, (3) community support for a trauma center, (4) number of severely injured patients (Injury Severity Score [ISS] >15) discharged from nontrauma acute care facilities, (5) number of Level 1 trauma centers, and (6) number of severely injured patients evaluated at trauma centers already in the trauma service area. Given overestimations of trauma centers required in rural areas and underestimations of centers already existent in urban areas, a second version (NBATS-2) was created in 2018 to incorporate predictive geospatial modeling. Benefits of this update include assessment of how established center volumes and payer mixes would be affected by the addition of a new trauma center. As the current understanding of trauma systems continues to evolve, tools like these will provide valuable insight into structure and organization unique to each region of the country.
Concurrent with the development of trauma systems has been the need for a reliable method of injury comparison. Scoring systems are typically based on either injury anatomy or the physiology demonstrated after one or more injuries are sustained. The Abbreviated Injury Scale (AIS) has been the most used anatomic system of injury classification since it was first described in 1971. Injuries are characterized by a six-digit taxonomy that includes the body region, type of anatomic structure, and specific anatomic detail of the injury. Table 17.1 demonstrates the body regions and the associated first digit code within the AIS lexicon that allow users of this system to know clearly the location of the injury. Perhaps of even more widespread use is the AIS severity code (frequently described as the post-dot code). This seventh digit describes the severity and potential risk of death for each injury in the AIS system. Post-dot codes range from 1 (minimal severity) to 6 (presumably fatal) and are frequently used to cohort injuries and to compare outcomes. The Association for the Advancement of Automotive Medicine frequently embarks on the rigorous process of refining the AIS to be sure that is stays current in its ability to accurately characterize injury.
| Ais First Digit | Body Region |
|---|---|
| 1 | Head |
| 2 | Face |
| 3 | Neck |
| 4 | Thorax |
| 5 | Abdomen |
| 6 | Spine |
| 7 | Upper extremity |
| 8 | Lower extremity |
| 9 | Unspecified |
The AIS represents the foundation for other scoring systems that are better able to account for the severity of multiple combined injuries. In 1974, Baker and colleagues presented the ISS, calculated by summing the squares of the AIS severity codes for the three most severely injured body regions. The ISS ranges from 1 to 75, with severity groupings being defined as minor injury (ISS less than 9), moderate injury (ISS between 9 and 16), serious injury (ISS between 16 and 25), and severe injury (ISS more than 25). The ISS has been commonly used throughout the literature to quantify the overall burden of injury sustained by a patient. As a further development in anatomic injury scoring, the Organ Injury Scale (OIS) released by the AAST has been incorporated into the more recent versions of the AIS. By introducing the concept of injury grades, the OIS has added greater anatomic detail for specific organs and incorporated the ability to better delineate organ injury severity. This OIS severity has been validated with the NTDB to optimize the associated risk of morbidity and mortality.
In addition to anatomic scoring systems, other scales have been developed that include the physiologic insult from injury. These physiologic scoring systems are more capable of identifying the overall condition and can also better guide real-time decision-making. One commonly used scale of this type is the Glasgow Coma Scale (GCS), which reflects level of consciousness. With scores ranging from 3 to 15, the GCS is composed of a measure of eye opening, verbal response, and motor function. The GCS, specifically the motor score alone, has been found to be reflective of outcomes after traumatic brain injury (TBI). The Revised Trauma Score is another well-studied physiologic scoring system that characterizes the condition of the injured patient by incorporating the GCS, systolic blood pressure, and respiratory rate. These scores have been of value for research purposes and have been successfully used to make triage decisions. To better demonstrate the way in which the GCS and Revised Trauma Score are designed, Tables 17.2 and 17.3 reflect how these scores are calculated.
| Eye opening | Spontaneous | 4 |
| To voice | 3 | |
| To pain | 2 | |
| None | 1 | |
| Verbal response | Oriented | 5 |
| Confused | 4 | |
| Inappropriate | 3 | |
| Incomprehensible | 2 | |
| None | 1 | |
| Motor response | Obeys commands | 6 |
| Localizes pain | 5 | |
| Withdraws to pain | 4 | |
| Flexion | 3 | |
| Extension | 2 | |
| None | 1 | |
| Total Glasgow Coma Scale score | 3–15 |
| Glasgow Coma Scale score | 13–15 | 4 |
| 9–12 | 3 | |
| 6–8 | 2 | |
| 4–5 | 1 | |
| 3 | 0 | |
| Systolic blood pressure (mm Hg) | >89 | 4 |
| 76–89 | 3 | |
| 50–75 | 2 | |
| 1–49 | 1 | |
| 0 | 0 | |
| Respiratory rate (breaths/min) | 10–29 | 4 |
| >29 | 3 | |
| 6–9 | 2 | |
| 1–5 | 1 | |
| 0 | 0 | |
| Total revised trauma score | 0–12 |
Immediately after a patient is injured, the trauma system engages the prehospital phase of care. The goal of the prehospital system is to move a patient to a location capable of providing definitive injury management as quickly as possible. The prehospital team plays an integral role in the management of the trauma patient because of the time-dependent nature of injury. The initial approach to the injured patient in the prehospital setting includes four key priorities:
Evaluate the scene.
Perform an initial assessment.
Make triage-transport decision.
Initiate critical interventions and transport the patient.
After-scene safety is ensured to protect our prehospital providers. The initial assessment should be rapidly completed. The initial assessment consists of a systematic approach to immediately identify life-threatening conditions that require urgent intervention. The ABC mnemonic guides the initial assessment, during which a irway, b reathing, and c irculation are sequentially evaluated and addressed. While the spine is protected, the airway is secured and assisted ventilation is provided as necessary. External hemorrhage is identified and immediately controlled while resuscitation is initiated.
Emergent interventions in the field can be immediately lifesaving, but optimal outcomes ultimately depend on quickly making an effective triage and transport decision. Using the “load and go” approach, all essential prehospital interventions can be provided while the patient is being transported. A recent NTDB review by Chen and colleagues demonstrated that trauma patients with prehospital hypotension (<90 mm Hg), GCS of ≤8, and nonextremity firearm injury have higher mortality with increasing prehospital time.
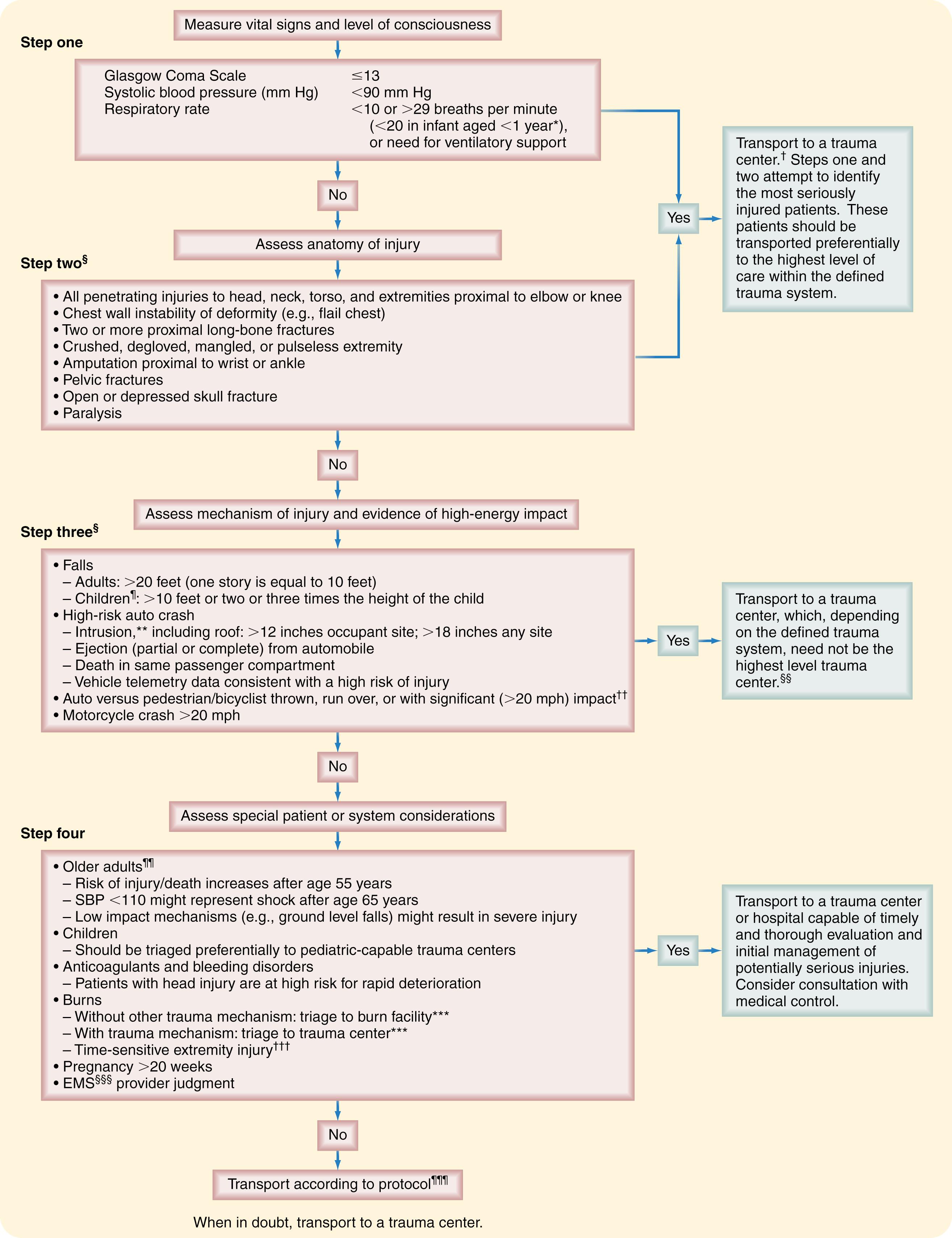
All prehospital teams know that immediate departure from the scene is paramount, but identifying where to go and how to get there can be more challenging. Well-defined protocols should guide the field triage process so that teams know immediately where to transport a patient. Fig. 17.2 demonstrates the Field Triage Decision Scheme, which was developed by the CDC and included in recent editions of the COT reference, Resources for the Optimal Care of the Injured Patient , and the Advanced Trauma Life Support (ATLS) , 10th edition 2018 update. Using physiologic status, mechanism of injury, and other indicators of a high-risk patient, this tool assists in determining which patients might benefit from care at a trauma center. Most prehospital agencies attempt to assess a patient rapidly and initiate the transport process while minimizing the scene time to less than 15 minutes.
The initial clinical concern that the prehospital team must assess is the airway. The “gold standard” for airway maintenance in the severely injured patient remains endotracheal intubation, typically using a rapid-sequence intubation (RSI) or drug-assisted intubation (DAI) technique. One must always assume that the patient has a spine injury and appropriately maintain spinal precautions. The utility of advanced airway management in the field has been questioned, with no high-quality prospective evidence in the literature. Previous studies have reported that airway management with endotracheal intubation is associated with increased mortality when compared to noninvasive techniques. Conversely, other investigators have suggested the benefit of advanced prehospital airway support in a select group of patients (i.e., neurologic outcome in severe TBI). In reality, the decision to establish a prehospital advanced airway is a complex decision, weighing the technical and physiologic consequences of RSI (i.e., cardiovascular collapse in hemorrhagic shock) against the possible benefits of airway protection and oxygen delivery. As an alternative, blind insertion supraglottic airway devices have become common and add great value in providing a bridge to a more definitive solution. Regardless of the approach implemented by the prehospital agency, personnel need to have the ability to manage all levels of airway compromise while transporting the patient to definitive care.
External hemorrhage control and initiation of resuscitation are critical needs during the prehospital phase of care. Direct pressure remains the mainstay of hemorrhage control, although tourniquet use has become more common in the management of exsanguinating extremity trauma. For some time, tourniquets were infrequently used because of concern about causing unnecessary muscle and nerve injury. Driven by military experience and advances in device development, tourniquets have demonstrated benefit in select situations. Recent publications now report a mortality benefit related to the use of prehospital tourniquets in the civilian sector. In response, the American College of Surgeons has developed the Stop the Bleed campaign, whereby laypeople are instructed in proper application of extremity tourniquets. Prehospital agencies now commonly include tourniquets on their standard equipment lists so that they may be used when a patient with uncontrolled extremity bleeding is encountered. Many commercial devices are available, and Fig. 17.3 illustrates an example of a tourniquet that can be used in the prehospital setting.

As hemorrhage is the primary cause of preventable trauma mortality, patients in shock require initiation of prehospital resuscitation concurrent with efforts toward temporary hemorrhage control. Prior studies have demonstrated that large-volume crystalloid-based resuscitation is detrimental, suggesting that blood products may be the superior resuscitative fluid. In a recent pragmatic, multicenter, cluster randomized trial of helicopter transported patients in hemorrhagic shock, packed red blood cells (PRBCs) administered with plasma conferred the greatest survival benefit, followed by plasma alone and PRBC alone. Among patients who would have qualified to receive blood products, administration of crystalloid increased mortality incrementally by dose. These data are in contrast to a second randomized controlled trial in which ground ambulance teams administered either 2 units of plasma or crystalloid alone to hypotensive patients en route to the hospital. No associated survival benefit was noted in the group receiving plasma-based resuscitation. Ultimately, prehospital plasma may be of greatest benefit to a select group of patients with moderate transfusion requirements, as the mortality-reducing effect was not seen in patients who went on to receive ongoing massive transfusion (>10 units PRBC in initial 24 hours). Although the ideal resuscitative scheme has yet to be identified, many of the challenges limiting prehospital transfusion are logistical in nature (i.e., supply, storage, cost). As a result, many prehospital agencies provide mixed crystalloid and product-based resuscitation practices, based on local resources.
The mainstay of the initial approach to the injured patient is the ATLS course. Since its development in 1980, ATLS has instructed more than 1 million students of trauma in 86 countries. The course has provided a structured, standardized approach to the injured patient that is based on the concept of rapidly identifying and addressing life-threatening conditions during the initial assessment of the patient. More specifically, ATLS conveys three important concepts that greatly enhance the ability to manage injured patients, regardless of where care is provided:
Treat the greatest threat to life first.
The lack of a definitive diagnosis should not delay the application of an indicated urgent treatment.
An initial, detailed history is not essential to begin the evaluation of a patient with acute injuries.
Following a defined order of assessment, life-threatening conditions are immediately addressed at the time of identification. This initial assessment, also termed the primary survey , follows the mnemonic ABCDE ( Fig. 17.4 ):
A irway and cervical spine protection
B reathing
C irculation
D isability or neurologic condition
E xposure and environmental control
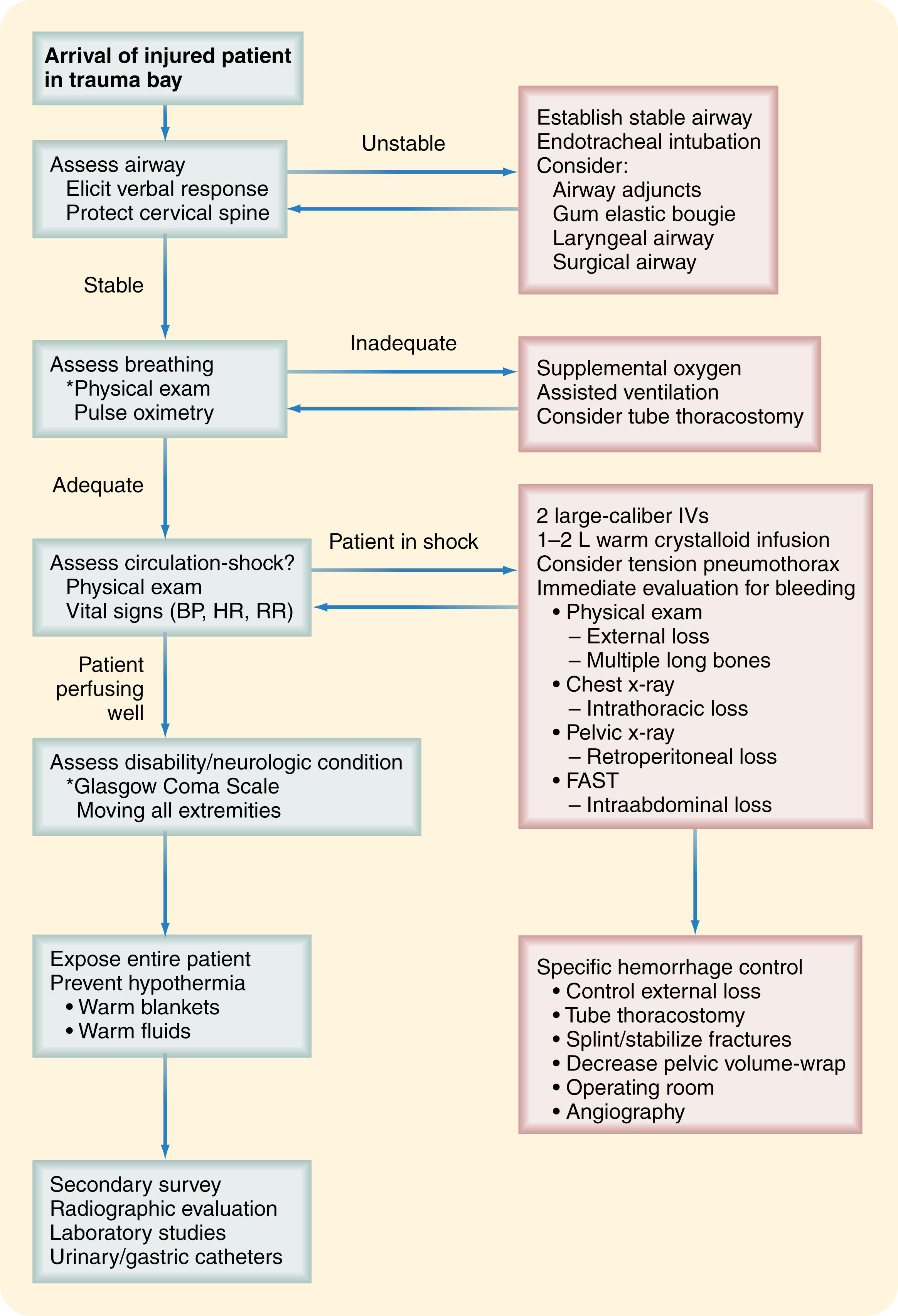
In addition, the primary survey can be repeated any time there is a change in condition. Despite being simple in design, the primary survey offers a tool that the surgeon can trust to identify what life-threatening condition exists and where to direct clinical effort. The following outlines describe the performance of the primary survey.
Upon arrival of the patient to the trauma bay, the status of the airway should be immediately assessed. Simply eliciting a verbal response provides the most meaningful information, as the ability to speak usually indicates adequate airway protection. Patients who cannot speak have either mental status depression or some obstruction to air flow, both of which are indications for airway management. Further indicators of airway compromise include noisy breathing, severe facial trauma (specifically with oropharyngeal blood or foreign body), and patient agitation. A determination of the adequacy of the airway, as well as the decision to obtain improved airway control, should be completed within seconds of arrival. After the initial assessment, frequent reassessment for deterioration and the development of airway compromise is paramount.
Until it is ruled out with an appropriate evaluation, all injured patients should be assumed to have an injury to the vertebral column and have the appropriate precautions maintained. This is of significant importance during the manipulation of the head and neck while the airway is being managed. Cervical spine protection includes the use of a hard cervical collar and the maintenance of the log roll technique for all movement of the patient. During airway assessment and management, the anterior portion of the cervical collar can be removed to optimize exposure, but manual stabilization from an assistant should be provided when the collar is not securely in place. Rigid long spine boards may be of value during transport of the patient but should be removed as soon as possible to avoid pressure-related wounds that can occur within a short time.
When the airway is deemed inadequate, a definitive airway must be established. The definitive airway of choice for most injured patients remains oral endotracheal intubation provided by RSI or DAI (ATLS 10th edition ) technique. While the patient is being prepared for intubation, adjuncts such as oropharyngeal and nasopharyngeal airways may assist in maintaining airway patency during preoxygenation. The patient is provided a sedative and fast-acting neuromuscular blocker, such as succinylcholine or rocuronium, to enhance glottic visualization maximally. Direct laryngoscopy and endotracheal intubation are performed, with care taken to avoid cervical spine motion. The appropriate position of the tube in the trachea is confirmed by chest auscultation, end-tidal carbon dioxide measurement, and a chest radiograph. The presence of experienced airway personnel is critical and, particularly in trauma centers, is often an important component of the trauma alert system.
Common adjuncts in the difficult airway scenario include the gum elastic bougie, video-assisted laryngoscopy, and blind insertion airway device. When the normal view of the glottis is obscured, the bougie may be placed with a limited view of the vocal cords, assisting with appropriate placement the endotracheal tube. Although prior studies have suggested improvement in success rates of intubation with bougie, a recent systematic review and metaanalysis concluded that equivalent rates of first-attempt intubation, intubation duration, and esophageal intubation were observed with techniques incorporating either bougie or stylet. The authors further note that available studies comprising the analysis include small sample sizes and heterogeneous types of providers performing the procedure.
Several devices are now available that provide the clinician a view of the upper airway anatomy that is displayed on a video monitor. Despite this mitigation of challenges related to the angle of the airway, recent data have yielded conflicting results as to any improvement in successful first-pass orotracheal intubation. In parallel to the utility of the bougie, a device is only as functional as the provider employing it. Whichever adjunct, or combination thereof, is selected in the setting of a difficult airway, familiarity with benefits, risks, and limitations of the tool is upon the person performing the procedure.
The blind insertion airway device offers an additional instrument to be applied when attempts at orotracheal intubation are unsuccessful. Devices such as the laryngeal mask airway, multi-lumen esophageal airway (Combitube), and laryngeal tube airway (King LT-D) are placed blindly and function by occluding the esophagus and the posterior pharynx, allowing assisted ventilation to pass selectively down the trachea.
As airway specialists are transitioning to advanced techniques, preparation for a surgical airway should begin. Before physiologic deterioration, a cricothyroidotomy should be performed when other approaches have failed. The inability to maintain oxygenation with a bag valve mask between intubation attempts is a reasonable indication for establishment of a surgical airway. A cricothyroidotomy ( Fig. 17.5 ) is performed in a three-step maneuver:
Spreading retraction with the nondominant hand of the tissues overlying the cricothyroid space (typically performed from patient’s right side).
Keeping lateral tension on the tissues, vertically incise in the tracheal midline, beginning at the thyroid cartilage and extending inferior to the cricoid cartilage.
Transversely incising the cricothyroid membrane, which can be palpated between the thyroid cartilage and cricoid ring, followed by insertion of a 6-0 endotracheal tube or tracheostomy appliance.
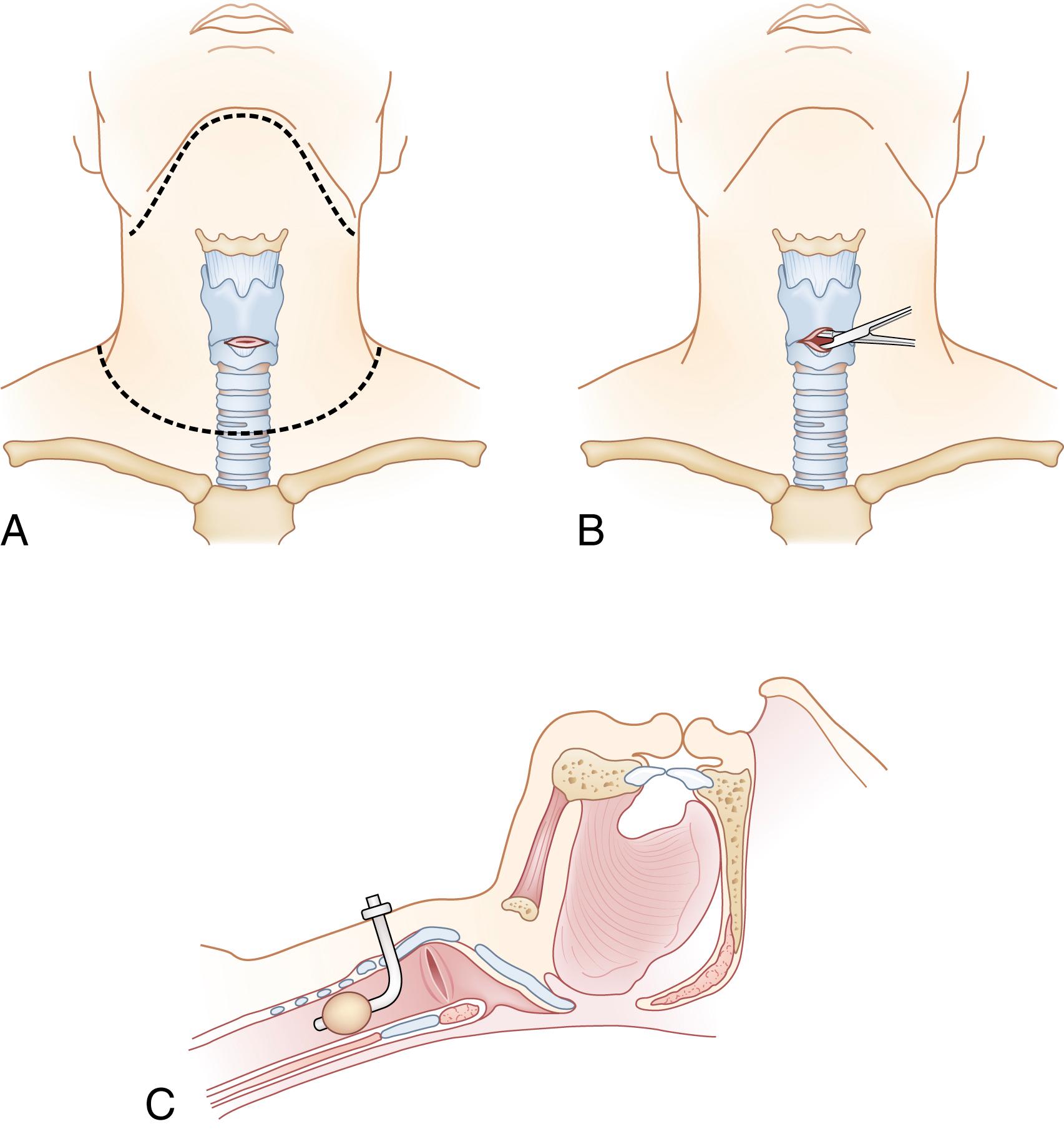
It is critical that the surgeon frequently palpate the underlying structures to guide the dissection and avoid injury to more lateral structures of the neck. Care must be taken also to avoid advancing an endotracheal tube past the carina, which is common in these situations. Tube position is immediately confirmed with lung auscultation and end-tidal carbon dioxide determination. Finally, patients suspected of having a laryngeal injury may have abnormal anatomy in the vicinity of the cricothyroid membrane and therefore require a tracheostomy rather than a cricothyroidotomy.
Following the management of the airway, breathing is evaluated by visualizing chest movement, auscultating breath sounds, and measuring oxygen saturation. Limited respiratory effort or dyspnea requires support of ventilation and further assessment of the chest. Ventilatory problems may be secondary to tension pneumothorax, massive hemothorax, or flail chest with pulmonary contusion. Tension pneumothorax may cause respiratory deterioration but may also be in the form of unstable hemodynamics or cardiovascular collapse. It is a clinical diagnosis that should be recognized on the primary survey without need for radiographic confirmation before treatment. Deviation of the trachea in the sternal notch with unilaterally absent or diminished breath sounds and cardiopulmonary compromise should immediately suggest tension pneumothorax. Thoracic decompression should be rapidly performed with a large-bore needle or tube thoracostomy, depending on the availability of equipment and supplies. Massive hemothorax also requires tube thoracostomy with evacuation of blood and reexpansion of the lung. Severe pulmonary contusion commonly requires aggressive mechanical ventilation, often with elevated levels of positive end-expiratory pressure. To avoid loss of positive end-expiratory pressure, one should resist repeated disconnection from the ventilator to suction or manually ventilate the patient, as oxygenation will only improve with an uninterrupted circuit.
The primary goal of a cardiovascular assessment is determining the presence or absence of shock. ATLS defines shock clinically as evidence of end-organ hypoperfusion present on physical exam. Clinical signs of shock are demonstrated in Box 17.3 . Although hypotension is a clear indicator of cardiovascular decompensation, patients may be in shock well before the onset of hypotension, given physiologic compensatory mechanisms. By far, the most common cause of shock in the injured patient is hemorrhage, and acute blood loss must be ruled out before other causes are considered. Table 17.4 indicates the different classes of hemorrhagic shock.
Agitation or confusion
Tachycardia
Tachypnea
Diaphoresis
Cool, mottled extremities
Weak distal pulses
Decreased pulse pressure
Decreased urine output
Hypotension
| Class I | Class II | Class III | Class IV | |
|---|---|---|---|---|
| Blood volume loss (%) | <15 | 15–30 | 30–40 | >40 |
| Heart rate | ─ | ─/↑ | ↑ | ↑↑ |
| Blood pressure | ─ | ─ | ─/↓ | ↓ |
| Pulse pressure | ─ | ↓ | ↓ | ↓ |
| Respiratory rate | ─ | ─ | ─/↑ | ↑ |
| Urine output | ─ | ─ | ↓ | ↓↓ |
| GCS | ─ | ─ | ↓ | ↓ |
| Base deficit (mEq/L) | 0 to −2 | −2 to −6 | −6 to −10 | −10 or < |
| Need for transfusion | Monitor | Possible | Yes | MTP |
Upon recognizing the presence of shock, ATLS recommends intravenous (IV) access with two large-bore, short, peripheral IV catheters, an intraosseous needle, or a central venous catheter and initial resuscitation with 1 L of warmed crystalloid solution. Patients who fail to respond appropriately to initial crystalloid resuscitation should undergo product-based resuscitation, recognizing that crystalloid resuscitation beyond 1.5 L increases risk of death.
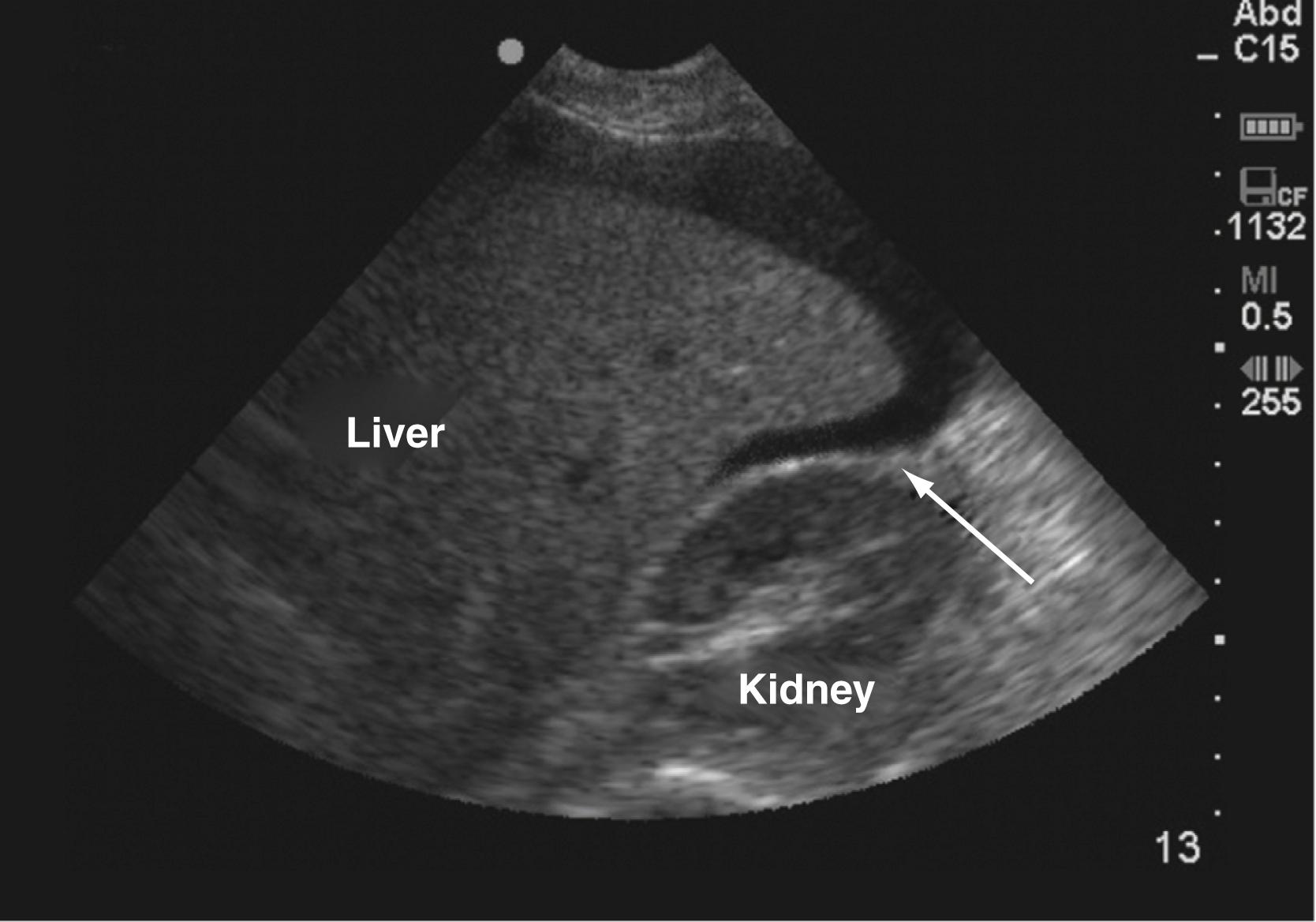
The patient must next undergo a rapid screen to identify the cause of life-threatening blood loss. There are essentially five major locations through which exsanguination may occur: chest, abdomen, retroperitoneum, pelvis, and/or long bone fractures. The initial physical examination identifies sources of external blood loss and long bone fractures. These are managed immediately with direct pressure and fracture splinting, respectively. Adjunctive imaging to the primary survey includes x-ray examinations (i.e., chest and pelvis) and ultrasound. A chest film quickly evaluates for hemothorax and a pelvic film will identify pelvic fracture. The focused abdominal sonography in trauma (FAST) scan is a rapidly obtainable ultrasound examination that assesses for intraperitoneal fluid. Specifically, the FAST scan assesses the hepatorenal, splenorenal, and pelvic spaces for fluid, which is presumed to be blood in the setting of trauma. The value of the FAST scan is that it can be performed quickly in the trauma bay and rapidly repeated, if necessary. As an example, blood in the hepatorenal space on FAST scan is demonstrated by Fig. 17.6 .
After the initial administration of IV fluid, patients are assessed for ongoing signs of shock. Those who respond by demonstrating a normalizing physiologic state then undergo a comprehensive evaluation to identify all injuries. A common pitfall during this time is to continue administering IV fluids at a high rate that may mask ongoing blood loss. As mentioned previously, failure to respond to the initial crystalloid bolus likely indicates continued bleeding, necessitating immediate intervention. Ongoing intrathoracic bleeding after chest tube placement may require thoracotomy. Intraabdominal bleeding in the hemodynamically unstable patient warrants emergent laparotomy. Pelvic fractures require immediate management of any increased pelvic volume with a binder or sheet, followed by operative or angiographic treatment with embolization for arterial hemorrhage.
During the primary survey, it is valuable to make a rapid determination of neurologic function. Of particular importance is globally characterizing neurologic function to assess for traumatic brain and spinal cord injuries (SCIs). The GCS score should be determined to identify deficits in eye opening, verbal ability, and motor responses to potentially reflect the degree of neurologic injury. When sedating medications are required, noting the baseline level of neurologic function before administration can be beneficial. The spinal cord is grossly assessed by visualizing movement of the extremities. While neurogenic shock should always be considered in the setting of hypotension with lack of extremity movement, the provider must be careful in attributing shock to an SCI due to the frequency of hemorrhage in the trauma patient. It is important to recognize that classic teaching requires a cervical or high thoracic spine injury to produce neurogenic shock. If a patient is able to move their upper extremities, the likelihood of neurogenic shock is greatly diminished.
All clothing is removed at this time to allow for an adequate examination, core body temperature measurement, and any required intervention. As hypothermia is one of the components in the “terrible triad of death” in trauma (coagulopathy, acidosis, hypothermia), efforts to restore physiologic body temperature with blankets, heating elements (i.e., Bair Hugger), elevated room/operating room (OR) temperature, and warmed resuscitative fluids are of critical importance.
After critical injury, select patients who experience cardiac arrest may benefit from resuscitative thoracotomy (RT) in the emergency department. First formally described by Cooley and Debakey over 50 years ago for penetrating cardiovascular trauma, RT provides opportunity for four therapeutic maneuvers: release of cardiac tamponade, temporary repair of cardiac injury, cross-clamping the distal thoracic aorta, and management of intrathoracic bleeding. Given risks to health care providers performing the procedure and overall low rates of salvage, multiple studies have attempted to define what groups of patients should be candidates for the procedure on the basis of injury mechanism and physiology at the time of presentation. Patients with the best outcomes after RT are those with penetrating thoracic injuries and signs of life (reactive pupils, spontaneous ventilation, carotid pulse, measurable or palpable blood pressure, extremity movement, or cardiac electrical activity) upon reaching the emergency department. Seamon and colleagues reviewed 72 studies with 10,238 patients, concluding that patients presenting after penetrating chest mechanism, with and without signs of life, survived at 21.3% and 8.3%, respectively. By converse, blunt trauma patients presenting with and without signs of life reveals 4.6% and 0.7% survival from RT, respectively. Moreover, an NTDB review of 11,380 patients undergoing RT revealed a 100% mortality in both blunt and penetrating mechanisms for patients above the age of 57. In these circumstances, bilateral thoracostomy tubes with conservative transfusion measures are likely more appropriate. RT should only be performed in locations with readily available surgical support for definitive repair of thoracic injuries if return of spontaneous circulation is achieved.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged over the past decade as a promising method of obtaining temporary hemorrhage control in the decompensating trauma patient. Although traditionally employed in the setting of abdominal aortic aneurysm repair, application for combat casualty care in noncompressible truncal hemorrhage was first described during the Korean War. With the evolution of this technology for rapid deployment in both military and civilian sectors, REBOA is now being used in approximately 51 domestic trauma centers (median 6 cases per center per year) in the setting of advanced shock and imminent cardiac arrest. Depending on the zone of trauma, REBOA is introduced through the common femoral artery, advanced proximal to level of injury, and inflated, effectively shunting blood to the heart and brain while also decreasing hemorrhage. Currently, there is no high-grade evidence to support indications or the superiority of REBOA beyond standard care, and a comparison of technique to RT introduces both survival and indication biases. Deployment of this technology should only take place within a trauma system capable of managing definitive surgical hemostasis and the multiple possible complications of placement (i.e., vascular injury, extremity ischemia, spinal cord ischemia). At present, protocols for utility are developed by multidisciplinary committee and may vary by institution.
ATLS defines the secondary survey as a thorough head-to-toe examination and patient history. This is often performed immediately after the primary survey in patients who are stable and not requiring emergent intervention. Findings identified during the secondary survey often prompt further evaluation with imaging or other diagnostic modalities. A more detailed neurologic evaluation can be completed at this time and abnormalities of the face and neck are identified. Posterior surfaces that are more difficult to visualize because of the cervical collar are now better examined. The torso is evaluated to identify evidence of pulmonary dysfunction, and findings consistent with peritonitis must be recognized. Seat belt marks or other superficial injury to the neck and abdomen may prompt further evaluation. The pelvis is assessed for tenderness and instability, with care taken to avoid excessive compression. A rectal examination with a nonbloody glove to assess the position of the prostate and the presence of gross gastrointestinal (GI) blood should be included. The extremities are manipulated to identify open or closed deformities and distal perfusion must be carefully assessed. Formal evaluation consisting of distal blood pressure measurements with comparison to uninjured extremities (i.e., ankle-brachial indices) is valuable to obviate further imaging for major vascular injuries. The patient is rolled to evaluate the spine for deformity or tenderness and the long spine board should be removed. In the setting of penetrating trauma, all possible areas of skin must be visualized, including those within body folds, scalp, posterior neck, mouth, axilla, perineum, and back. Marking of penetrating injuries with radiopaque markers can be extremely helpful if subsequent imaging studies are obtained.
The concept of damage control arose in contrast to the traditional approach of definitive injury repair at index operation. It was noted that a portion of patients in the latter group would develop progressive intraoperative physiologic derangement with exacerbation of hypothermia, coagulopathy, and metabolic acidosis. Therefore, damage control emerged as a method of halting this rapid deterioration by expeditious hemostasis, including application of packs, management of GI contamination with repair or resection, and temporary abdominal closure. The patient was then transported to the intensive care unit, and definitive reconstruction could be delayed until resuscitation had been completed. Rotondo and associates first coined the term “damage control” to describe this approach to management in a series of 46 patients operated for penetrating abdominal injury. While actual survival rates were similar between damage control and definitive laparotomy groups (55% vs. 58%, respectively), a significant improvement in survival was noted in a subset of patients with major vascular injury and two or more visceral injuries (77% vs. 11%, P < 0.02). Although damage control began as a method to manage severe abdominal injuries, it is now universally used in the chest, pelvis, and extremities.
Functioning in tandem with damage control surgery, massive transfusion protocols (MTPs) have emerged from the military experience to reveal improved survival with transfusion of equivalent blood component ratios (1:1:1—plasma, platelets, PRBC) in order to approximate whole blood. This approach to the severely injured patient was termed damage control resuscitation and defined by permissive hypotension, facilitation of rapid hemostasis with early balanced transfusion, treatment of coagulopathy, and minimization of crystalloid. To inform initiation of MTP, the Assessment of Blood Consumption score provides a 4-point metric (penetrating mechanism, positive FAST, arrival systolic blood pressure 90 mm Hg, and arrival pulse >120 bpm) whereby clinicians may request product coolers based on prehospital (if available) or initial vital signs. A score of at least 2 was predictive of MTP need (75% sensitivity, 86% specificity) and a delay in initiation is associated with a 5% increase in mortality per minute. Many trauma centers have adopted this strategy and now have well-defined MTPs.
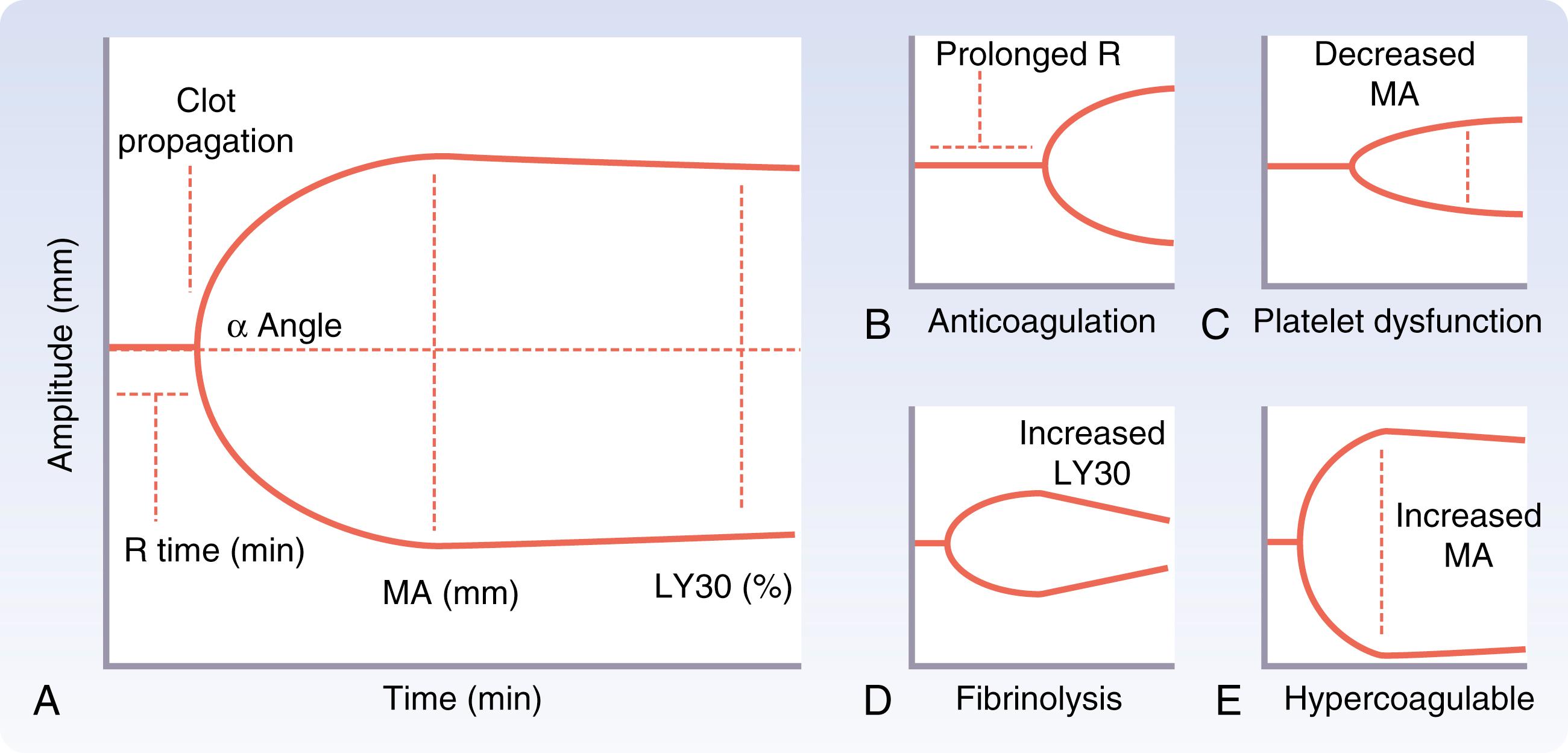
In certain locations, thromboelastography (TEG) provides adjunctive guidance to ongoing MTP and is rapidly obtainable as a point-of-care metric. Originally developed approximately 70 years ago for assessment of inherited bleeding disorders, TEG has historically been employed in liver transplant and cardiac surgery. In traditional analyzers, clot formation is assessed based on resistance transduced from a pin in a small quantity (360 μL) of whole blood. As the blood oscillates, a real-time graphic is produced, providing a dynamic representation of clot generation. Component deficiencies are illustrated as morphologic changes to the clot cylinder ( Fig. 17.7 ). Potential advantages to utilization of TEG-based resuscitation include rapid results for individualized component transfusion, overall conservation of blood products, and a survival benefit with fewer deaths due to hemorrhagic shock in the first 6 hours after injury.
Lastly, an additional treatment adjunct to MTP in damage control resuscitation is tranexamic acid (TXA). TXA is a synthetic derivative of lysine with high affinity for lysine binding sites on plasminogen, thus inhibiting fibrinolysis via antagonism of plasmin binding to fibrin surfaces. It has been shown previously to reduce the need for blood transfusion in elective surgery by one third. The Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage (CRASH)-2 trial randomized 20,211 injured patients to either early administration of TXA (within 8 hours) versus placebo. Patients who received TXA demonstrated a decrease in all-cause mortality compared with placebo (14.5% vs. 16%, P = 0.0035) and a reduction in risk of death due to bleeding (4.9% vs. 5.7%, P = 0.0077). Notably, there was no observed difference in rates of vascular occlusive events between the treatment and placebo groups (1.7% vs. 2.0%, respectively). While the study had limitations due to the inclusion of large numbers that did not require transfusion, it has led to TXA becoming a standard part of the initial resuscitation within many prehospital systems and trauma centers.
Even in the setting of optimal care, TBIs result in substantial morbidity and account for approximately one-third of all trauma-related mortality, resulting in an annual cost of $75 billion to the U.S. economy. Those who survive often experience permanent disability that ranges from mild deficits to conditions requiring permanent total care. Outcomes faced by patients who sustain polytrauma are often dictated predominantly by the TBI. As injury epidemiology has evolved, falls are now the most common cause of brain injuries, with those at the extremes of age being most vulnerable. Although further high-quality evidence for TBI is needed, comprehensive guidelines for management are described by the Brain Trauma Foundation, American College of Surgeons, EAST, and WTA.
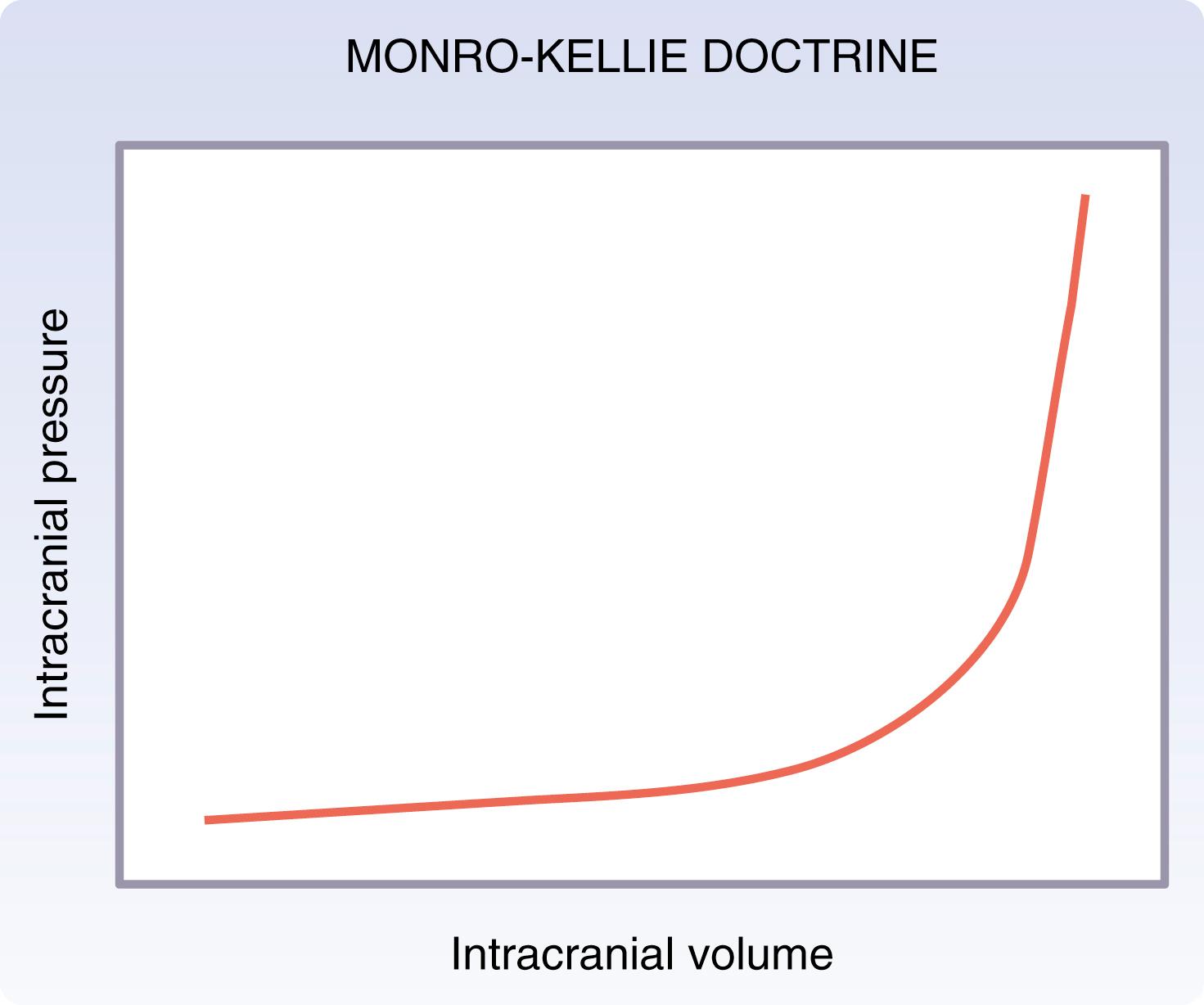
At the tissue level, brain injuries are the result of either direct transmission of energy, the accumulation of blood within the cranium, or a combination of the two. Energy transmitted to the cranium and the underlying brain tissue can cause direct injury both at the location of contact and on the contralateral side (coup contrecoup). Further, the shearing of blood vessels at the time of injury can result in the accumulation of blood within the cranium. As is the case with most tissue, injured brain develops inflammation and edema after trauma that can be worsened by ongoing ischemia. According to the Monro-Kellie doctrine, any increase in the volume of intracranial contents (from extravascular blood or edema) results in an elevation of intracranial pressure (ICP) with an associated decrease in the volume of other tissues (i.e., brain parenchyma, intravascular blood, and cerebrospinal fluid [CSF]). As seen in Fig. 17.8 , an increase in intracranial volume ultimately results in an exponential increase in ICP, thereby worsening cerebral perfusion pressure (CPP), oxygenation, and increasing the risk of herniation.
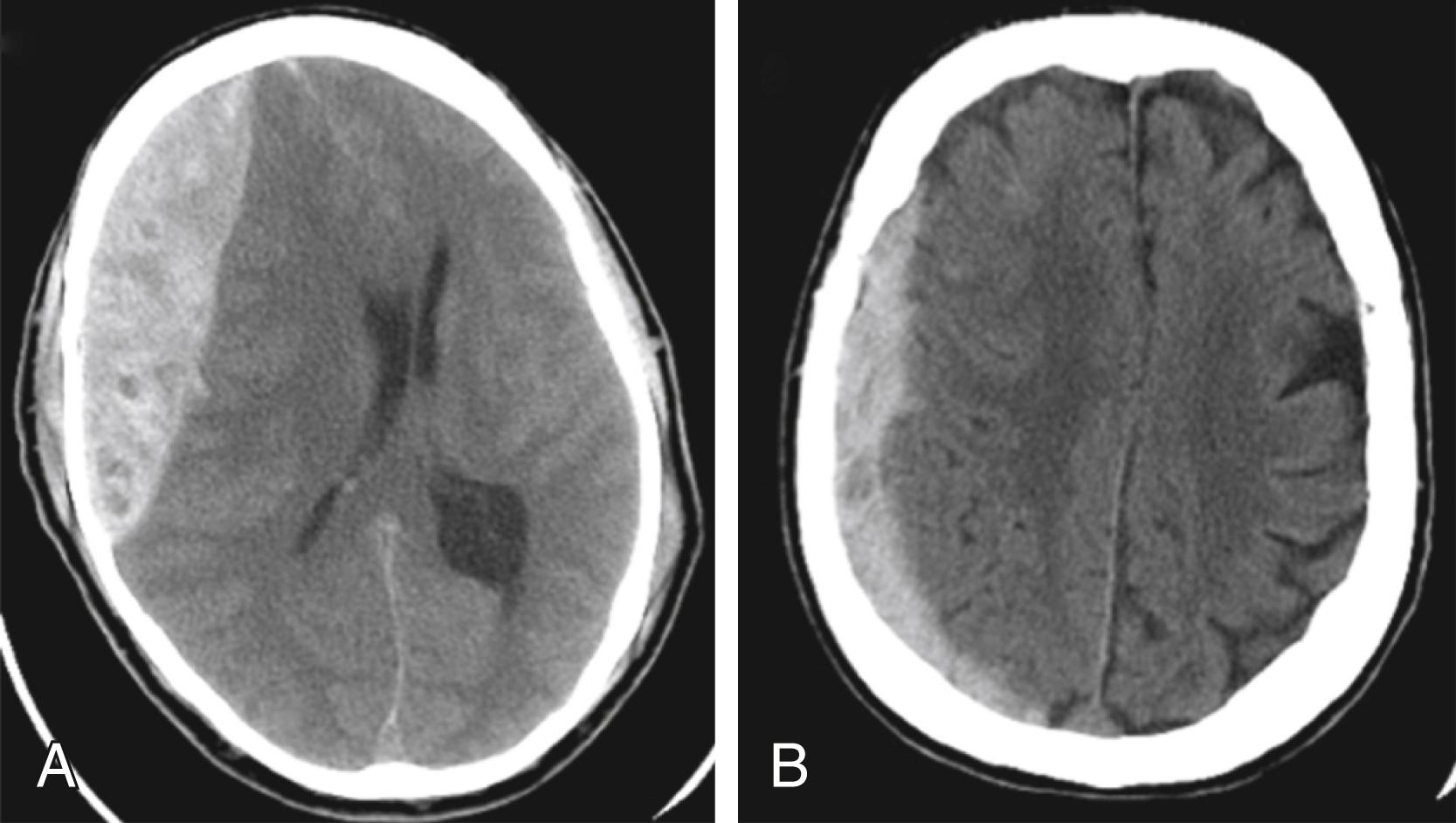
In terms of specific TBI, epidural hematomas ( Fig. 17.9 ) typically result from a lateral fracture of the cranium, causing bleeding from the middle meningeal artery or a nearby vessel. Classically, the clinical course consists of an initial loss of consciousness followed by a lucid interval, during which time the hematoma expands. Upon reaching a significant size, the epidural hematoma causes profound neurologic deterioration. Recognition of this clinical course early may result in treatment with decompression, leading to a favorable outcome. Fortunately, the underlying brain tissue is often not severely injured in the setting of an epidural hematoma. This is in distinction to subdural hematomas, which commonly are associated with severe underlying brain tissue injury (see Fig. 17.9 ). Subdural hematomas are commonly caused by tearing of the bridging veins deep to the dura mater and superficial to the arachnoid mater. Although the hematoma itself can be compressive, it is usually the underlying contusion and axonal injury that predict the outcome after these injuries. Bleeding within the subarachnoid space is indicative of diffuse bleeding from brain tissue and in itself is not deleterious. Despite this, subarachnoid hemorrhages are not benign, and surveillance is mandated to identify deterioration. Parenchymal contusions of brain tissue result from the direct transmission of energy to the cranium and underlying brain as well as from movement of the brain within the rigid cranial vault, resulting in contrecoup injury. Finally, diffuse axonal injury describes the phenomenon of axonal disruption of from the neuronal body secondary to severe rotational forces. Imaging often underestimates the severity of diffuse axonal injury, revealing only punctate hemorrhages and loss of gray and white matter differentiation. Commonly, diffuse axonal injury becomes evident when patients demonstrate poor neurologic status in the setting of underwhelming imaging studies, although ultimate functional prognosis remains difficult to predict based on this finding.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here