Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Primary small bowel malignancy accounts for 0.6% of all new cancer cases, and 0.2% of cancer deaths in the United States. Despite the low incidence relative to other malignancies, the incidence of small bowel malignancy has been increasing from 1.1 per 100,000 persons in 1975 to 2.5 per 100,000 persons in 2015, largely owing to the increasing incidence of neuroendocrine tumor (NET) and duodenal adenocarcinoma. Only 32% of primary small bowel malignancy is confined to the primary site at the time of diagnosis, probably due to its nonspecific presentation, and the poor sensitivity of fluoroscopy or routine abdominopelvic computed tomography (CT) for primary small bowel neoplasm. This chapter will discuss current small bowel imaging techniques and review the imaging appearance of common small bowel masses: NET, adenocarcinoma, gastrointestinal stromal tumor (GIST), lymphoma, and metastasis ( Table 31.1 ).
| Neoplasm | Location | Growth Pattern | Contrast Enhancement | Obstruct-Ion a | Other Features |
|---|---|---|---|---|---|
| Adenocarcinoma | Periampullary, proximal jejunum | Mucosal to transmural | Variable, usually less than mucosa | Common | Apple core or annular constricting. |
| Carcinoid | Distal ileum | Submucosal | Usually intense and homogenous | Rare | Small: often have spiculated mesenteric mass. Hypervascular liver metastases. |
| NHL | Ileum | Transmural | Usually = or > than mucosa | Rare | Often large; may show aneurysmal dilation, often with adenopathy. |
| GIST | Jejunum | Submucosal | Usually intense homogeneous | Rare | Usually no adenopathy. May show liver or mesenteric metastases. |
a Obstruction indicates frequency of small bowel obstruction. GIST, gastrointestinal stromal tumor; NHL, non-Hodgkin lymphoma.
Traditional imaging techniques to assess for small bowel neoplasm, such as small bowel follow-through or small bowel enteroclysis, have been largely supplanted by cross-sectional techniques, such as CT or magnetic resonance (MR) enterography. CT and MR are widely available and provide better sensitivity for primary small bowel neoplasm, while also allowing for detection of extraintestinal abnormalities such as mesenteric, peritoneal, nodal, or hepatic metastasis.
CT enterography (CTE) is superior to routine CT for detection and characterization of small bowel pathology. For CTE, 1350 to 2000 mL of enteral contrast is ingested over 1 hour to distend the small bowel. CTE requires neutral enteral contrast with Hounsfield unit attenuation similar to water, such as methylcellulose, mannitol, polyethylene glycol, or a proprietary preparations such as 0.1% barium sulfate suspension (VoLumen, E-Z-EM, New York, NY, USA) or flavored thickened agents, such as Breeza for neutral abdominal imaging. After bowel distension is achieved with enteral contrast, intravenous iodinated contrast enhanced images of the entire abdomen and pelvis are acquired in one to three phases (late arterial, venous, and delayed). The late arterial phase (35- to 50-second delay) allows for detecting arterial phase hyperenhancement of the bowel mucosa or tumor. The venous phase (70- to 80-second delay) and delayed phase (2- to 5-minute delay) help assess for the presence of metastasis and improve the characterization of liver lesions, hemorrhage, and fibrosis.
MR enterography (MRE) is more sensitive and specific for small bowel diseases than CTE, without the use of ionizing radiation. However, MRE is more expensive, is more subject to artifacts, and requires technical sophistication for performance and interpretation. Like CTE, MRE requires enteral contrast for bowel distention. A biphasic contrast that is hypointense on T1-weigthed images and hyperintense on T2-weighted images is preferred. Less expensive biphasic contrast agents such as polyethylene glycol, methylcellulose, or water may be used. Antiperistaltic agents are recommended to reduce motion artifact from bowel peristalsis. Glucagon, a common antiperistaltic agent, may be administered intravenously, intramuscularly, or subcutaneously at the start of the study and before postcontrast phases. , The typical MRE protocol includes coronal T2-weighted single-shot fast spin-echo (SSFSE or HASTE), and coronal single-shot free precession sequences (FIESTA, FISP, FFE). Pre- and postcontrast coronal, three-dimensional fat-suppressed gradient echo sequences (VIBE, LAVA, THRIVE) are acquired in late arterial, venous, and delayed phases. Axial or coronal diffusion-weighted imaging may help detect hepatic, mesenteric, peritoneal, and nodal metastasis. Coronal single-shot free precession CINE sequences may help assess motility.
CT or MR enteroclysis is an alternative to enterography, where contrast is instilled in the bowel via nasojejunal tube and achieves more optimal distension than oral contrast. In general, enterography is easier to perform and better tolerated than enteroclysis and is the preferred option in most centers.
Video-capsule endoscopy and double-balloon endoscopy performed by gastrointestinal (GI) physicians compliment the radiologic techniques. Capsule endoscopy is routinely performed for suspected small bowel disease. It depicts the small bowel mucosa very well, but has limitations including poor visualization of submucosal tumors ( Fig. 31.1 ), limited angle of view, risk of obstruction at a stricture, incorrect localization of the site of disease, and the requirement to view several thousand images. Double-balloon endoscopy allows for visualization and sampling of mucosal lesions but is technically challenging. , Double-balloon endoscopy has a risk of bowel perforation, with a complication rate of approximately 1% to 4%. Due to limited availability, it is only used in select cases.
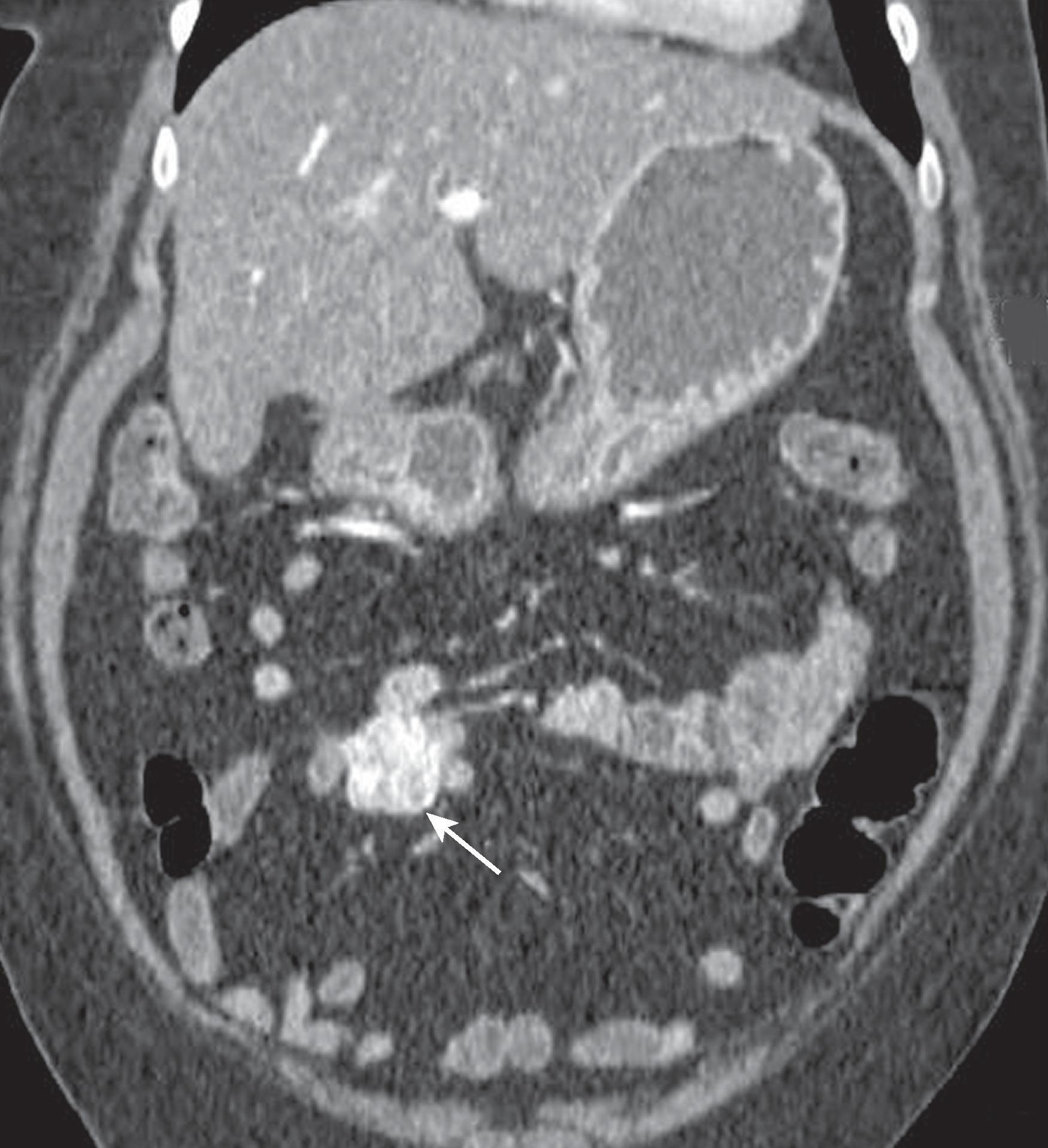
Gastroenteropancreatic NET, also known as carcinoid tumor, arises in the GI tract or pancreas. The incidence of mid gut NET is 1.05 per 100,000 persons, a sixfold increase from 1974 to 2012, perhaps due to improved detection. NET is now the most common primary malignancy of the small bowel. Most small bowel NETs occur in the distal jejunum or ileum, and multifocal primary tumors may present in 20% of patients. Small bowel NETs are staged based on size, depth of invasion, regional lymph node metastasis, or distant metastasis, and are graded based on mitotic count and Ki-67 index. Even small NET may metastasize, and 68% of patients present with metastases ( Fig. 31.2 ). Metastases occur in the lymph nodes, liver, mesentery, peritoneum, retroperitoneum, ovaries, bones, and lung in decreasing order of frequency.
Patients with small bowel NET may present with abdominal pain, diarrhea, flushing, bowel obstruction, GI bleed, nausea, vomiting, and wheezing. About 60% of patients with NET develop carcinoid syndrome from oversecretion of serotonin. Carcinoid syndrome develops once there are hepatic metastases that allow serotonin to bypass the portal venous system of the liver, or if there is liver dysfunction, high tumor load, or peritoneal and distant metastases with systemic venous drainage.
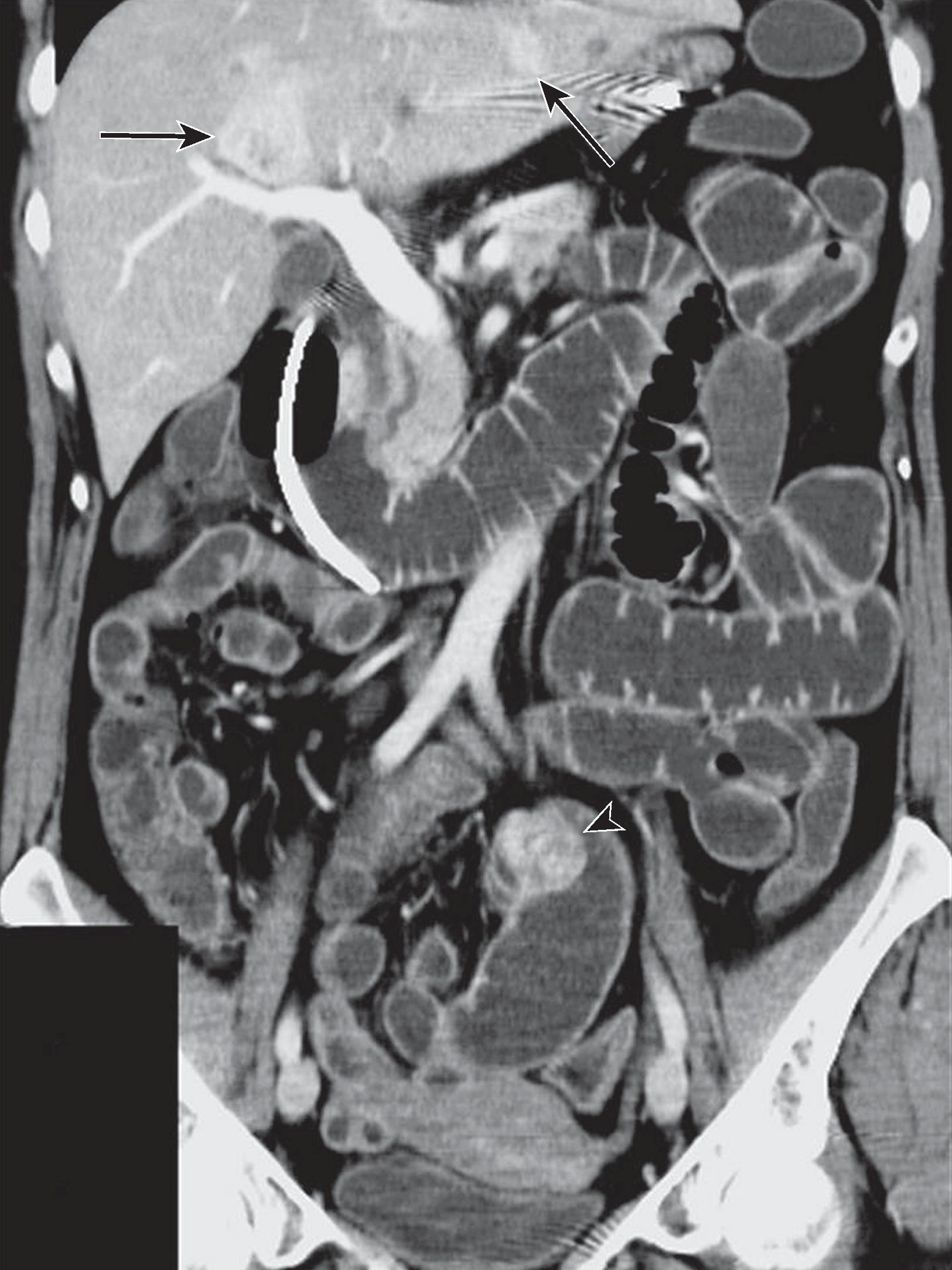
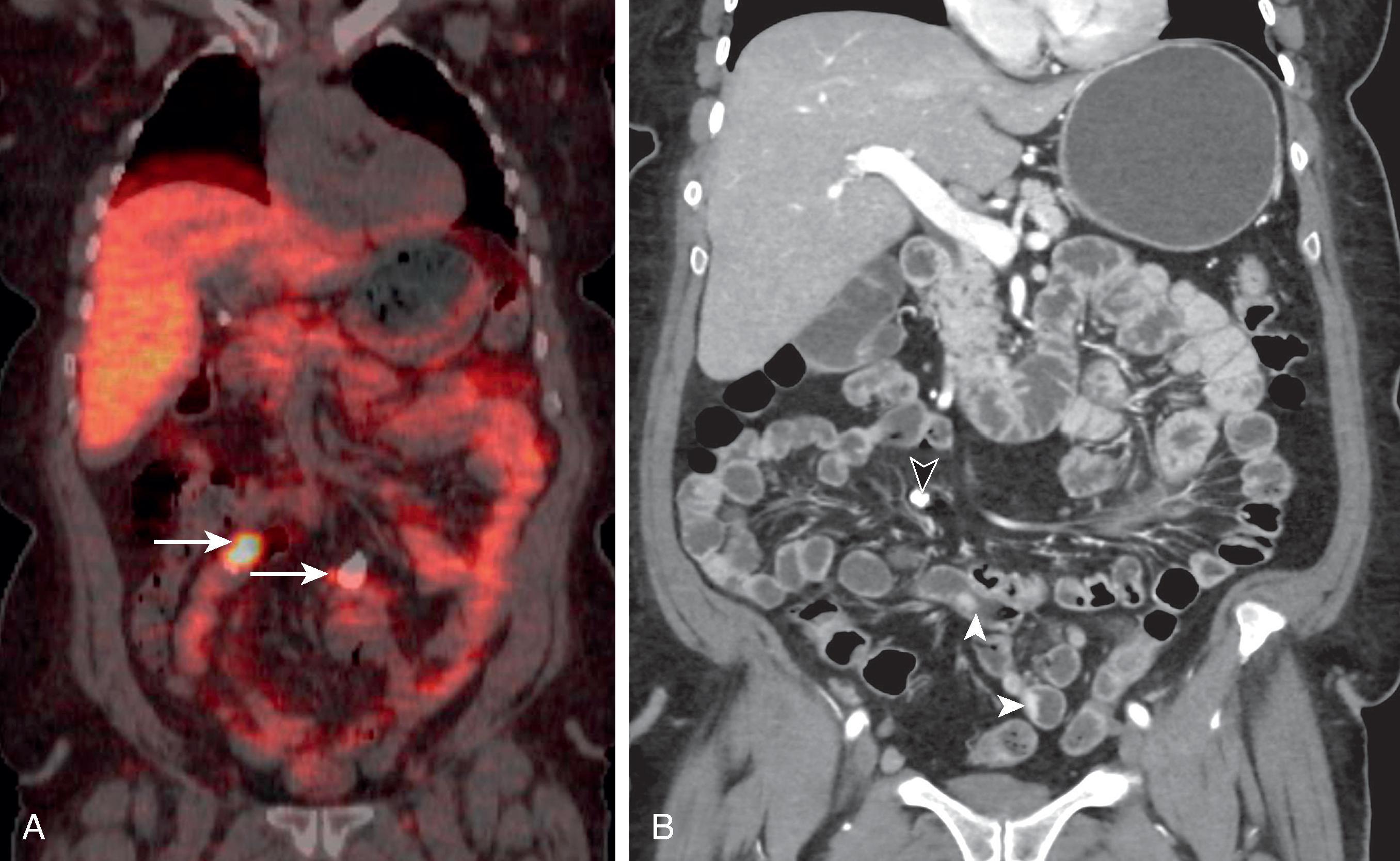
The primary small bowel NET is typically small (often<1 cm) and difficult to detect with CT or MR ( Fig. 31.3 ). Traditionally, functional imaging such as 111 In-pentetreotide single-photon emission computed tomography (SPECT)-CT was performed to localize primary and metastatic NET. Positron emission tomography (PET)-CT, using 68 Ga–tetraazacyclododecane-tetraacetic acid (DOTA) analog as the radiopharmaceutical agent, has changed the paradigm in functional imaging of NET. This technique is used to detect primary and metastatic NET (see Fig. 31.3 ). A prospective trial showed that 68 Ga-DOTATATE PET-CT detected 96% of NET of the bowel compared with only 14% detected by 111 In-pentetreotide SPECT-CT. 68 Ga-DOTA PET-CT has been shown to identify additional sites of tumor and has lower effective radiation dose, superior image quality, and shorter exam time compared with conventional octreotide imaging. Referral to an oncology center should be considered for patients with known or suspected NET if 68 Ga-DOTATATE PET-CT is not available locally.
When visible on CT or MR, the primary NET is usually seen as a plaquelike or polypoid mass or nodular foci of wall thickening. These masses can serve as a lead mass to intussusception. Tumor infiltration of the bowel wall and adjacent mesentery may ensue and result in fibrosis and kinking of small bowel loops. On magnetic resonance imaging (MRI), NET is typically isointense to muscle on T1-weighted imaging and iso- to mildly hyperintense to muscle on T2-weighted images. Mesenteric metastases present as spiculated masses close to the primary bowel tumor. Calcification may be seen in 70% cases. Desmoplastic reaction is common and may distort the mesentery and cause fixation of adjacent bowel loops.
Hepatic metastases are best demonstrated on multiphase contrast-enhanced CT or MR, or diffusion-weighted MR. Small metastases are typically hypervascular, while larger masses may have peripheral hyperenhancement that can vary with the phase of contrast.
Complete surgical resection is the optimal therapy. The main predictors of resectability are the degree of mesenteric desmoplasia and vascular occlusion and patient comorbidity. Tumor debulking is performed even if there is metastatic disease since surgery reduces the risk of bowel obstruction or vascular occlusion. Newer treatments based on therapeutic radionuclides, such as 177 Lu-DOTATATE, may lead to improved outcomes in patients with NET. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here