Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pleural effusions are common clinical problems occurring in more than 1 million patients each year. In some settings, up to 22% of these effusions are caused by malignant disease and more than 100,000 malignant effusions require treatment annually. Affected patients with advanced neoplastic diseases experience considerable morbidity as a result of these pleural fluid collections.
Pleural effusion results from a derangement in the normal physiology such that there is increased production of pleural fluid or a change of its composition with or without a reduction in the absorption of the fluid. This may be to the result of primary or secondary tumors of the pleura with seeding of the intrapleural space and lymphatic obstruction. Free-floating tumor cells block absorption of fluid and produce vasoactive substances that in turn increase the production and block the absorption of intrapleural protein and fluid.
In adults, 95% of neoplastic pleural effusions arise from a metastatic source, with lung and breast carcinoma accounting for 75% of all cases. Other common causes are lymphoma, gastric cancer, and ovarian cancer. Approximately half of patients with breast cancer develop a pleural effusion within their lifetime, as compared with one fourth of patients with lung cancer and one third of patients with lymphoma.
Most pediatric effusions, on the other hand, are benign. If malignant, lymphomas or leukemias account for half with the remainder a mix of tumors such as neuroblastoma, Wilms tumor, and germ cell neoplasms.
Adenocarcinomas whose primary is unknown constitute a distinct entity in patients in whom the source of the pleural malignancy is never found. They are associated with exposure to environmental tobacco smoke.
Patients with malignant pleural effusions are typically symptomatic and complain of dyspnea, cough, or chest pain. The discomfort often is independent of respiration but is aggravated by activity. With invasive pleural metastases, the affected nerve roots may radiate pain. Like pericardial effusions, rapid fluid accumulation amplifies the symptoms. For at least 10% of patients, the dyspnea is multifactorial and does not improve after drainage. Physical findings indicating pleural effusion are decreased tactile fremitus and dullness to percussion on examination of the posterior chest, accompanied by decreased breath sounds. As the effusion increases in size, there may be hyperresonance on percussion immediately above the fluid level because of compression and overdistention of the lung. Bronchial breath sounds may be prominent. As effusions become massive, tracheal deviations from mediastinal shifts are detectable. Malignant pleural effusions rarely cause hemodynamic compromise from tension hydrothorax.
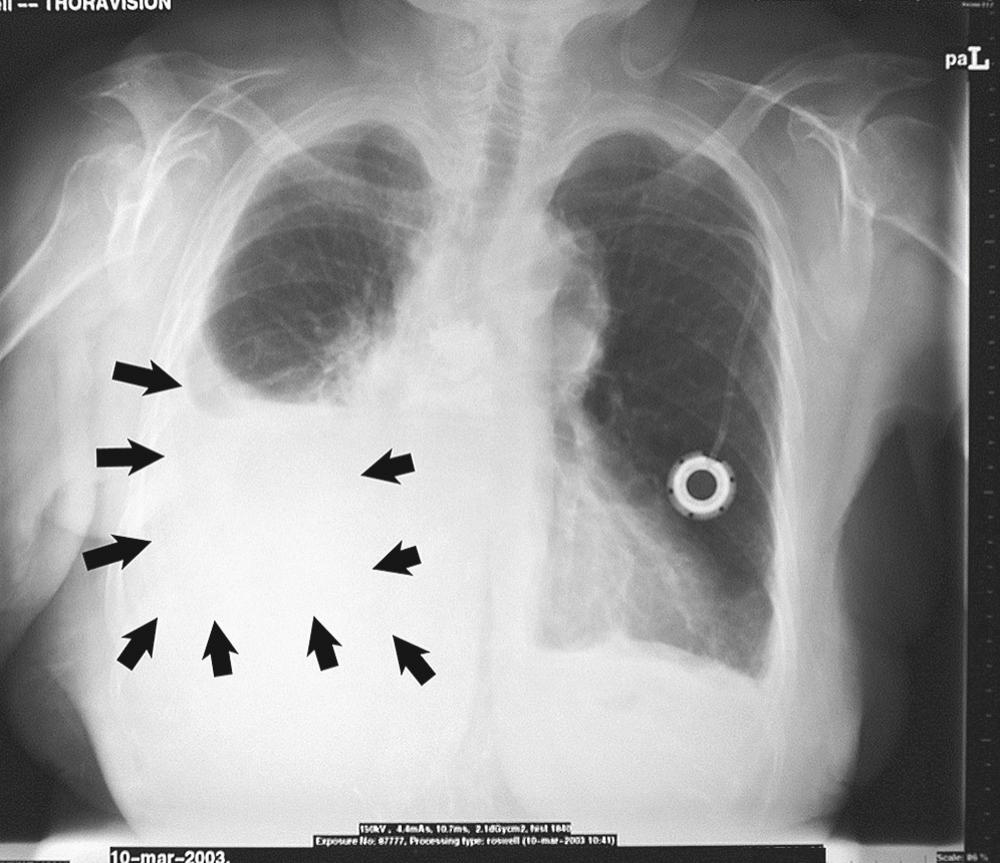
Early evidence of pleural effusion by chest radiograph is costodiaphragmatic sulcus blunting caused by as little as 125 to 250 mL of fluid, depending on the quality of the film ( Fig. 30-1 ). Occasionally, a spurlike shadow projects into the fissure. Massive effusions are uncommon; however, when they occur, they are more likely malignant. In patients in whom the finding is uncertain, a decubitus film might show shifting of the fluid. Effusions wider than 10 mm by decubitus film often are tapped successfully.
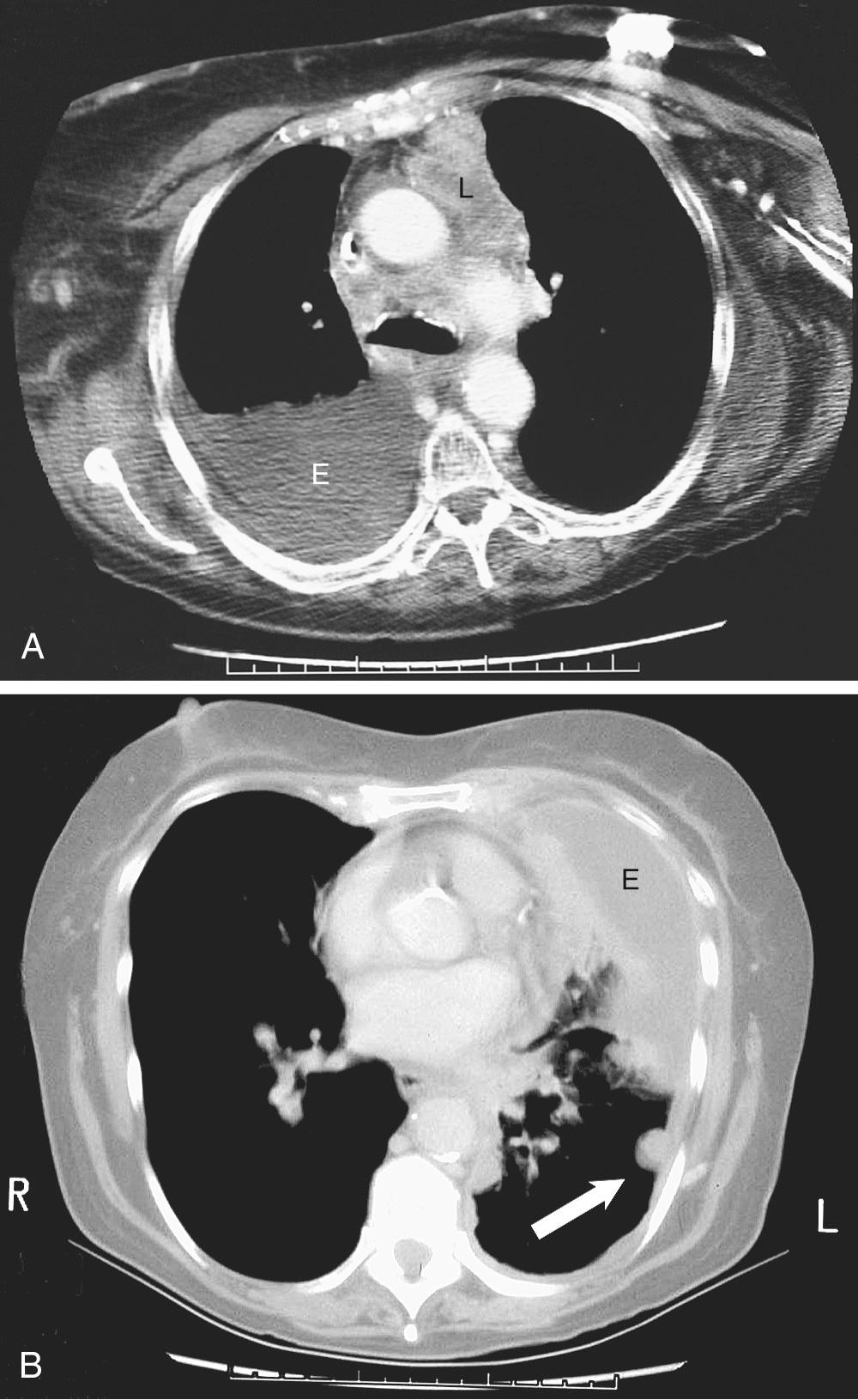
There are specific computed tomography (CT) criteria for pleural effusions. In general, loculation, pleural thickening, pleural nodules, and extrapleural fat of increased density are present only in exudative effusions. Multiple pleural nodules and nodular pleural thickening are generally limited to effusions of malignant origin ( Fig. 30-2 ). Pleural thickening greater than 1 cm also is a reliable criterion. Magnetic resonance imaging (MRI) has limited use, but it may be slightly more useful for certain pleural tumors such as lipoma; it also can be helpful in determining the extent of invasion for mesothelioma. Thoracic ultrasound shows the optimal site for diagnostic thoracentesis of a small pleural effusion. It also helps in the selection of ideal entry points for thoracoscopes or pleural biopsy instruments by avoiding adhesions. In addition, ultrasound has moderate accuracy in predicting the malignant nature of the effusion.
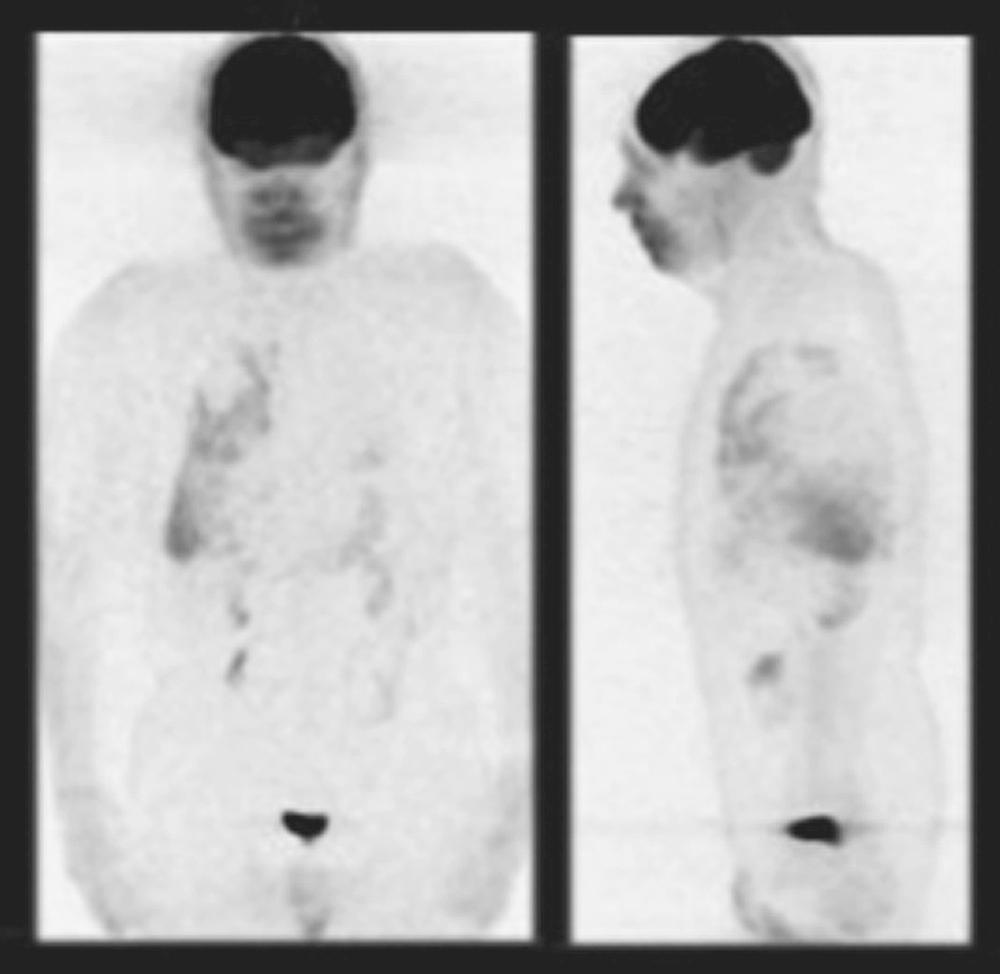
Positron emission tomography (PET)–CT scans may detect early pleural metastases before other imaging tests. If nuclear imaging reveals pleural activity and pleural fluid is scant, diagnostic thoracoscopy is necessary to exclude pleural disease provided this staging is relevant. Limited data suggest a high degree of diagnostic accuracy in differentiating benign from malignant pleural effusions. Figure 30-3 shows an image of a PET scan demonstrating a malignant pleural effusion.
The diagnosis of pleural effusion is confirmed by thoracentesis. Withdrawing only a small portion of pleural fluid is indicated occasionally when full lung reexpansion is unlikely or as a prelude to a definitive drainage procedure. It is usually preferable to attempt drainage of as much pleural fluid as possible ( Fig. 30-4 ).
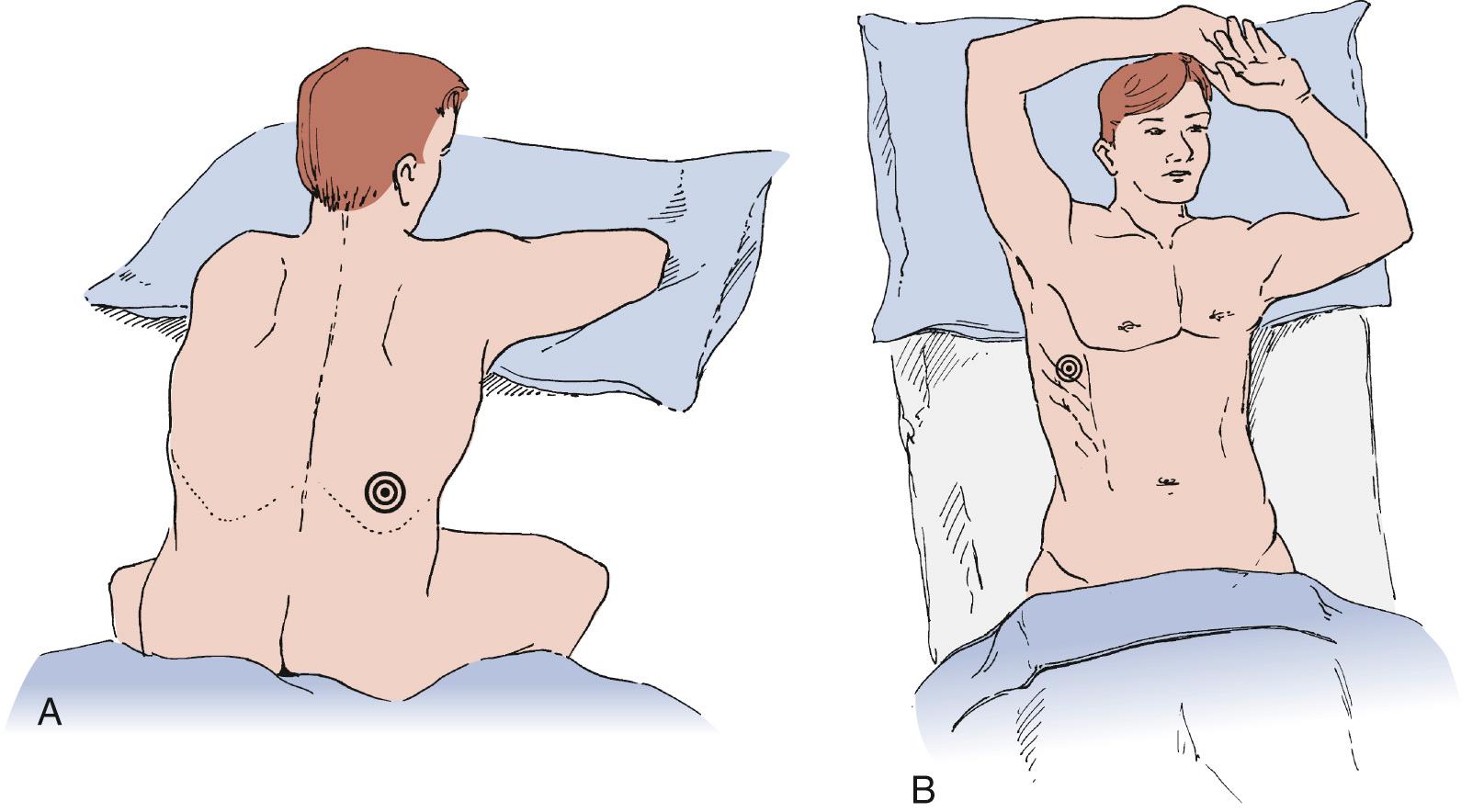
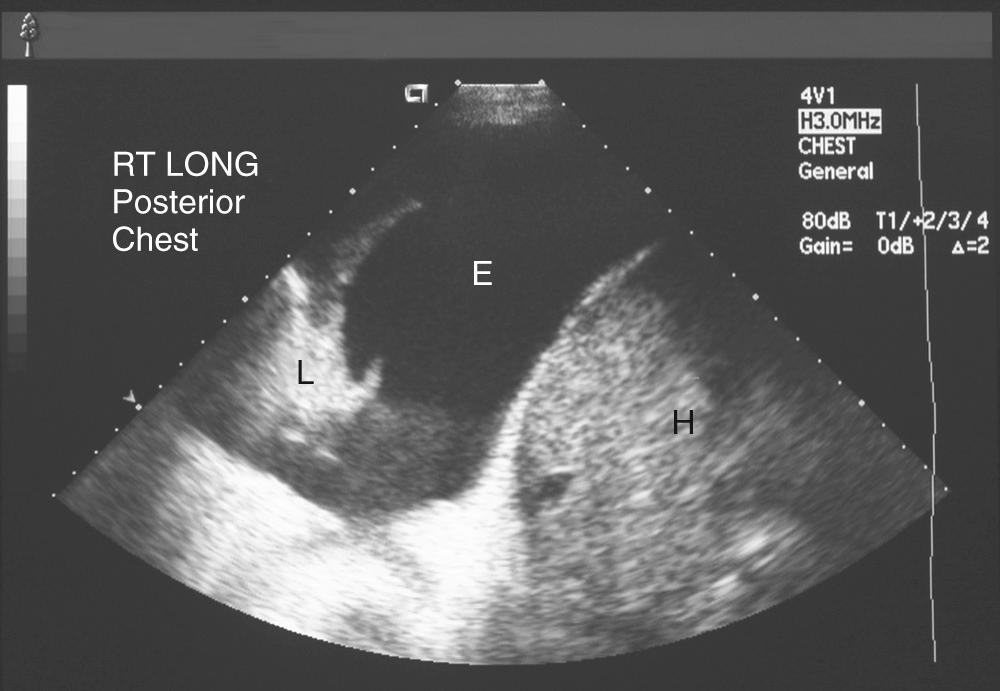
Several commercial kits facilitate drainage by allowing insertion of soft catheters with side holes to completely evacuate the fluid. The catheters can be readjusted during drainage in case the lung temporarily occludes the holes. Needles with retractable blunt obturators (e.g., Turkel needle) also prevent lung injury during pleural entry. Physical examination can guide a pleural tap. On the other hand, ultrasound or other imaging can optimize needle or chest tube placement for effusion drainage in more complex situations ( Fig. 30-5 ). This is particularly useful for a small effusion or pleural disease of long duration complicated by lung consolidation or pleural loculation. Although marking of the area of “deepest” fluid is useful, it is important to perform the thoracentesis with the patient in the same position as during the imaging. Attempts to completely aspirate chronic effusions should be undertaken carefully. High vacuum applied to the pleural space, by either syringe aspiration or vacuum bottle, is sufficient to rupture alveoli and cause a complicated hydropneumothorax.
Practically all malignant pleural effusions are exudates. Laboratory criteria to establish exudative effusions are based on absolute values or ratios to systemic parameters. One of the most useful sets of criteria used to classify effusions as exudative was described by Light: pleural fluid/serum total protein ratio greater than 0.5, pleural fluid/serum lactate dehydrogenase (LDH) ratio greater than 0.6, and pleural fluid LDH greater than 200 U/liter (greater than two thirds of the laboratories' upper limit of normal for serum).
Low glucose (<60 mg/dL) and low pH (<7.20) levels are common in malignant pleural effusions. This is attributed to glucose usage and acid production by the malignant cells, leukocytes within the pleural fluid, and also increased pleural membrane metabolism. Investigators have alternatively implicated abnormal pleural membrane transport of glucose, carbon dioxide, and hydrogen ion. Pleural fluid amylase is elevated in approximately 10% patients with malignant pleural effusions, even without pancreas gland disease. In fact, the most common cause of an amylase-rich pleural effusion is neoplasm. The cell count can also indicate malignancy. For some investigators, bloody fluid is the strongest positive predictor of malignant effusion. Fluid viscosity has also been studied as a diagnostic test.
Many investigators focused their explorations on staining patterns of cells obtained from pleural fluid to confirm malignancy, and, if present, correctly classify its origin. Unfortunately, markers are not yet sufficiently sensitive and specific, but they generally show twice the positivity of cytology alone (80% versus 40%). These tests usually are used after standard cytologic screening and in combination with cytogenetic and other miscellaneous tests to establish a diagnosis. A more complete list is displayed in Table 30-1 .
| Ref | Test | Benign Mesothelial | MM | General CA * | Adeno CA † | Other CA ‡ | Other Type |
|---|---|---|---|---|---|---|---|
| AFP | ++ | Hepatocellular | |||||
| Alcian Blue | 0 | + | |||||
| B 72.3 | 0 | ++ | +++ | ||||
| B-19 | ++ | Prostate | |||||
| BCA-225 | 0 | ++ | |||||
| BER-EP4 | 0 | 0 | ++ / +++ | +++ | |||
| CA 15.3 | +++ | Breast | |||||
| CA 19.9 | +++ | Gastric | |||||
| Calretinin | +++ | +++ | 0 | 0 | |||
| CD44 s | +++ | – | |||||
| CD44 v 3-10 | 0 | + | |||||
| CEA | 0 | +++ | ++ | +++ | Colorectal | ||
| Desmin | ++/+++ | 0 | 0 | ||||
| E-cadherin | 0 | +++ | ++ | ||||
| EMA | – | +++ | +++ | ||||
| GATA | 0 | +++ | Breast | ||||
| GLUT1 | – | +++ | |||||
| Keratin | 0 | +++ | + | +++ | |||
| Ki67 | 0 | ++ | |||||
| Leu M1 | ++ | ||||||
| MCA | ++ | Breast | |||||
| MCA-b-12 | – | +++ | |||||
| MOC31 | 0 | +++ | |||||
| Mucicarmine | + | ||||||
| Mucin | 0 | + | |||||
| N -cadherin | ++ | +++ | + | ||||
| p53 | –/0 | + | |||||
| UEA | 0 | + | |||||
| TIMP-2 | ++ | ||||||
| Vimentin | + | ||||||
| CKMNF 116 | + |
* Nonspecific diagnosis of cancer.
Some of these markers, such as p53, predict a worse prognosis for malignant effusions that are p53-negative. Another marker, Ki-67, is associated with a worse prognosis when its labeling index is low.
Vascular endothelial growth factor (VEGF) has been found to be a useful discriminator for malignant effusions. Other special chemistry values, displayed in Table 30-2 , are occasionally useful to categorize malignancy. The sensitivities and specificities vary by specific cell types.
| Ref | Assay | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Ca 19-9 | 36 | 83 | |
| Ca15.3 | 80-95 | 93 | |
| Calprotectin | 100 | 83.15 | |
| CEA | 52 | 77 | |
| CYFRA 21-1 | 91 | 90 | |
| MUC-1 > 0.126 | 64 | 95.7 | |
| MUC-5AC > 0.028 | 72 | 98 | |
| Sialic acid > 0.075 | 68 | 77 | |
| Sialyl EA | 64 | 95 | |
| TNF ratio | 84 | 90 | |
| TSA | 80 | 67 | |
| CYFRA 21-1 | 47 | 92 | |
| MN/CA9 | 89 | 91 | |
| Mammaglobin by RT-PCR | 82 | 75 |
In the past few years, epigenetic biomarkers for cancer have been studied. These include markers to differentiate benign from malignant pleural effusions. These markers often have the advantage of easier sample processing and, if validated, may impact the field significantly.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here