Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Malaria remains an overwhelming problem in tropical developing countries, accounting for an estimated 216 million cases and 445,000 deaths in 2016. Nearly 40% of the world's population is at risk for acquiring malaria. In sub-Saharan Africa, most severe cases and deaths occur in children younger than 5 years and in pregnant women.
The introduction of chloroquine and dichlorodiphenyltrichloroethane (DDT) at the end of World War II brought dramatic new power to malaria control efforts worldwide. With postwar economic recovery and a renewed spirit of international cooperation, optimism ran high that the widespread use of these new compounds would eliminate malaria, and in 1955, the World Health Organization (WHO) launched its campaign to eradicate the disease. This goal proved overly optimistic, and the centrally organized DDT spraying programs at the core of the campaign were discontinued in 1967. The campaign, nevertheless, brought regional successes that coincided with other factors to reduce malaria incidence in many areas of the world (e.g., in Asia) ( Fig. 274.1A ).
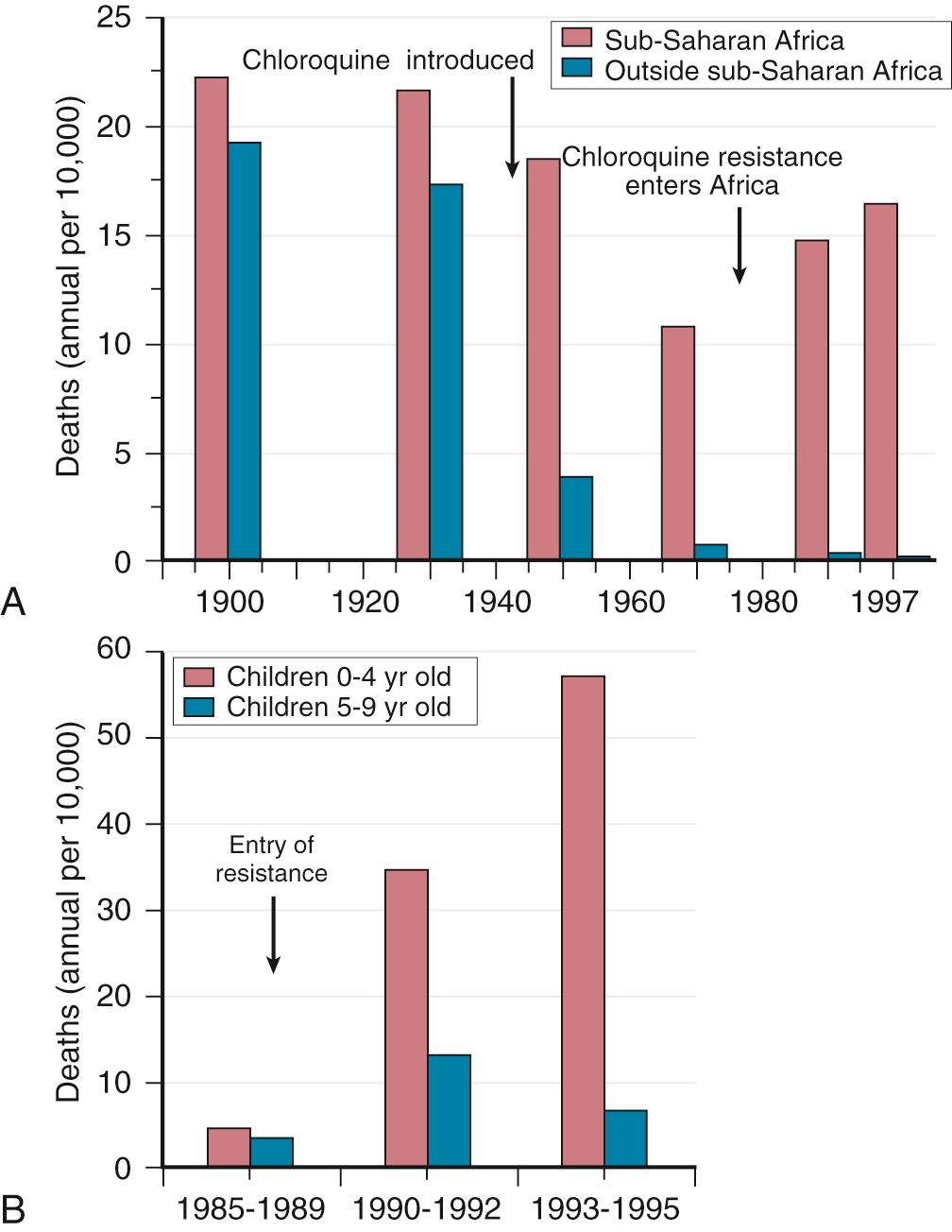
A stark exception to this general progress has been sub-Saharan Africa, where malaria remains deeply entrenched. Even the most committed spraying and eradication programs in endemic areas of this region could not defeat malaria's efficient transmission by the Anopheles gambiae mosquito. The wide availability and use of chloroquine did, however, boost the health of young African children who suffer most from Plasmodium falciparum, the species responsible for the deadliest forms of malaria. As chloroquine became increasingly available in the 1950s to 1970s, death rates from malaria in Africa began to drop, approaching half the level of the prechloroquine years.
Unfortunately, the massive use of chloroquine (hundreds of tons sufficient for hundreds of millions of treatments annually) in the 1980s selected for chloroquine-resistant P. falciparum strains in Southeast Asia that entered and spread across Africa. In the 1980s and 1990s, malaria resurged and death rates increased. The impact of chloroquine resistance was especially evident in young children, who do not have the partially protective immunity to malaria that usually develops after repeated episodes of the illness ( Fig. 274.1B ). In the absence of a fully effective vaccine, success against malaria in Africa will continue to depend on effective drugs, such as artemisinin-based combination therapies (ACTs), that are reliable, affordable, and readily available. Although increased international support and funding for prevention, control, and elimination reinvigorated the efforts of malaria control programs, decreasing the incidence of malaria by an estimated 18% globally between 2010 and 2016, the number of cases and deaths have plateaued in the past several years. Continued progress against the disease will require strengthened community commitments and health infrastructures, supported by new and improved drugs, advances in diagnostics, more effective vaccines, and better insecticides and vector control measures.
Plasmodium parasites belong to the Apicomplexa group of protozoa, which includes other pathogens, such as Babesia, Toxoplasma, and Cryptosporidium species. Apicomplexa are distinguished morphologically by the presence of a specialized complex of apical organelles (i.e., micronemes, rhoptries, and dense granules) involved in host cell invasion (see Fig. 274.4A ). Four Plasmodium species are classified as human malaria parasites: P. falciparum, P. vivax, P. ovale, and P. malariae. Some malaria parasites of other primates (e.g., P. knowlesi, P. cynomolgi, and P. simium ) can also infect humans under natural conditions. Indeed, with the recently appreciated extent of human infections from P. knowlesi, a natural pathogen of macaque monkeys, this parasite has been proposed to be a “fifth human malaria parasite” responsible for significant morbidity and mortality in Malaysia.
In 1880, Alphonse Laveran first observed malaria parasites in a human blood sample, witnessing the exflagellation of microgametes that usually emerge in the mosquito. It was eventually established that parasites in the bloodstream reproduce asexually in the haploid state ( Fig. 274.2 ). During erythrocytic development, a small minority of parasites undergo a switch to sexual-stage development. The resulting male and female gametocytes are the forms that are taken up by and infect anopheline mosquitoes, as proved by Ronald Ross and Battista Grassi in the 1890s. Gametocytes emerge from erythrocytes in the mosquito midgut as male and female gametes that cross-fertilize to form diploid zygotes, which in turn differentiate into ookinetes that burrow through the midgut wall. Each ookinete develops into an oocyst containing up to 1000 sporozoites, which emerge and are then carried by the insect hemolymph to invade the salivary glands. These processes in the mosquito require an incubation period of about 1 to 2 weeks.
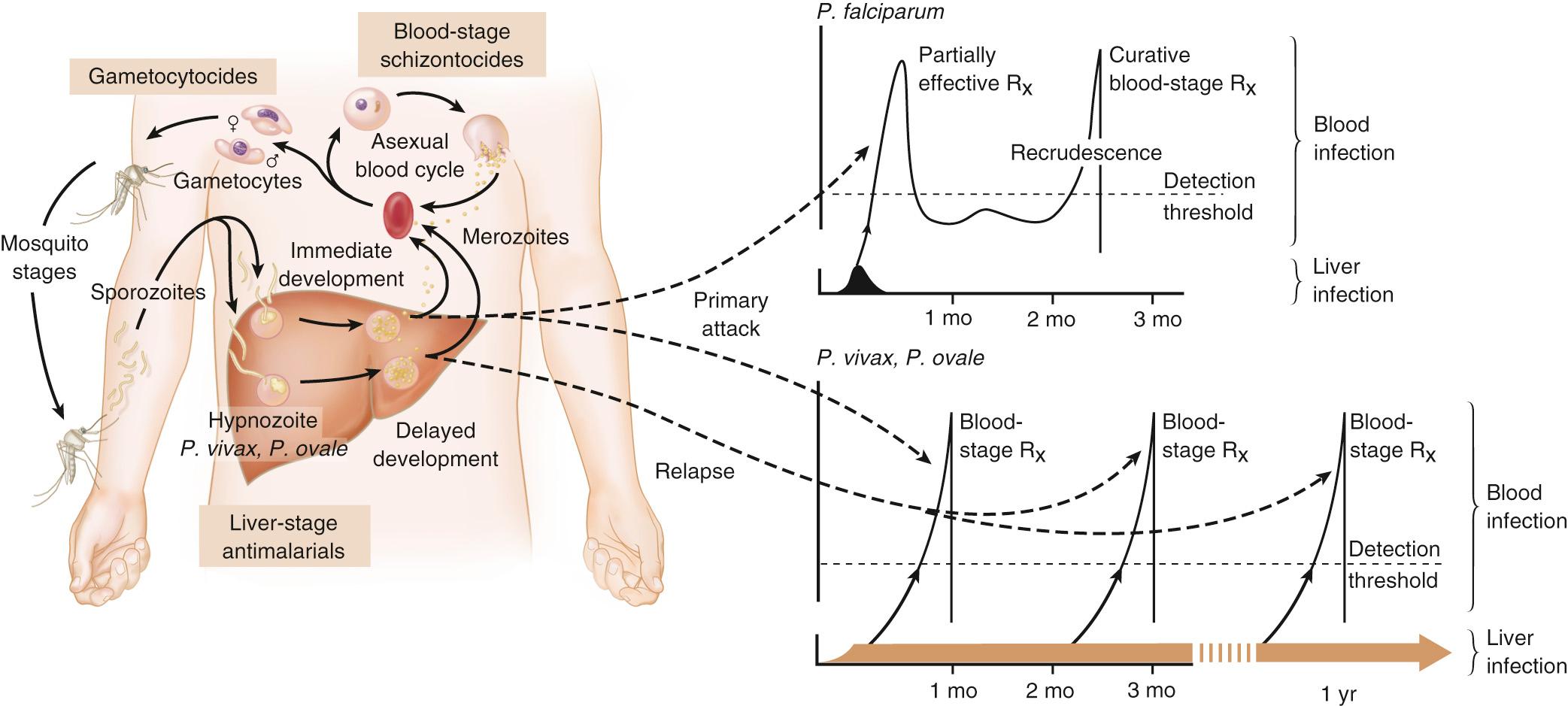
Female mosquitoes inject sporozoites into humans while probing the dermis in preparation for taking a blood meal. Shortt and Garnham demonstrated in 1948 that sporozoites must first invade and replicate in hepatocytes before they can differentiate into merozoites capable of entering the intraerythrocytic cycle. The injected sporozoites typically take several hours to travel through dermal tissues and migrate across host cell barriers before they enter blood and lymphatic systems and are carried to the liver. A molecular motor installed between the sporozoite plasma membrane and a double, inner membrane complex powers motility in this journey, whereas sporozoite surface proteins that are linked to this motor provide traction for gliding and crossing cellular barriers in tissue transit and invasion.
Invasion of sporozoites into hepatocytes takes place by a coordinated series of steps, including host cell contact, signaling events with discharge of calcium, release of ligands and processing molecules from apical organelles, and active entry of the sporozoite into an induced parasitophorous vacuole in the hepatocyte cytoplasm. The host tetraspanin molecule CD81 is important for sporozoite entry into hepatocytes. Two studies have shown that the class B type 1 scavenger receptor SR-B1, a known coreceptor with CD81 for invasion of hepatocytes by hepatitis C virus, also promotes efficient Plasmodium infection of hepatocytes. A likely role for SR-B1 is the organization of CD81 into tetraspanin-enriched microdomains that are preferred membrane areas for sporozoite entry. Because SR-B1 is vital in providing cholesterol to the hepatocyte by high-density lipoprotein (HDL)–cholesteryl ester uptake, its exploitation by Plasmodium and hepatitis C virus represents the evolutionary selection of a dependable invasion pathway by these pathogens. SR-B1's role in HDL–cholesteryl ester uptake and activation of the liver fatty acid–binding protein (L-FABP) carrier also supports the transformation and massive growth requirements of a parasite inside its host hepatocyte. Individual infected hepatocytes support the development of 10,000 to 30,000 merozoites, a process that is not associated with symptoms. All P. falciparum and P. malariae parasites complete their liver-stage development in about 1 to 2 weeks. P. vivax and P. ovale liver stages also can develop promptly or can remain latent as hypnozoites in the liver for months to years before emerging to produce relapses of malaria (see Fig. 274.2 ).
Once a merozoite egresses by protease activity from its host hepatocyte (or from its host erythrocyte in the bloodstream cycle), it engages loosely with an uninfected erythrocyte and then reorients so that its apical end faces the cell surface. The merozoite then drives itself into the erythrocyte through a ring-shaped, electron-dense junction that moves from the front to the back end of the merozoite by the power of an actin-myosin motor. An envelope of invaginated membrane surrounds the merozoite as it enters, forming the parasitophorous vacuole once invasion is complete. These steps of invasion are supported by cell-signaling events, energy-dependent migration, and discharge of contents from the rhoptries, micronemes, dense granules, and perhaps other apical compartments. In P. falciparum, invasion can be supported by multiple different interactions between parasite molecules and erythrocyte surface molecules, including glycophorins. Recent work has demonstrated the essential role of a ligand-receptor interaction between the P. falciparum RH5 (PfRH5) protein and the Ok blood group antigen, basigin. A number of studies have also established a dependence of P. vivax merozoites on interaction with erythrocyte Duffy antigen receptor for chemokines (DARC), but this may not be an absolute requirement considering that P. vivax infections occur in DARC-negative populations of Africa.
Within erythrocytes, merozoites develop from ring forms into trophozoites and then schizonts over 24 hours ( P. knowlesi ), 48 hours ( P. falciparum, P. vivax, and P. ovale ), or 72 hours ( P. malariae ). After breaking down their host cell membrane by enzymatic digestion, 24 to 32 merozoites enter the bloodstream and are each capable of infecting a new erythrocyte. Cycles of invasion and growth in erythrocytes produce a parasite biomass that enlarges rapidly, causing fever and leading to pathologic processes, such as erythrocyte loss (anemia), sequestration of infected erythrocytes in microvascular beds (cerebral malaria), and adverse sequelae of inflammatory cascades and cytokine release.
Malaria presents as an acute febrile illness that is often but not always characterized by the classic malaria paroxysm: chills and rigors, followed by fever spikes up to 40°C (104°F), and then profuse sweating that can ultimately give way to extreme fatigue and sleep. Paroxysms last several hours, can occur with a regular periodicity coinciding with the synchronous rupture of blood schizonts, may alternate with relatively asymptomatic periods, and are associated with high levels of tumor necrosis factor (TNF). Paroxysms can occur in 24-hour, tertian 48-hour, or quartan 72-hour cycles, or in other more complicated patterns. TNF may originate from monocytes stimulated by glycosylphosphatidylinositol moieties or other substances released on schizont rupture.
Malaria can be acutely malignant and painful or more indolent and undermining. It predisposes African children to bacteremia and increases the morbidity and mortality associated with other diseases by stressing host systems and producing effects such as dehydration, anemia, and some degree of immune suppression. Malaria is tremendously debilitating to health and impedes economic development through its adverse effects on fertility, population growth, saving and investment, worker productivity, absenteeism, premature mortality, and medical costs. A single episode of malaria has been estimated to result in a loss of 5 to 20 working days, and an agricultural family afflicted by malaria may be up to 60% less productive than a family without malaria.
P. falciparum malaria can be much more acute and severe than malaria caused by other Plasmodium species ( Fig. 274.3 ). Although P. vivax can cause serious and fatal illness, by far the largest fraction of deaths directly attributable to malaria are caused by severe complications of P. falciparum infection, including cerebral malaria, severe anemia, respiratory distress, renal failure, and severe malaria of pregnancy. Important contributory factors include metabolic acidosis, hypoglycemia, and superimposed bacterial infections. Fatal P. falciparum infections are often associated with the failure of multiple organ systems.
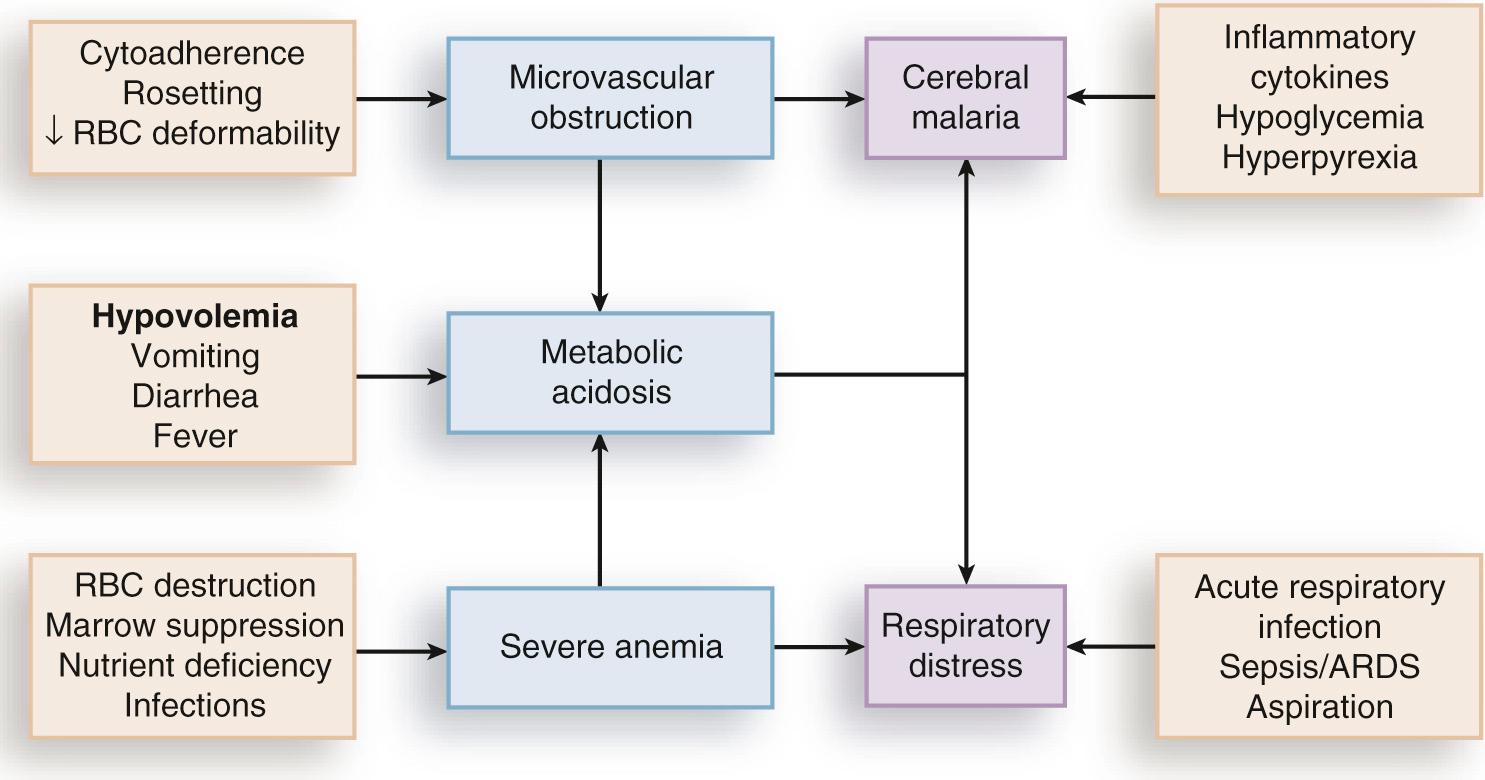
An important feature of the pathogenesis of P. falciparum is the ability of its mature trophozoite and schizont forms to sequester in the deep venous microvasculature. This sequestration is promoted by a number of processes: the adherence of infected erythrocytes to endothelial cells ( Fig. 274.4F and G ); rosetting—the binding of infected erythrocytes to uninfected erythrocytes (see Fig. 274.4H ); reduced erythrocyte deformability (see Fig. 274.4C and D ); and platelet-mediated clumping of infected erythrocytes. In malaria of pregnancy, infected erythrocytes accumulate within the proteoglycan matrix of placental intervillous spaces. P. falciparum –infected erythrocytes can thus accumulate throughout the body, including the heart, lung, liver, brain, kidney, intestine, dermis, bone marrow, and placenta. Uninfected erythrocytes, monocytes, platelets, and deposits of thrombin and fibrin are often found in association with these infected erythrocytes.
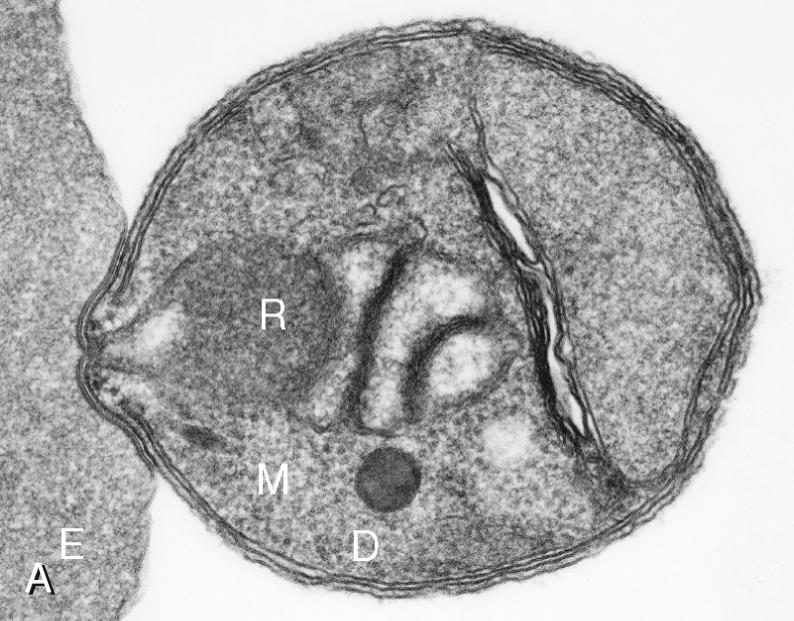
By sequestering in microvessels, P. falciparum may avoid filtration and destruction by the spleen and thus multiply to high densities. The survival and propagation of parasites may be enhanced when they sequester in the low-oxygen-tension environment of postcapillary venules. Attachment points to endothelium have been shown by electron microscopy to be dense protrusions, termed knobs, on the surface of infected erythrocytes (see Fig. 274.4D and E ), where antigenically variant cytoadherence proteins ( P. falciparum erythrocyte membrane protein 1 [PfEMP-1] variants) are anchored. Attachment at knobs (see Fig. 274.4F ) supports cytoadherence in vitro and sequestration in vivo (see Fig. 274.4G ). Under flow conditions, cytoadherence events are reminiscent of leukocyte adhesion, involving distinct phases of tethering, rolling, and stable adhesion.
PfEMP-1 is central to malaria pathogenesis. PfEMP-1 is a family of antigenically variant proteins encoded by the multicopy var gene family. Approximately 60 different var genes are present in the haploid genome of each parasite, encoding PfEMP-1 variants with unique antigenic and cytoadherence properties. A single PfEMP-1 variant is thought to be predominantly expressed on the surface of an individual infected erythrocyte, whereas all others are silenced. Switches in expression between individual members of the var gene family occur at an estimated rate of 2% to 18% per cell per generation and produce the antigenic variation in P. falciparum populations during the course of an infection. These switches may be structured to safeguard sequential expression among variant antigenic genes and thereby promote longevity of infection.
The various PfEMP-1 proteins exposed on knobs have binding domains that adhere to host molecules, including CD36, intercellular adhesion molecule 1 (ICAM-1), thrombospondin, platelet endothelial cell adhesion molecule (PECAM/CD31), chondroitin sulfate A (CSA), and endothelial protein C receptor, which is involved in the deadly sequestration of parasitized erythrocytes that leads to cerebral malaria. In addition to the association of cerebral malaria with subsets of PfEMP-1 variants that adhere to brain endothelium, other pathologic conditions have also been linked to particular PfEMP-1 variants and host receptors. CD36 is an important cytoadherence receptor expressed on microvascular endothelium, as well as on monocytes and platelets, and is thought to mediate the sequestration of parasites as well as the host immune response to infection. Infected erythrocytes that bind CSA expressed by syncytiotrophoblasts sequester selectively in placental tissue and are responsible for malaria of pregnancy.
Some PfEMP-1 variants are also important parasite ligands in rosetting because they can adhere to complement receptor 1 (CR1) and blood group A antigen on uninfected erythrocytes. A human CR1 polymorphism that reduces P. falciparum rosetting was found in one study to protect against severe malaria ; data from other studies of rosetting and disease severity have, in some cases, shown an association and in others have not. PfEMP-1 variants that bind to glycoprotein CD36 on the surface of platelets are also thought to play an important role in the platelet-mediated clumping of infected erythrocytes.
P. falciparum can infect erythrocytes of all ages, promoting heavy parasite burdens. High parasite densities, increased parasite multiplication rates, and evidence of high parasite biomass (e.g., intraleukocytic pigment, mature trophozoites, and schizonts) on peripheral blood smears are associated with increased severity of malaria and death. Such high peripheral blood parasitemias are not observed with the severe pathology of vivax malaria. P. vivax parasites selectively infect reticulocytes and accumulate as developing sexual stages (gametocytes) and mature replicative stages (schizonts) in the bone marrow and liver.
The classic histopathologic finding of fatal cerebral falciparum malaria is the intense sequestration of infected erythrocytes in cerebral microvessels (see Fig. 274.4G ), often accompanied by “ring” hemorrhages, perivascular leukocyte infiltrates, thrombin deposition, activated platelets, and immunohistochemical evidence for endothelial cell activation. In one autopsy series of patients who died from cerebral malaria, 94% of brain microvessels contained adherent infected erythrocytes, compared with 13% of patients who died from noncerebral malaria. In another study, 7 of 31 (24%) African children who received a clinical diagnosis of cerebral malaria were found at autopsy to have nonmalarial causes of coma, underscoring the possibility that other illnesses may mimic the cerebral malaria presentation in areas where incidental parasitemia is common.
Sequestration of P. falciparum –infected erythrocytes stimulates the local production of inflammatory cytokines, such as TNF, elevated levels of which may correlate with disease severity. These cytokines and other inflammatory mediators also upregulate adhesion molecules, such as ICAM-1, in the cerebral microvasculature, which may lead to further sequestration of infected and uninfected erythrocytes, leukocytes, and activated platelets. Impaired nitric oxide bioavailability may also contribute to endothelial dysfunction by increasing microvascular tone, endothelial cell adhesion molecule expression, cytokine production, and infected erythrocyte sequestration. These processes may cause varying degrees of functional obstruction and consequently impair local delivery of oxygen and glucose. However, obstruction by infected erythrocytes and other blood elements does not generally produce neurologic sequelae akin to those that follow the physical occlusion in thrombotic stroke, because most patients with cerebral malaria who recover can do so rapidly within 48 hours and without such consequences. Systemic sequestration of metabolically active parasites, blood cells, and platelets likely contributes to the metabolic acidosis and thrombocytopenia commonly seen in severe malaria. Metabolic acidosis, hypoglycemia, hyperpyrexia, and nonconvulsive status epilepticus can contribute significantly to the cerebral malaria presentation, as suggested by the rapid clinical improvement of some patients after fluid resuscitation, blood transfusion, dextrose infusion, fever reduction, and anticonvulsant therapy in addition to antimalarial treatment.
Hypoglycemia in malaria can cause coma and convulsions and thus may contribute substantially to the morbidity and mortality associated with cerebral malaria. The pathophysiologic mechanisms of hypoglycemia in children and adults seem to be different. In children, insulin levels are appropriate and hypoglycemia is associated with impaired hepatic gluconeogenesis and increased consumption of glucose by hypermetabolic peripheral tissues. Large amounts of glucose are also consumed by intraerythrocytic parasites. In adults, hypoglycemia is often associated with hyperinsulinemia, which may result from pancreatic islet cell stimulation by parasite-derived factors or parenteral quinine or quinidine therapy, or both. Depletion of liver glycogen stores after decreased food intake during the prodromal period may also contribute to hypoglycemia.
The pathophysiology of malarial anemia is multifactorial and complex. The intravascular lysis and phagocytic removal of infected erythrocytes contribute to anemia but do not account for the dramatic reductions in erythrocyte mass that can accompany acute P. falciparum malaria episodes. Additional processes have therefore been implicated in the pathogenesis of malarial anemia. Excess removal of uninfected erythrocytes may account for up to 90% of erythrocyte loss and may be mediated by processes (e.g., oxidative stress) that accelerate the senescence and reduce the deformability of erythrocytes. The contribution of impaired erythropoietic responses to malarial anemia is significant and probably involves general processes also found in other diseases. Release of inflammatory cytokines (e.g., TNF) is associated with impaired production of erythropoietin, decreased responsiveness of erythroid progenitor cells to adequate levels of erythropoietin, and increased erythrophagocytic activity. These pathogenic processes account for the normochromic normocytic anemia seen in malaria and explain the notable absence of a robust reticulocyte response. Although microcytosis and hypochromia are seen in malaria, these findings are often attributable to thalassemias and iron deficiency in endemic areas. Bacteremia, nutritional deficits (e.g., vitamin A and B 12 deficiencies), concomitant infections (e.g., hookworm and Schistosoma ), and genetic polymorphisms (glucose-6-phosphate dehydrogenase [G6PD] deficiency) have also been associated with greater degrees of anemia in malaria episodes, presumably by lowering the baseline from which hemoglobin levels acutely decline. In endemic areas where chloroquine resistance is prevalent, the inability of young children to clear their parasitemias with chloroquine contributes to their higher baseline prevalence of anemia when compared with children treated with more effective drugs.
The most significant pulmonary manifestation directly attributable to P. falciparum is noncardiogenic pulmonary edema. Sequestration of infected erythrocytes in the lungs is thought to initiate regional production of inflammatory cytokines that increase capillary permeability, leading sequentially to pulmonary edema, dyspnea, hypoxia, acute lung injury, and acute respiratory distress syndrome. Pulmonary edema is common with severe malaria in adults but infrequent in children, and it is not associated with pleural effusion. Iatrogenic fluid overload and acute renal failure may contribute to the development or worsening of pulmonary edema. Although pulmonary edema usually occurs after other features of severe disease (e.g., coma, acute renal failure) become manifest, it may occur at any time during the clinical course, even when the patient appears to be recovering on antimalarial therapy. Dyspnea and increased respiratory rate are features of impending pulmonary edema and often precede other clinical (e.g., use of accessory muscles of respiration) and radiologic (e.g., generalized increase in interstitial markings) signs.
Pulmonary manifestations of deep breathing and respiratory distress associated with severe malaria may also arise from metabolic acidosis, severe acute respiratory infections, sepsis-related acute respiratory distress syndrome, aspiration (especially with diminished consciousness or convulsions), and nosocomial pneumonia. Cerebral pathologic processes may result in abnormal breathing patterns, including Cheyne-Stokes respirations and respiratory failure.
Metabolic acidosis is a common feature of severe malaria and is associated with significant lactic acidemia in up to 85% of cases. Metabolic acidosis is principally caused by reduced delivery of oxygen to tissues, from the combined effects of anemia (decreased oxygen-carrying capacity), sequestration (microvascular obstruction), and hypovolemia (reduced perfusion) resulting from fluid losses caused by fever, decreased oral intake, vomiting, and diarrhea. These effects produce a shift from aerobic to anaerobic metabolism and cause lactate levels to increase. The following factors may also contribute to metabolic acidosis: production of lactate by anaerobic glycolysis in sequestered parasites ; reduction of hepatic blood flow, leading to diminished lactate clearance ; induction of lactate production by TNF and other proinflammatory cytokines ; impairment of renal function ; and ingestion of exogenous acids (e.g., salicylate) or unknown constituents of traditional herbal remedies for fever.
Placental malaria results in maternal morbidity and mortality, intrauterine growth retardation, premature delivery, low birth weight, and increased newborn mortality. Selective accumulation of infected erythrocytes in the placenta involves their interaction with syncytiotrophoblastic CSA, which is possibly complemented by interactions with other molecules, such as hyaluronic acid and immunoglobulins. This is in contrast to the sequestration of infected erythrocytes in the systemic microvasculature, where CD36 is the major endothelial receptor. Parasites that accumulate in the placenta express PfEMP-1 variants that bind CSA but not CD36. Evidence suggests that women experiencing a malaria episode from CSA-binding parasites during their first pregnancy lack immunity to the PfEMP-1 antigenic variants presented by these strains and, despite immunity to CD36-binding variants from previous infections, are highly susceptible to the new placental infection. Placental malaria in subsequent pregnancies is typically less severe than in the first pregnancy, presumably because of a woman's previous exposure to CSA-binding parasites.
Infections with P. vivax and P. ovale can be considered similar to each other from a clinical perspective. P. vivax infections can be tremendously debilitating and are sometimes associated with serious complications, including acute lung injury and splenic pathology. Splenic rupture has been associated with acute and chronic infections and can occur spontaneously or with minor trauma, including manual examination of the spleen. Although more commonly associated with P. vivax malaria, splenic rupture has been associated with all four human malaria parasites. Anemia is frequently observed as a consequence of acute or chronic infections, or as a result of repeated acute infections. Suppressed erythrocyte production and hemolysis of both infected and uninfected erythrocytes have been implicated in the pathogenesis of P. vivax malarial anemia. Although not often fatal, P. vivax infections have recently been associated in Papua New Guinea and Indonesia with severe disease manifestations, including cerebral malaria, severe anemia, and respiratory distress.
P. vivax merozoites selectively invade reticulocytes. Because these cells account for only a small proportion of the total erythrocyte mass, parasitemias in P. vivax infections are usually less than 1% even when the pathology of vivax malaria is severe. P. vivax parasites can accumulate as developing sexual stages (gametocytes) and mature replicative stages (schizonts) in the bone marrow and liver. Recent evidence suggests that P. vivax can cytoadhere to lung endothelial cells and can cause inflammation and injury by sequestering in the pulmonary microvasculature. P. vivax parasites may avoid splenic entrapment by increasing rather than decreasing erythrocyte deformability.
The quartan malaria of P. malariae usually presents with fever and paroxysms similar to those of P. vivax but with a 3-day rather than 2-day periodicity. P. malariae often establishes parasitemias that are below the level of detection by microscopy. Patients can remain infected and asymptomatic for many years before presenting with fevers, malaise, and splenomegaly decades after they have left an endemic area. Chronic P. malariae infection can lead to nephrotic syndrome in young children living in endemic areas. This complication has features of an immune complex–mediated glomerulonephritis.
A large focus of human malaria caused by P. knowlesi occurs in Malaysia, where high case-fatality rates have been reported. P. knowlesi is indistinguishable from P. malariae on blood smear examination, showing both immature and mature forms in the circulation. Unlike P. malariae, however, P. knowlesi replicates every 24 hours and can cause daily fever spikes and hyperparasitemias that are life threatening. In addition to hyperparasitemia, severe P. knowlesi malaria cases have been associated with metabolic acidosis, hepatorenal dysfunction, respiratory distress, severe anemia, and refractory hypotension.
Fatal P. falciparum malaria has been a potent evolutionary force in shaping the human genome. Evidence for the natural selection of genetic polymorphisms can be found in the ethnic and geographic distributions of hemoglobin variants, thalassemias, G6PD deficiencies, erythrocyte membrane proteins, and cytokines and other mediators of inflammation and immunity.
The geographic distributions of hemoglobin variants overlap considerably with those of P. falciparum malaria. Case-control and longitudinal cohort studies have associated malaria protection with hemoglobin S (HbS) heterozygosity (HbAS; sickle cell trait), in which the sixth amino acid of one β-globin chain is changed from glutamate to valine. The genetic fitness of HbS homozygosity (HbSS; sickle cell disease) is very poor in sub-Saharan Africa, whereas the prevalence of HbAS individuals can be more than 25% in some areas. The HbS mutation thus exists as a balanced polymorphism: the malaria protective benefit afforded to HbAS heterozygotes offsets the childhood deaths of HbSS homozygotes. The hemoglobin C (HbC) mutation is in the same sixth position as the HbS mutation but differs in that the amino acid is changed from glutamate to lysine. A number of case-control studies in West Africa have associated HbC with malaria protection.
Most studies have associated HbAS protection against malaria with reductions in parasite density. However, some epidemiologic studies have found similar parasite densities in HbAA and HbAS children, very high parasite densities in some HbAS children, and occasional cases of severe malaria in HbAS children. These observations are not fully explained by proposals that infected HbAS erythrocytes are more likely to sickle or support reduced parasite growth rates under conditions of low oxygen tension in microvessels, by enhanced phagocytosis of infected HbAS erythrocytes by macrophages, or by translocation of sickle cell erythrocyte microRNAs that may negatively regulate parasite growth in the infected host cell. Other mechanisms of protection that include additional genetic or environmental factors are likely operating in HbAS children. Unlike for HbAS, malaria protection by HbC has not been associated with reduced parasite densities in vivo or significant impairment of parasite multiplication in vitro, suggesting that HbC erythrocytes support normal invasion and development of P. falciparum.
In other studies of possible mechanisms of protection, freshly drawn and infected HbAS and HbAC erythrocytes were found to be impaired in their adherence to microvascular endothelial cells and monocytes, two interactions critical to the development of severe malaria. Abnormal display of the parasite's main virulence factor and antigenically variable cytoadherence ligand, PfEMP-1, on the surface of infected erythrocytes offers one explanation for these findings. In this model of protection, reduced cytoadherence of infected HbAS and HbAC erythrocytes enables them to sequester in microvessels and achieve high parasite densities while lessening the inflammatory consequences of cytoadherence and thus reducing the chances of progressing to severe disease. Evidence indicates that aberrant remodeling of host cell actin, compromised formation of knobs, and reduced PfEMP-1–mediated cytoadherence are the result of a redox imbalance inherent to hemoglobinopathic and fetal erythrocytes. PfEMP-1–specific antibodies, which are acquired rapidly in endemic areas, may synergize with HbS and HbC to further weaken cytoadherence interactions and ameliorate disease severity.
Hemoglobin E (HbE) is another hemoglobin variant characterized by the substitution of lysine for glutamate at the 26th amino acid of β-globin. This mutation also introduces an alternative splice site that reduces the amount of β-globin produced, thereby conferring a β-thalassemia phenotype to HbE erythrocytes. Unlike HbS and HbC, HbE is found predominantly in Cambodia and neighboring countries in Southeast Asia. Some epidemiologic studies have found HbE to protect against malaria, whereas others have not. Although HbE has been suggested to decrease the multiplication rate of P. falciparum, parasite densities do not differ between HbAA and HbE patients with malaria.
The thalassemias arise from deletion of one or more of the four genes encoding the α-globin chains or mutations or deletions in one of the two genes encoding the β-globin chains of hemoglobin. These hemoglobinopathies are generally benign in the heterozygous state and are associated with varying degrees of hypochromic microcytic anemia. Further loss of expression in the homozygous state causes severe disease and can be incompatible with life. Mutations associated with thalassemias are thus believed to exist as balanced polymorphisms in human populations; indeed, some of them (e.g., α-thalassemia) have been associated with protection from severe malaria. Although P. falciparum development can be supported by thalassemic erythrocytes, some studies have demonstrated impaired growth, especially under conditions of oxidative stress. Other studies have demonstrated that infected thalassemic erythrocytes bind increased amounts of antibody from both nonimmune and immune sera, which suggests the possibility of enhanced opsonization in vivo. These mechanisms are difficult to reconcile with several more recent studies from Africa, which show that both heterozygous and homozygous α-thalassemias protect against severe malaria without reducing parasite densities in vivo. Other evidence suggests that α-thalassemia may protect against severe malaria in Africa by the same mechanism proposed for HbS and HbC: namely, abnormal PfEMP-1/knob display, decreased cytoadherence, and reduced activation of endothelium. Increased microerythrocyte counts in α-thalassemia homozygotes have been proposed to contribute to protection against severe malarial anemia in Papua New Guinea by a mechanism that reduces loss of erythrocytes and hemoglobin during P. falciparum infection.
Hemoglobin F (HbF, α 2 /γ 2 ) is a normal hemoglobin variant expressed by the fetus in utero and by the infant during the first few months of life. The expression of HbF dramatically declines after the third month of life as adult hemoglobin A (HbA, α 2 /β 2 ) replaces it. The uncommon presentation of malaria in neonates younger than 6 months led to the hypothesis that HbF contributes to malaria protection, along with maternal antibody. Proteases responsible for digesting host cell hemoglobin in the food vacuole of the parasite may work less efficiently on HbF than HbA. Impaired antioxidant capacity of HbF-containing erythrocytes has also been proposed to contribute to malaria protection. More recently, abnormal PfEMP-1/knob display has been associated with HbF. In a new model of infant protection, the combined effects of HbF and maternal PfEMP-1–specific immunoglobulin G substantially diminish the ability of infected erythrocytes to bind and activate microvascular endothelium, thus impeding the development of malaria in infants.
G6PD is a cytoplasmic enzyme that is essential for an erythrocyte's capacity to withstand oxidant stress, such as that exerted by the developing malaria parasite. The G6PD gene is located on the X chromosome and is therefore present in only one copy in males. In heterozygous females carrying a mutant gene, mosaic populations of G6PD-deficient and G6PD-normal erythrocytes are produced from hematopoietic cells that have one or the other X chromosome inactivated. G6PD deficiency is the most common enzymopathy in humans, with more than 300 allelic polymorphisms identified to date. The most common polymorphism in Africa (the A − allele, 10%–50% enzyme activity) has been associated with malaria protection in children and pregnant women.
In a large case-control study performed in populations of West and East Africa, male hemizygotes and female heterozygotes carrying the A − allele were reported to be 58% and 46% protected against severe malaria, respectively. However, a more recent and larger study, which included a reanalysis of data from the earlier study, found that male hemizygotes, but not female heterozygotes, carrying the A − allele are protected against severe malaria. These findings agree with the facts that male hemizygotes carry erythrocytes uniformly deficient in G6PD, whereas female heterozygotes carry mosaic populations of G6PD-normal and G6PD-deficient erythrocytes. Enhanced phagocytosis of infected G6PD-deficient erythrocytes has been proposed to play a role in malaria protection. Although such phagocytosis is consistent with greater protection of males than females by G6PD deficiency, it does not account for the presence of similar parasite densities in G6PD-deficient males and females. Alternative mechanisms of protection may therefore operate in vivo.
A 27-base pair deletion in band 3 (the major anion transporter in erythrocytes) causes Southeast Asian ovalocytosis and leads to reduced membrane deformability. These properties may be associated with reduced parasite invasion rates of ovalocytes in vitro and reduced parasitemias in heterozygous individuals. How these findings relate mechanistically to the dramatic reduction in cerebral malaria episodes among Southeast Asian ovalocytosis heterozygotes has not yet been established.
In studies of PIEZO1 mutations and hereditary xerocytosis, cerebral malaria from Plasmodium infection was prevented in an engineered mouse model. Prevalence of the PIEZO1 allele, E756del, in about one-third of Africans may reflect an association of this allele with malaria resistance.
Blood group O antigen has been associated with protection against severe malaria and rosetting, a phenotype of infected erythrocytes that correlates with severe disease in Africa. A mechanism of reduced rosetting in type O erythrocytes, compared with type A, B, or AB erythrocytes, has been associated with this protective effect.
DARC is an erythrocyte receptor for P. vivax merozoite invasion. Erythrocytes lacking DARC are resistant to invasion, but P. vivax infections nevertheless occur among DARC-negative individuals. These facts are consistent with the low observed incidence of P. vivax malaria in regions of sub-Saharan Africa, where erythrocyte DARC negativity is highly prevalent. The mechanism for P. vivax infection of DARC-negative individuals is unknown. Reduced DARC expression on erythrocytes has been identified as a protective polymorphism against P. vivax malaria in Papua New Guinea. DARC negativity does not protect against malaria from P. falciparum.
Acquired immunity against malaria (premunition) is not a sterilizing immunity against parasitemia. Instead, it is an immunity that protects against symptomatic and severe disease without necessarily preventing the continued presence of malaria parasites in the bloodstream. It is an immunity that increases with age, cumulative number of episodes of malaria, and time spent living in an endemic area. Parasitemia in individuals with premunition is usually low, whereas nonimmune individuals may develop high parasitemias (up to 80%) after infection. As individuals gain the experience of numerous infections in their lifetime, they can be chronically infected yet have only mild symptoms or none at all; that is, they develop “disease-controlling” immunity. In highly endemic areas, children have multiple bouts of malaria each year and suffer greatly under its morbidity and mortality when they are young, generally less than 5 to 10 years old, depending on the transmission level. Pregnant women, especially primigravidae, are an important exception to this general rule of disease-controlling immunity from previous malaria episodes and thus can be highly susceptible to antigenically new CSA-binding parasites that sequester in the placenta (see “ Malaria of Pregnancy ” earlier).
Acquired immunity to malaria is believed to be short-lived without the continual stimulation of various antigenic exposures from different P. falciparum strains. Although individuals who reside outside an endemic area for more than a year or two can develop symptomatic or severe malaria, or both, after their return, one study found evidence that acquired immunity to P. falciparum malaria persists after several years of nonexposure in African immigrants living in France.
Neonates appear to be resistant to malaria during the first few months of life. This immunity may be conferred by transplacentally acquired maternal immunoglobulin G, although the presence of fetal hemoglobin within erythrocytes likely plays a role as well (see “ Hemoglobin F ” earlier).
Splenomegaly often accompanies malaria and is thought to indicate an important role of the spleen in parasite clearance. Removal of uninfected erythrocytes by the stimulated spleen, however, may contribute to anemia. In asplenic individuals, P. falciparum malaria can progress extremely rapidly to high parasitemias that include mature forms not usually found circulating in the bloodstream.
The immunity of endemic human populations to severe malaria is complex and not well understood. Although antibodies and T-cell responses develop against a number of parasite antigens during natural infection, none of them has been found to be superior to age or parasite exposure as correlates of protective immunity. Studies in which humans were infected with a single inoculum of P. falciparum in the use of malariotherapy for tertiary syphilis showed that erythrocyte infection rose and fell in successive waves of decreasing peak parasitemia ( Fig. 274.5 ). Individuals who eventually cleared their infection were protected against subsequent reinfection by the same parasite strain but not protected against reinfection with a different P. falciparum strain.
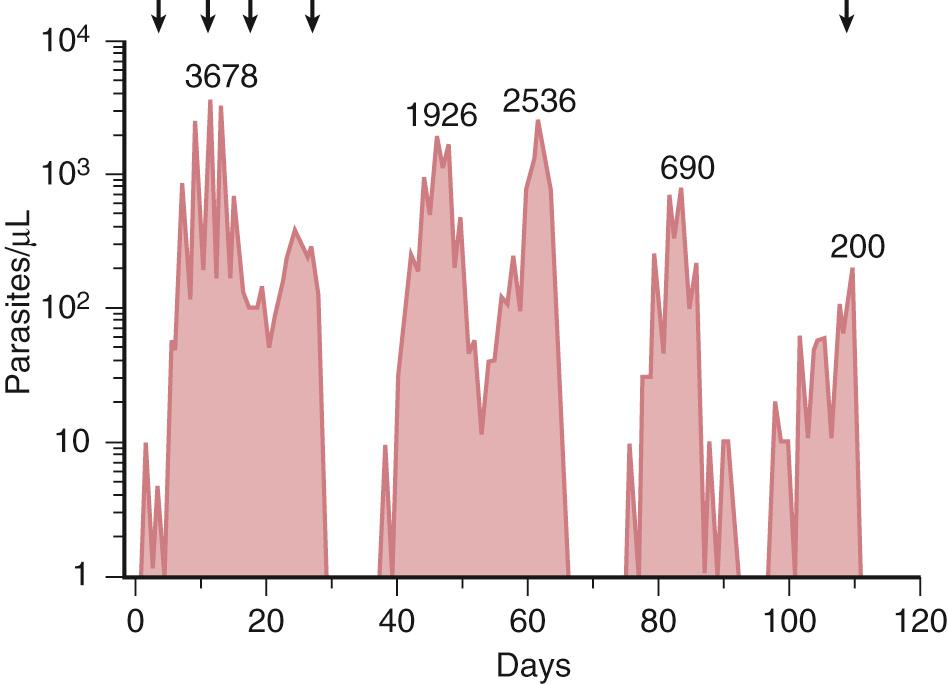
Antigen switching results in new waves of parasitemias that escape the antibody response already produced against previous waves, manifest clinically as recurrent or relapsing fevers reminiscent of those seen in Borrelia recurrentis relapsing fever or Trypanosoma brucei rhodesiense African sleeping sickness, and may not be cleared for months to years. An important component of the eventual acquisition of disease-controlling premunition after repeated episodes of malaria is the development of an antibody repertoire that can recognize a full spectrum of PfEMP-1 variant antigens. Studies in endemic areas have shown that the ability of serum to recognize diverse heterologous parasite strains increases with age and that children tend to be infected with parasites against which they have no preexisting antibody. The mechanisms by which variant-specific antibodies act in acquired immunity may include antibody-dependent cellular cytotoxicity, opsonization for uptake and destruction by splenic macrophages, and interference with PfEMP-1–mediated cytoadherence interactions.
Malaria occurs mostly in tropical regions of sub-Saharan Africa, Asia, Oceania, and Latin America ( Fig. 274.6 ), but its distribution is continually changing. The Malaria Atlas Project ( https://www.map.ox.ac.uk ) and the Centers for Disease Control and Prevention (CDC; https://www.cdc.gov/malaria/travelers ) provide up-to-date information on the geographic distribution of malaria. Information on drug-resistant malaria is also available from the publication CDC Health Information for International Travel 2018 ( https://wwwnc.cdc.gov/travel/page/yellowbook-home ).
P. falciparum and P. vivax infections account for most cases of human malaria. P. falciparum and P. malariae are found worldwide. P. vivax is infrequent in most of sub-Saharan Africa but is common elsewhere. P. ovale occurs in Africa and in foci within Asia and Oceania and is often present with other Plasmodium species as a mixed infection. Although most P. knowlesi infections of humans have been reported from Borneo and peninsular Malaysia, cases have also been reported from other areas of Southeast Asia, including the Philippines, Singapore, Thailand, and Myanmar.
Malaria is transmitted person to person by anopheline mosquitoes. Its endemicity depends upon competent mosquito vectors, a reservoir of infected humans, and conditions that bring them into proximity. The Anopheles gambiae complex of species and Anopheles funestus transmit malaria with notoriously high efficiency and are the predominant vectors in sub-Saharan Africa, where environmental conditions favor their robust reproduction and transmission of parasites to large numbers of people. Highest levels of transmission typically occur during the wet season in endemic areas.
Malaria epidemics can result from the movement of people with no immunity into an endemic area (e.g., nomadic traders, seasonal forest laborers, and military personnel), the breakdown of control measures in areas under previously successful management programs, or unusually heavy rainfalls that can place indigenous populations at risk for higher-than-normal transmission. Man-made environmental alterations (e.g., agricultural and waterworks projects, damming of rivers, and deforestation) can lead to increases in malaria transmission by creating new mosquito habitats. Malaria may be imported into areas previously free of the disease as a result of immigration of populations from endemic areas (e.g., migrating workers, persons displaced by natural disasters or civil strife, and resettlement of refugees).
In the United States, changes that include new agricultural and animal husbandry practices, improved housing with screens, water management with swamp drainage, and a radically altered landscape with urban development led to a steady decline in malaria after the mid-19th century. Final pockets of transmission were removed by the mid-20th century with the help of focused water management and insecticide spraying. Malaria diagnosed in the United States today is therefore almost always acquired in a malaria-endemic country by a returning traveler or immigrant. Because of parasite or host factors, immigrants may harbor parasites for months to years and not be recognized as possible sources of transmittable infection. In 2015, the CDC received reports of 1517 confirmed cases of imported malaria. P. falciparum, P. vivax, P. ovale, and P. malariae species were identified in 67%, 12%, 4%, and 3% of cases, respectively. Less than 1% of cases were infected by two species, and the infecting species was unreported or undetermined in 13% of cases.
Autochthonous transmission, although infrequent, typically occurs when parasitized individuals infect competent vectors ( Anopheles albimanus, Anopheles quadrimaculatus, and Anopheles freeborni ) that remain common in the United States. “Airport malaria” occurs when infected mosquitoes arrive from an endemic country on an aircraft from which they escape to bite local residents. Because mosquitoes travel short distances, infections of local residents tend to occur near airports. The spraying of insecticide within aircraft leaving endemic areas reduces the incidence of airport malaria. Malaria may also be acquired from needles shared among drug users, blood transfusion, or solid-organ transplantation. These blood and organ donors are usually asymptomatic persons from endemic areas with low-level parasitemia. The incidence of transfusion-acquired malaria is reduced when returned travelers and former residents of malaria-endemic areas are required to wait 1 and 3 years, respectively, before donating blood. Malaria may also be acquired congenitally.
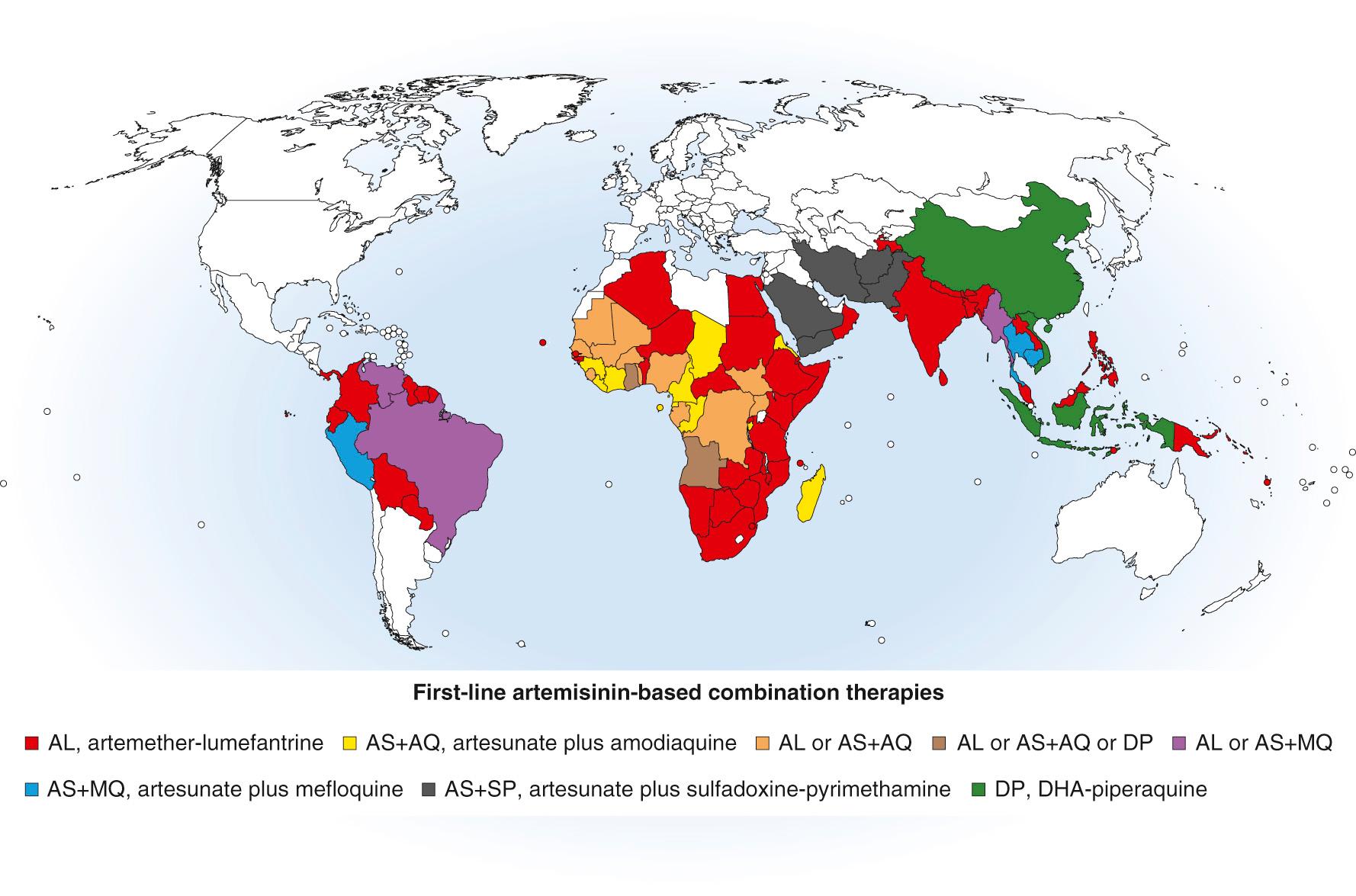
The widespread and complicated patterns of P. falciparum resistance to antimalarials has resulted in various ACT regimens becoming the first- or second-line drug regimens in most of the world's malaria-endemic countries (see Fig. 274.6 ).
Chloroquine-resistant P. falciparum malaria is widespread in sub-Saharan Africa, Asia, and Latin America. It has also been reported in areas of the Middle East, including Iran, Yemen, Oman, and Saudi Arabia, but not in Mexico, other regions of Central America west of the Panama Canal, Haiti, or the Dominican Republic. High-grade resistance of P. vivax malaria to chloroquine has been reported in Papua New Guinea and Indonesia. Case reports of P. vivax malaria not responsive to chloroquine treatment have also been reported from India, Myanmar, and Central and South America. Chloroquine-resistant P. malariae has been reported in Sumatra, Indonesia. In some regions of Africa and China where chloroquine availability has ceased, chloroquine-sensitive P. falciparum strains have gradually returned to a greater prevalence.
Amodiaquine-resistant P. falciparum has been reported in several regions of Africa and Asia. Mefloquine-resistant P. falciparum malaria now occurs along the various border regions of Thailand, Myanmar, southern China, Cambodia, Laos, and Vietnam, with scattered cases reported in the Amazon Basin and Africa.
Resistance to sulfadoxine-pyrimethamine (SP) is widespread through much of Southeast Asia, the Amazon Basin, and Africa. The prevalence of SP resistance varies among African regions, with some areas of West Africa showing relatively low rates of resistance. Malaria strains resistant to cycloguanil (the active metabolite of proguanil) have been reported since the late 1940s and can exhibit different degrees of cross-resistance to pyrimethamine. Interindividual variations of conversion to cycloguanil may also contribute to clinical success or failure of proguanil treatment.
Resistance to atovaquone-proguanil was previously reported only with its use in prophylaxis, but with increasing use for the treatment of uncomplicated malaria, frank treatment failures have been reported from several countries. Reduced susceptibility to quinine has been reported mostly in Southeast Asia but also in sub-Saharan Africa and South America.
Concerns about the emergence of resistance to artemisinin derivatives were first raised with reports of treatment failures with artesunate-mefloquine and artemether-lumefantrine in Thai and Cambodian malaria control programs. These failures may be associated with slow parasite clearance rates in response to artemisinin derivatives in vivo, which have now been carefully documented in Cambodia, Thailand, Vietnam, Myanmar, Laos, and southern China (see Fig. 274.6 ). These findings have invoked the specter of current ACT regimens becoming less effective throughout Southeast Asia, prompting WHO to launch a “Global Plan for Artemisinin Resistance Containment” in 2011 and an “Emergency Response to Artemisinin Resistance in the Greater Mekong Subregion” in 2013. These observations have also raised the possibility that rapid parasite clearance, a hallmark benefit of artemisinins in the treatment of severe malaria, may become less dependable after artemisinin dosing in this region.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here