Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The ability to accurately measure blood flow and oxygen saturations across the human late-gestation fetal circulation offered by advances in magnetic resonance imaging (MRI) has provided new insights into the impact of congenital heart disease (CHD) on the developing fetus. MRI has demonstrated redistribution of blood flow and interruption of the normal streaming of blood resulting from the obstructions to flow and abnormal connections that characterize CHD. Abnormal fetal hemodynamics have been linked to the dysmaturation of the lungs and brain that are typical of newborns with more severe forms of CHD. We have also learned how diminished cardiac output in fetuses with single-ventricle physiology is associated with reduced fetal perfusion of the placenta and impaired placental oxygen exchange, which may account for the growth restriction typical of late-gestation fetuses with these types of cardiac malformations. Recent data indicate that an impaired maternal-fetal environment may have an important impact on congenital cardiac surgical results. These observations are likely to be of relevance as we seek to understand better the risk factors for adverse outcomes of neonatal cardiac surgery and develop new therapies to alter the natural history of CHD prior to birth.
Much of what is known about fetal cardiovascular physiology has been learned through invasive experiments performed in sheep. Elaborating on methods for exteriorizing and catheterizing the fetal lamb developed by Joseph Barcroft and Geoffrey Dawes at Cambridge and Oxford universities, Abraham Rudolph and his coinvestigators at the Cardiovascular Research Institute at the University of California, San Francisco, defined our modern understanding of the distribution of blood flow and oxygen transport across the fetal circulation. Rudolph's flow measurements were achieved through the selective injection of radioactive microspheres into different venous compartments that were subsequently trapped in the microcirculation of the end organs supplied by the fetal heart. By measuring the relative activity of the different tracers in each of the various fetal organs and applying the Fick principle to measure umbilical flow, blood flow in each of the major vessels was calculated. These flow measurements were combined with blood pressure measurements and oximetry performed by conventional blood gas analysis of samples obtained using catheters placed in the fetal vessels. Based on observations regarding differences between the species, including the size of the fetal brain and hematocrit, Rudolph then predicted the distribution of blood flow and oxygen saturations across the human fetal circulation. To summarize, the fetal circulation operates in parallel, with shunts at the ductus venosus, foramen ovale, and ductus arteriosus, which allow well-oxygenated blood returning from the placenta to pass directly to the most metabolically active fetal organs, the heart and brain, while deoxygenated blood is directed back to the placenta. In the fetus, the blood with the highest oxygen saturation of approximately 85% is found in the umbilical vein and, downstream, the oxygen saturation is 10% to 15% higher in the left heart (65%) than the right (55%) (see ). In fetal sheep, this is achieved by a remarkable streaming of blood emerging from the ductus venosus and left hepatic vein toward the foramen ovale, while more deoxygenated blood passes from the infrahepatic inferior vena cava and right hepatic vein toward the tricuspid valve.
In fetal sheep, the right ventricle provides the more dominant contribution (approximately two-thirds) toward the combined ventricular output, the majority of which passes into the ductus arteriosus and descending aorta. Rudolph suggested that the right ventricle was also dominant in the human, with about 15% of the combined ventricular output passing into the pulmonary circulation, which remains vasoconstricted in lambs because of the low oxygen saturation of the blood supplied to the pulmonary arteries. Of the left ventricular output, the majority passes into the head and upper limbs, returning to the right atrium via the superior vena cava (SVC), while a small proportion passes across the aortic isthmus to join the ductus arteriosus flow in the descending aorta. Rudolph estimated that descending aortic flow accounted for approximately 40% of the combined ventricular output in the human fetus, with 25% being returned to the placenta via the umbilical veins. Foramen ovale flow was calculated to comprise 20% of the combined ventricular output, with 3% being supplied to the coronary circulation. Similar estimates of the distribution of the normal fetal circulation have subsequently been reported based on ultrasound measurements of blood flow made in human fetuses. However, the measurement of fetal vessel flow by ultrasound is challenging due to difficulties in accounting for variations in flow velocity across the vessel lumen, problems with making accurate vessel area measurements, and limitations in obtaining an adequate angle of insonation. These limitations have resulted in limited data regarding the redistribution of flow that is expected to result from CHD in the fetus. Instead, investigators have focused on other Doppler ultrasound measures, such as peak velocity or pulsatility index, to assess the impact of obstructions to flow or changes in downstream vascular resistance to help interpret fetal cardiovascular physiology in human fetuses with CHD. Similarly, changes in fetal oxygenation have been inferred from Doppler assessments of changes in placental and cerebral vascular resistance, and so such data also have major limitations.
While technical challenges arising from artifacts resulting from fetal motion and difficulties in obtaining adequate signal from small blood vessels remain, noninvasive MRI techniques for use in human pregnancies have recently been developed. The data from these studies resemble the invasive flow and oximetry measurements that Rudolph first reported in fetal sheep 50 years ago. This approach is currently only feasible in late-gestation fetuses and consists of cine phase contrast vessel flow measurements and oximetry based on magnetic resonance (MR) relaxometry.
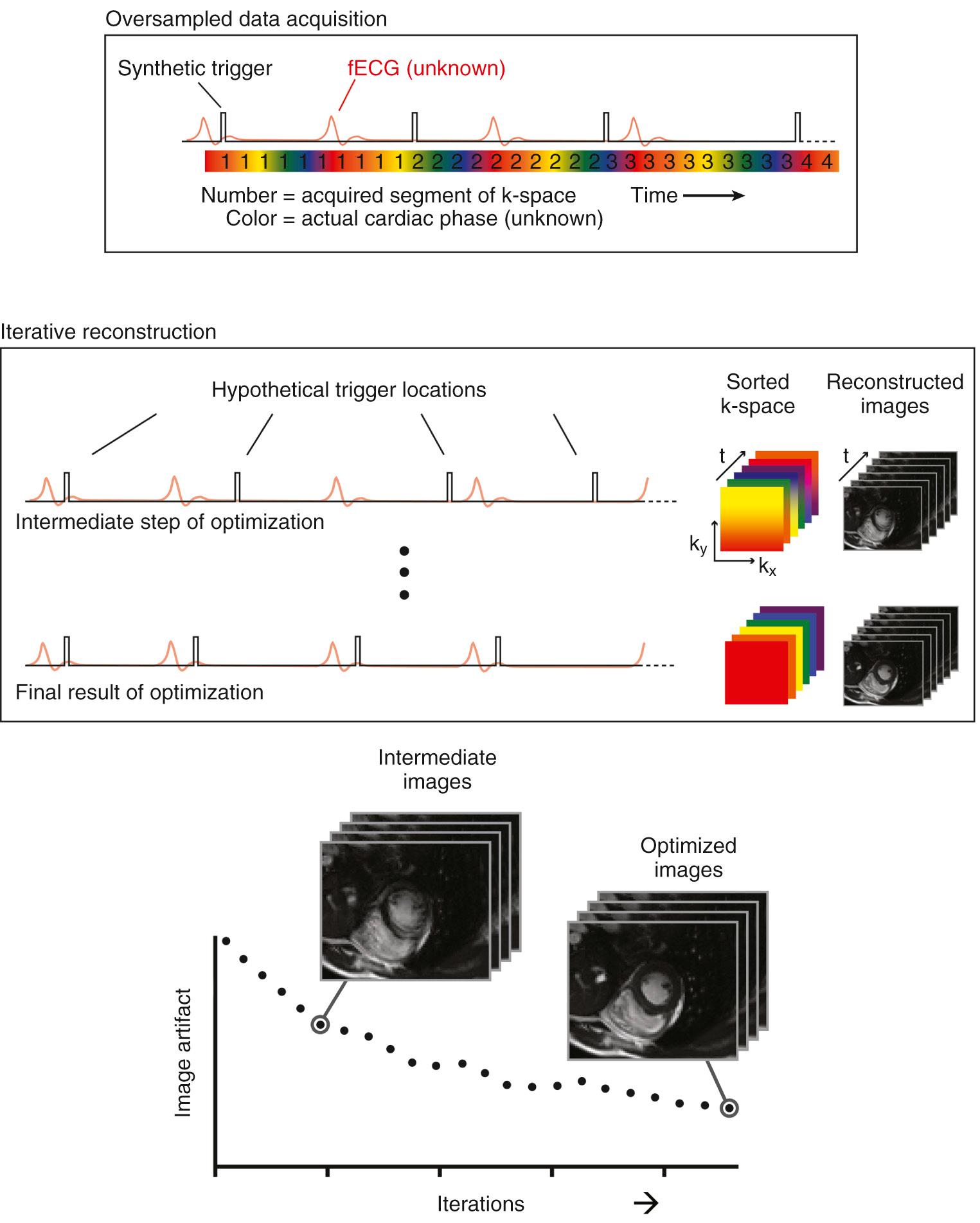
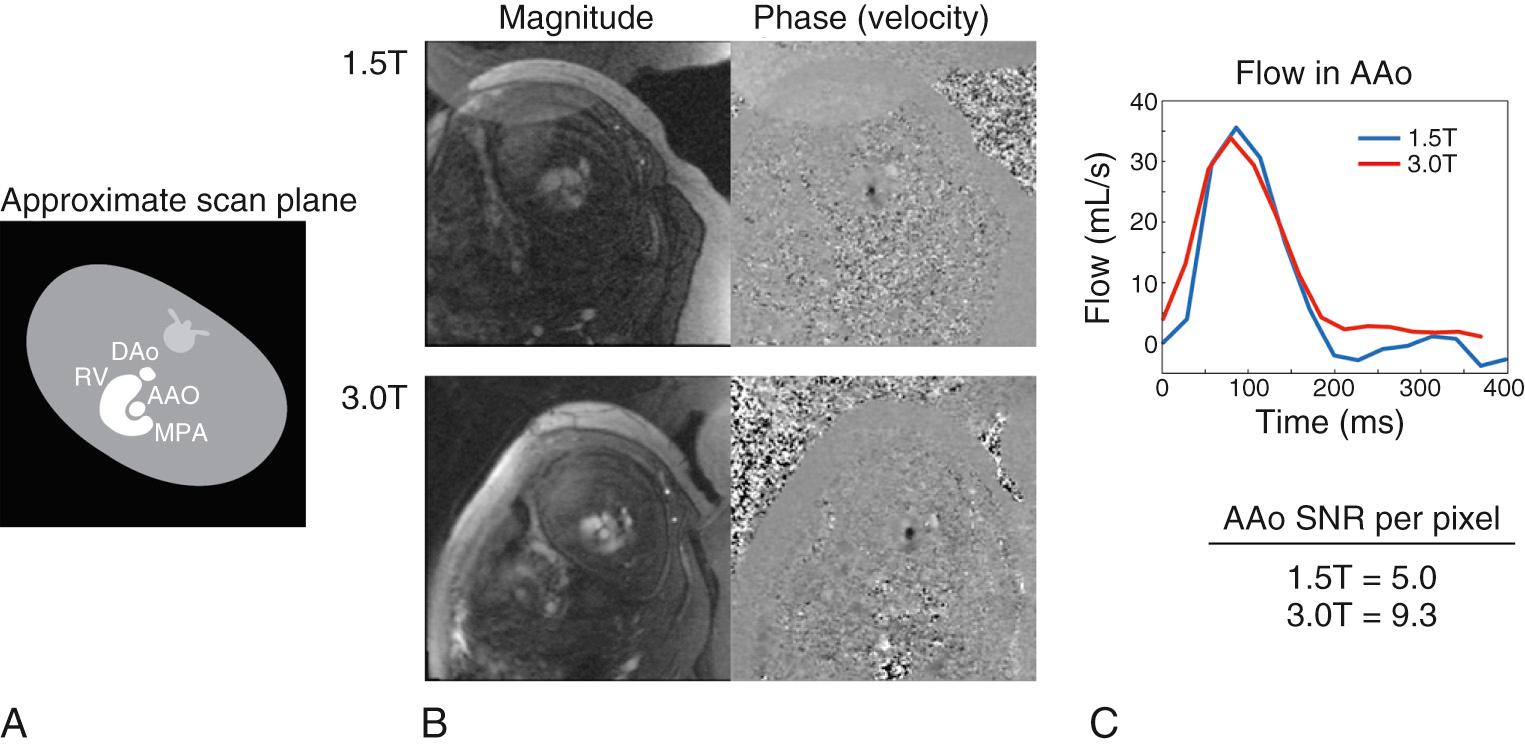
As the electrocardiographic signal usually used for temporal calibration of cardiac MRI is not easily available from the fetus, alternative methods for achieving high-resolution flow measurements have been developed. Metric optimized gating is a technique that uses a postprocessing solution to achieve cardiac triggering. To summarize, the MRI data are acquired using an artificial QRS complex, which has been programmed into the imaging acquisition, as shown in Fig. 7.1 . The data are then reconstructed through a range of candidate heart rates, with the heart rate that was present during that particular acquisition identified through the lack of artifact in the resulting images. Examples of cine phase contrast acquisitions obtained in the ascending aorta at 1.5 and 3.0 T using metric optimized gating are shown in Fig. 7.2 , which demonstrates the improvement in signal-to-noise ratio with higher magnetic field strength. The imaging plane is obtained in the short axis of the vessel, allowing for “through plane” quantification of flow. The accuracy of the flow measurement depends on achieving a true short-axis orientation, which can be achieved by obtaining two orthogonal long-axis views using anatomic survey images through the thorax and upper abdomen. Following metric optimization of the phase contrast acquisition, a region of interest is placed around the vessel using standard postprocessing software in order to quantify flow, which can be indexed to fetal volume (or weight) based on segmentation of the fetal envelope. When acquiring phase contrast flow imaging it is important to have adequate spatial resolution, which entails providing at least 8 voxels across the vessel cross-section, and adequate temporal resolution—in the range of 50 ms or better.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here