Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intraoperative lymphatic mapping and sentinel lymph node dissection (SLND) has replaced axillary lymph node dissection (ALND) as a minimally invasive and highly accurate staging procedure for early invasive breast cancer. The status of the axillary lymph nodes is an important prognostic indicator for overall survival in breast cancer. It is the presence or absence of lymph node metastases, the number of tumor-positive nodes, and the size of the primary tumor that determine the pathologic stage. The prognostic information derived from ALND has been sufficiently crucial such that clinically uninvolved lymph nodes have been removed for staging with no therapeutic benefit. The therapeutic role of ALND and selective management of the axilla for minimally invasive breast cancer are addressed in Chapter 35 .
Surgeons, radiologists, and pathologists have had a long-standing interest in the draining lymphatics of malignant disease. Tumors have unique lymphatic drainage patterns (e.g., Virchow node for gastric cancer, Delphian node for thyroid cancer). The urologist Ramon Cabanas coined the term sentinel node (SN/SLN) as a specific group of lymph nodes associated with the superficial epigastric vein for penile carcinoma. Preoperative lymphangiograms were used to direct the anatomic dissection to resect this specific lymph node center. In his series, a positive lymph node center was identified in 15 patients, and complete inguino-femoro-iliac dissection demonstrated no additional positive nodes in 80% (12 of 15). Penile carcinoma was staged with preoperative lymphangiograms to identify the specific nodal center at risk for metastases, and bilateral biopsy of the sentinel node (SN) center with inguino-femoro-iliac dissection was performed only when tumor cells were discovered in the SN center. On the basis of these results, when the sentinel nodal center was negative, no further nodal dissection was indicated.
The SN concept is founded on the principle that the afferent lymphatic channel draining a primary tumor courses to the first, “sentinel,” lymph node in that specific regional lymphatic basin ( Fig. 33.1 ). Morton and colleagues at the John Wayne Cancer Institute (JWCI) first tested the SN hypothesis in an animal model and then validated it in patients with melanoma.
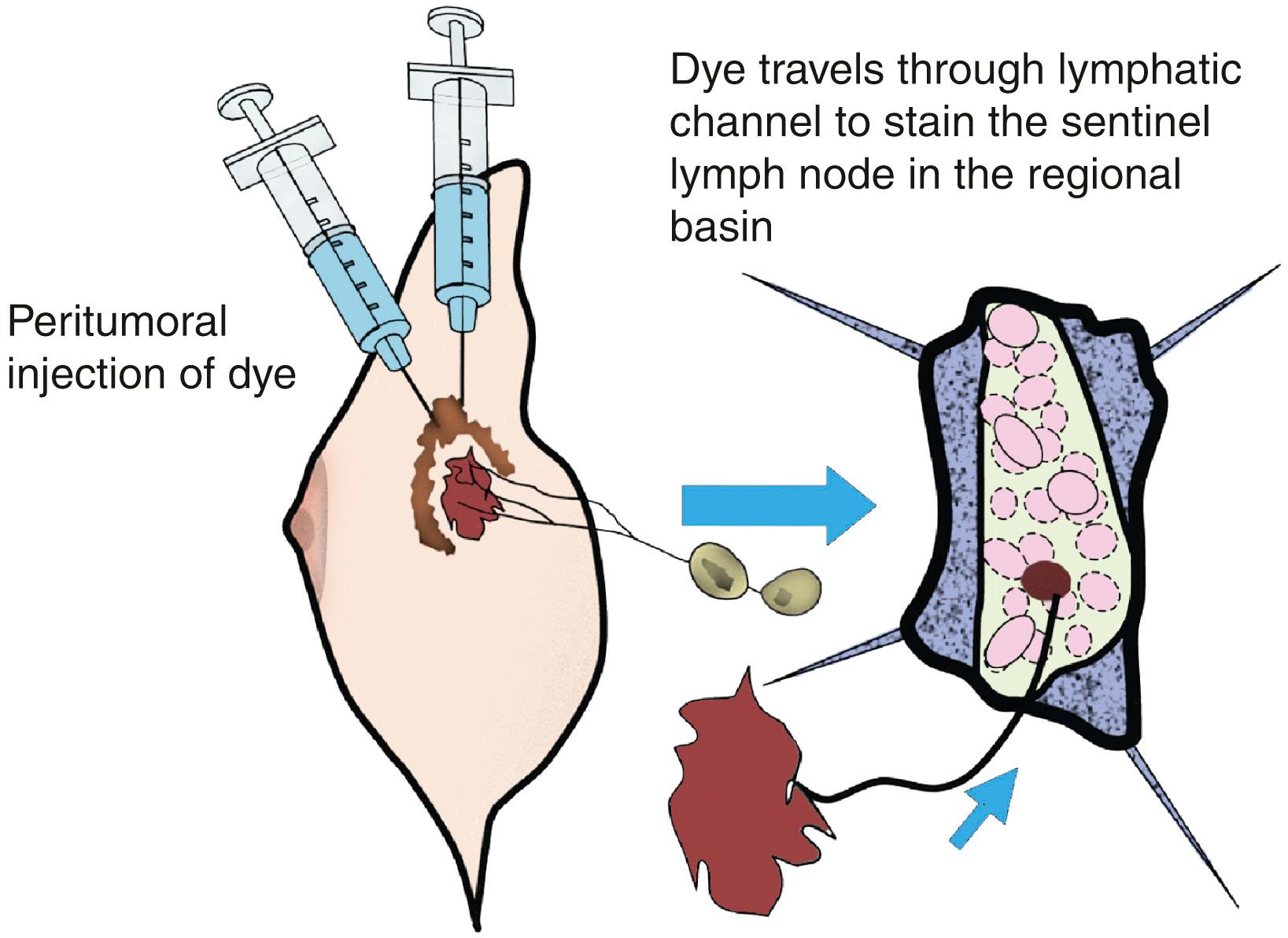
Investigators next asked if this minimally invasive technique could be applied to staging of breast cancer. Radiographic imaging to stage the axilla had been attempted to identify nodal metastases in breast cancer, but no predictable preoperative technique had emerged with sufficient sensitivity and specificity to replace formal ALND. Interestingly, Kett and Lukacs had reported that the first regional lymph node, the “Sorgius node,” could be identified in breast cancer using direct mammalymphography. Contrast material, Lipiodol Ultra-Fluide, was injected into a lymphatic collecting channel that had been visualized after intradermal injection of patent blue violet into the areola. Benign lymph nodes could be distinguished from malignant nodes by the pattern of distribution of contrast material in the node. This technique did not attain general acceptance for diagnosing malignant nodes in breast disease. It was labor intensive, time consuming, and required serial roentgenography preoperatively for at least 24 hours. In addition, an ALND had to be performed regardless of the preoperative imaging studies, to isolate the suspected lymph nodes. Postoperative roentgenograms of the excised axillary nodes were necessary to distinguish nodes that contained metastatic deposits from those that did not. Simplification of the technique with indirect lymphography was attempted but still required multiple projections of the axilla taken at regular intervals, followed by surgery 24 to 48 hours later. The correlation of pathologically positive nodes with radiographically opacified nodes was poor, and the technique was abandoned.
Armed with this historical information and the success of intraoperative lymphatic mapping for melanoma, three technical approaches for SN identification in breast cancer evolved: dye-directed lymphatic mapping, isotope-based radiolocalization, and the combination of vital dye and isotope techniques.
In October 1991 the feasibility of lymphatic mapping and sentinel lymphadenectomy in breast cancer was investigated in the hope of developing a more accurate staging procedure with lower morbidity than ALND. Several hypotheses for SLND in breast cancer were proposed and tested in a prospective manner. The discoveries made by answering each hypothesis have brought us to the current acceptance of observation of the axilla in SN-negative patients. Although these studies established a technique and showed the feasibility of SLND, new questions emerged from these initial studies that were the basis of national clinical trials and rigorous scientific research (see later discussion).
In the absence of any prior experience with intraoperative lymphatic mapping for breast cancer, a learning period was required to define the technical aspects of the procedure. Several factors were identified that determined its ultimate success. These included patient selection, injection method, dissection technique, and histopathologic evaluation of the SN.
Could an SN be identified intraoperatively in breast cancer? In 1991 there was no established protocol for intraoperative lymphatic mapping for breast cancer. The purpose of this first pilot study was to establish a method of intraoperative lymphatic mapping and SLND in breast cancer and to determine the feasibility, safety, and accuracy of the procedure for early-stage breast cancer. To accomplish this, intraoperative lymphatic mapping and SLND were followed by a completion level I, II, or, in some, III ALND in all patients with invasive breast cancer, even those with advanced tumors and grossly involved nodes who we now know are not candidates for the procedure. The technical variables that were evaluated included the quantity of isosulfan blue dye (0.5–10 mL), the appropriate site of injection (tumor vs. parenchyma vs. biopsy site), and time interval from injection to axillary incision (1–60 minutes) required for successful identification of the SN.
There were two phases to this study: the early phase (I), or the technical development period, and the second phase (II), a refinement of a technique. During phase I, the ability to identify the SN was 58.6% with an accuracy of 94.3%. As the technical variables were refined and the method evolved in phase II, the identification rate was 78% with 100% accuracy. The probability of excising a tumor-bearing SN was significantly higher with SLND (61.9%) than with random axillary node sampling (17.5%; P < 0.0001).
This study must be put into the proper context of the time in which it was reported. It was a pilot study to determine whether the SN concept could be applied to breast cancer in the absence of any established criteria or guidelines. The ability to find the SN was lower than hoped for and disheartening, but the observation that, when discovered, it truly reflected the status of the axilla was exciting. This was sufficient to persist in further investigation.
To improve accuracy, a modification of the technique was discovered by evaluating the causes for a false-negative node in the pilot study. Of the five false-negative SNs, four could have been identified by intraoperative frozen section analysis or by postoperative immunohistochemistry (IHC). The second study considered whether focused histopathologic evaluation of the SN with serial section and IHC could improve the SN identification rate and reduce the false-negative rate.
ALND alone was compared with SLND followed by completion ALND. In the SLND group, the SN was evaluated by frozen section hematoxylin and eosin (H&E) and permanent sections with H&E and IHC, whereas the non-SNs were evaluated by H&E alone. Axillary metastases were identified in 29.1% of the ALND group and 42% of the SLND group ( P < 0.03). The difference resulted from the increased sensitivity in the detection of micrometastases by H&E and IHC in the SLND group versus the ALND group (16% vs. 3%, respectively; Table 33.1 ).
| ALND (n = 134) | SLND (n = 162) | |
|---|---|---|
| Median number of excised axillary nodes/basin (range) | 19 (8–40) | 21 (6–46) |
| Median number of positive axillary nodes/basin (range) | 1 (1–27) | 2 (1–33) |
| Number of patients with axillary metastasis | 39 (29.1%) | 68 (42%) a |
| ≤2 mm by H&E | 4 (3%) | 15 (9.2%) b |
| ≤2 mm by H&E or IHC | 4 (3%) | 26 (16%) c |
| ≤2 mm by IHC | 0 | 11 (6.8%) |
This study demonstrated that frozen section diagnosis, multiple levels of SN analysis, and IHC increased the accuracy of axillary staging. Micrometastases were easier to identify in the SN with multiple levels and IHC, although the clinical significance of IHC-detected micrometastases has not been shown to affect survival. This topic is discussed further later in this chapter.
The two initial studies established the methodology, feasibility, safety, and histopathologic processing of the SN. ALND still remained the established method of staging for the treatment of breast cancer in 1994. For an alternative approach to be considered, the new technique required prospective and systematic validation. From July 1994 to October 1995, 107 patients with potentially curable breast cancer underwent SLND with the mature method. The SN was identified in 93.5%, and 66.7% of those with a tumor-positive SN had no other tumor-bearing axillary lymph nodes ( Table 33.2 ). The status of the axilla relative to the tumor size is shown in Table 33.3 .
| Variable | No. of Patients |
|---|---|
| SLND procedures | 107 |
| Detecting sentinel node | 100 (93.5%) |
| Tumor-involved axillae | 42 |
| Sentinel node positive | 42 |
| With macrometastases | 23 |
| With micrometastases | 19 |
| Detected by H&E | 10 |
| Detected by IHC | 9 |
| Primary Tumor Size | No. of Patients | Sentinel Node Positive | Sentinel Node Negative | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| T1 | 64 | 22 | 34.4 | 42 | 65.6 |
| T1a | 4 | 1 | 25 | 3 | 75 |
| T1b | 18 | 8 | 44.4 | 10 | 55.6 |
| T1c | 42 | 13 | 31 | 29 | 69 |
| T2 | 34 | 18 | 52.9 | 16 | 47.1 |
| T3 | 2 | 2 | 100 | 0 | 0 |
| All | 100 | 42 | 42 | 58 | 58 |
There were no false negatives, with a specificity and sensitivity of 100%. Axillary staging with SLND was validated for this defined patient population using strict technical guidelines for identification and evaluation of the SN. Since then, a number of prospective clinical trials have confirmed the feasibility of intraoperative lymphatic mapping, its accuracy, and its oncologic safety in clinically node-negative breast cancer.
The SN hypothesis was not immediately accepted but challenged as an artifact of increased SN histopathologic processing and not a reflection of the status of the axilla. This challenge led to the initiation of the pivotal study of lymphatic mapping in breast cancer and proof of principle that the SN is the first regional node draining the primary tumor.
Complete histopathologic evaluation of SNs and non-SNs was performed with serial sectioning, H&E, and IHC in all cases of H&E-negative SNs. H&E staining identified a tumor-bearing SN in 32% of patients, and IHC upstaged an additional 14.3% ( Table 33.4 ). In 60 patients whose SNs were negative by H&E and IHC, 1087 non-SNs were examined at two levels by IHC. Only one additional tumor-positive node was identified. The SN was sufficient for staging and therapy in 57.3% of patients because they had no additional nodal disease ( Table 33.5 ).
| H&E | IHC | IHC Conversion Rate (%) | P Value | |
|---|---|---|---|---|
| Lymph node evaluation | ||||
| Tumor in sentinel lymph node | 0/157 | 10/157 | 6.4 | <0.0001 |
| Tumor in nonsentinel lymph node | 0/1087 | 1/1087 | 0.1 | |
| Patient evaluation | ||||
| Patient with sentinel node metastases | 0/70 | 10/70 | 14.3 | <0.0001 |
| Patient with sentinel lymph node metastases only | 0/60 | 1/60 | 1.7 |
| Tumor Status | No. of Patients (%) |
|---|---|
| Lymph node positive | 59 (57.3) |
| Lymph node negative | 44 (42.7) |
| Sentinel node positive only | 25 (24.3) |
| Sentinel node and nonsentinel node positive | 18 (17.5) |
| Nonsentinel node positive only | 1 (1) |
| Total | 103 (100) |
This study provided evidence that the SN is the first draining lymph node from the primary tumor and is not an artifact of histopathologic evaluation. The SN hypothesis was validated and has been confirmed by independent investigators.
Standardization of the SLND technique, validation with completion ALND, and complete histopathologic assessment of the SN and non-SNs confirmed the accuracy and prognostic value of SLND. The natural segue in the incorporation of SLND was the study to determine whether ALND could be avoided when the SN was negative. The purpose of the next study was to determine the complication rate and the local recurrence rate in women who had a tumor-free SN who did not undergo ALND. This was the first study to eliminate ALND in SN-negative patients.
Women with tumors of 4 cm or less underwent SLND for staging as the only axillary treatment if the SN contained no tumor cells. Completion ALND was performed when the SN contained metastatic cells. Patients did not receive axillary irradiation. SLND identification rate was 99% ( Table 33.6 ). Complications occurred in 20 patients (35%) undergoing ALND after SLND, but in only two patients (3%) undergoing SLND alone ( P = 0.001). The complications in both groups were minimal, primarily wound seromas. There were no locoregional recurrences at 39 months. Subsequent studies support these findings. The axillary recurrence rates are under 1% in the majority of large studies.
| No. of Patients/Total Patients | Percent of Patients | |
|---|---|---|
| Sentinel node identified | 124/125 | 99.2 |
| Sentinel node negative | 67/124 | 54 |
| Sentinel node positive | 57/124 | 46 |
| >2 mm | 26/57 | 45.6 |
| ≤2 mm | 31/57 | 54.4 |
| H&E identified | 9/31 | 29 |
| IHC identified | 22/31 | 71 |
SLND can eliminate the need for ALND in patients who have negative SNs, because removal of negative axillary lymph nodes does not alter disease outcome. The role of SLND alone for SN-negative breast cancer patients has been investigated in large national and international trials (see later discussion).
During the 1970s and 1980s, radiocolloid lymphoscintigraphy was shown to be a versatile and simpler technique than mammalymphography, but the prognostic value of axillary lymphoscintigraphy alone was insufficient to avoid axillary dissection for staging. In the early 1990s, experimentation with radiolocalization of the SN on the skin surface that could direct surgical removal with a gamma detection probe was initiated in an animal model. Gamma probe–guided SN identification had some theoretical advantages: the location of the SN could be identified, surgery could be confined to a small area in a nodal basin, the SN could be distinguished from non-SNs by quantitative counts, and extraaxillary SNs could be detected.
In 1993 Krag and colleagues reported a pilot study in 22 patients that established intraoperative radiolocalization of an SN in breast cancer. Unfiltered technetium-99m–labeled ( 99m Tc) human sulfur colloid (0.5 mCi suspended in 0.5 mL saline) was injected in a 180-degree arc oriented toward the axilla, successfully identified a radioactive node in 82%, and reflected the status of the axilla in all these cases.
In 1997 Veronesi and colleagues reported a large series of patients who had subdermal injection of 99m Tc-human colloidal albumin, preoperative lymphoscintigraphy, and intraoperative gamma probe detection of the SN. The SN was identified in 98% of cases and accurately predicted the status of the axilla in 97.5%. In the large majority of early-stage breast cancer patients, the combination of preoperative lymphoscintigraphy with intraoperative radiolocalization could locate an SN in the axilla with a high degree of accuracy.
The group from the Moffitt Cancer Center adapted the discoveries made from blue dye lymphatic mapping and isotope radiolocalization and reported on the combined technique in 62 women. The authors predicted that the ability to identify the SN by combined technique would be greater. In fact, in their hands, the SN was localized successfully in 92% of patients. A hot spot could be identified on the skin of the axilla and therefore limit the axillary exploration, minimizing tissue disruption. Radiocolloid identified more SNs, but in no case did a “hot” nonblue node contain micrometastatic disease. No skip metastases were identified in this small series.
Following these lead studies of blue dye alone, radioisotope alone with or without preoperative lymphoscintigraphy, or combined dye and nuclide, investigators from many large academic centers and smaller community hospitals in the United States and throughout the world rapidly experimented with variations on the techniques. These studies demonstrate a high accuracy rate and a low false-negative rate ( Table 33.7 ). The value of the technique is inherent to the successful identification of the correct SN that reflects the status of the axilla.
| Author | Year | No. of Patients | Success (%) | Sensitivity (%) | NPV (%) | False Negative (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| Vital Dye Technique | |||||||
| Guiliano | 1994 | 174 | 66 | 88 | 94 | 4.3 | 96 |
| Guiliano | 1997 | 107 | 94 | 100 | 100 | 0 | 100 |
| Guenther | 1997 | 145 | 71 | 90 | 96 | 9.6 | 97 |
| Dale | 1998 | 21 | 66 | 100 | 100 | 0 | 100 |
| Koller | 1998 | 98 | 98 | 96 | 94 | 5.8 | 97 |
| Flett | 1998 | 68 | 82 | 83 | 93 | 17 | 95 |
| Morgan | 1999 | 44 | 73 | 83 | 91 | 16.7 | 94 |
| Imoto | 1999 | 88 | 74 | 86 | 89 | 13.7 | 94 |
| Kern | 1999 | 40 | 98 | 100 | 100 | 0 | 100 |
| Morrow | 1999 | 50 a | 88 | 95 | — | — | 96 |
| Ilum | 2000 | 161 | 60 | 86 | 87 | 14.3 | 93 |
| Motomura | 2001 | 93 b | 84 | 81 | 93 | 19 | 95 |
| Radioisotope Technique | |||||||
| Krag | 1993 | 22 | 82 | 100 | 100 | 0 | 100 |
| Veronesi | 1997 | 163 | 98 | 95 | 95 | 4.7 | 98 |
| Pijpers | 1997 | 37 | 81 | 100 | 100 | 0 | 100 |
| Krag | 1998 | 157 | 93 | 95 | 98 | 4.9 | 98 |
| Krag | 1998 | 443 | 93 | 89 | 96 | 11.4 | 97 |
| Crossin | 1998 | 50 | 84 | 88 | 98 | 12.5 | 98 |
| Borgstein | 1998 | 130 | 94 | 98 | 98 | 2.2 | 98 |
| Offodile | 1998 | 41 | 98 | 100 | 100 | 0 | 100 |
| Snider | 1998 | 80 | 88 | 93 | 98 | 7 | 99 |
| Rubio | 1998 | 55 | 96 | 88 | 95 | 11.8 | 96 |
| Veronesi | 1999 | 376 c | 99 | 93 | 94 | 6.7 | 97 |
| Miner | 1999 | 57 | 97 | 92 | 98 | 7.7 | 98 |
| Feldman | 1999 | 75 | 93 | 81 | 92 | 19 | 94 |
| Moffat | 1999 | 70 | 89 | 90 | 96 | 10 | 97 |
| Zurrida | 2000 | 376 | 99 | 93 | 94 | 6.7 | 97 |
| Fraile | 2000 | 132 | 96 | 96 | 97 | 4 | 98 |
| Rink | 2000 | 123 | 94 | 92 | 96 | 7.7 | 97 |
| Mariani | 2000 | 197 d | 97 | 86 | 92 | 13.7 | 95 |
| Combined Technique | |||||||
| Albertini | 1996 | 62 | 92 | 100 | 100 | 0 | 100 |
| Barnwell | 1998 | 42 | 90 | 100 | 100 | 0 | 100 |
| O'Hea | 1998 | 59 | 93 | 85 | 92 | 15 | 95 |
| Kollias | 1999 | 117 | 81 | 94 | 97 | 6.5 | 98 |
| Morrow | 1999 | 42 e | 86 | 97 | — | — | 96 |
| van der Ent | 1999 | 70 | 100 | 96 | 98 | 3.7 | 99 |
| Hill | 1999 | 104 f | 93 | 89 | 91 | 10.6 | 95 |
| Mariani | 2000 | 197 d | 97 | 86 | 92 | 13.7 | 95 |
| Doting | 2000 | 136 | 93 | 95 | 96 | 5.1 | 98 |
| Imoto | 2000 | 59 | 93 | 92 | 94 | 8 | 96 |
| McMasters | 2000 | 562 | 90 | 94 | 98 | 5.8 | 98 |
| Smillie | 2001 | 106 | 84 | 95 | 96 | 6 | 98 |
| Motomura | 2001 | 138 g | 95 | 100 | 100 | 0 | 100 |
| Tafra | 2001 | 529 | 87 | 87 | 95 | 13 | 96 |
b Indigocyanine green arm only.
c Includes subgroup of 54 patients who had patent blue dye and isotope.
d Analysis of 197 of 284 cases with axillary lymph node dissection.
e Blue dye and radioisotope arm.
f Subgroup of 104 of 500 cases with axillary lymph node dissection.
Morton and colleagues first defined the SN as the first lymph node that receives afferent lymphatic drainage from the primary tumor. As the techniques have varied to include radioisotope, dye, or a combination, the definition of the SN has become more complex. Operational definitions of the SN have evolved from technology. These include the first blue node, the hottest node, the suspicious palpable node, and the node to which a blue lymphatic tracks. Problems are encountered when the SN is defined by technique. Radioactive lymph nodes are detected by lymphoscintigraphy and gamma detection probes. Lymphoscintigrams can visualize primary and secondary echelon nodes, as well as nonaxillary sites. The brightest nodes, however, may be bright because of the distance from the gamma counter, not because they are the first to receive metastases. Likewise, the gamma detection probe finds the hottest node that may be the node to which open lymphatic channels have been diverted rather than the less radioactive SN, because tumor has occluded the lymphatics and obliterated the nodal tissue. The physiologic principle described by Morton, however, has not changed and governs the SN concept. The definition of the SN in breast carcinoma is the first node or nodes to which lymph drainage and metastasis occurs from the primary tumor. The SN is usually an axillary node, but it can in rare cases be found in nonaxillary locations. The breast is a parenchymal organ that has the potential to drain to multiple nodal basins. Do breast parenchymal, subcutaneous, dermal, and/or periareolar lymphatics flow to the same axillary nodes that govern lymphatic drainage? When blue dye was injected intraparenchymally in the same quadrant as the primary tumor or in a discordant quadrant from the tumor and radioisotope was injected intradermally over the tumor, the SN was blue and hot in 93.9% of cases with concordant quadrant injections and 92.5% in discordant quadrant injections. In another study, peritumoral blue dye and subareolar isotope resulted in 97% blue nodes that were 98% concordant for blue-hot nodes. These studies confirm the SN concept and suggest that there may be a primary nodal drainage pattern from the breast and that this most likely can be identified by a variety of techniques.
In 1995 the first multicenter trial was initiated to evaluate the ability of surgeons with varied experience in SLND to identify and to accurately stage the axilla using the radioisotope technique. Eleven surgeons entered 443 patients into the study. The SN was identified using a gamma detection probe. A completion level I and II ALND was performed in all cases. A level III dissection was performed for suspicious nodes. Failure to identify a hot spot before excision of the SN was associated with a previous excisional biopsy, age 50 years or older, and medial tumor location. The SN was identified in 88.6% of cases. The accuracy was 96.8%, the negative predictive value was 95.7%, and the sensitivity was 89%. The false-negative rate ranged from 0% to 28.6%. All 13 false-negative SNs occurred with medial hemisphere tumors. The SN was located in a region outside the axilla in 8% of cases. Variations in success were attributed to both surgeon experience and patient characteristics.
This study was followed by the 42-center Department of Defense Multicenter Trial of Breast Lymphatic Mapping, which required surgeons to participate in a 2-day course using an intraparenchymal injection of blue dye and radioisotope. Patients were randomized to SLND alone if the SN was negative or SLND followed by ALND. Preoperative lymphoscintigraphy was performed in 84% of cases and demonstrated at least one SN in 66%. The nodal drainage pattern was to the axilla in 99.9%, axilla only in 78%, internal mammary in 14%, internal mammary only in 2%, and other sites in 5%. The success rate for identifying the SN was 92% at the Moffitt Cancer Center, 91.4% at other university centers, and 85.2% in community/regional centers. The SN was positive in 32% of patients, and the only site of metastases in 63%. Skip metastases were identified for a false-negative rate of 4%. There were no axillary recurrences in the SLND-alone group at 16 months. This multicenter trial demonstrated successful mapping, a low skip metastasis rate, and a reliable performance rate for both university and nonuniversity institutions, thus confirming not only the accuracy of SLND but its widespread feasibility.
In another multicenter trial, surgeons from academic institutions and the private sector attended a formal lymphatic mapping course with hands-on experience, in a training laboratory facility first. The surgeons achieved a 90% identification rate and a 4.3% false-negative rate when they had performed more than 30 cases. Multivariate analysis demonstrated the poorest success rate in older patients (≥50 years) and by inexperienced surgeons (≤10 cases). Identification was not affected by type of prior surgery (fine-needle aspiration, core biopsy, excision), filtered or unfiltered 99m Tc, time from injection to surgery, tumor size, or tumor location. The false-negative rate was worse for central lesions ( P < 0.02).
In one of the largest multicenter studies evaluating the optimal SLND technique, 99 surgeons enrolled in the University of Louisville Breast Cancer Sentinel Lymph Node Study and performed SLND in 806 patients. The surgeons were provided flexibility in choosing the technique. Each case was evaluated for patient and tumor characteristics, identification rate, false-negative rate, and technique used. There was no difference in SN identification for dye alone or dye plus isotope (86% vs. 90%, respectively). The false-negative rate was significantly lower with the dual agents (11.8% vs. 5.8%, P < 0.05). The isotope-dye combination resulted in more SNs removed (2.1 vs. 1.5, P < 0.0001). In this large study, the optimal technique with the lowest false-negative rate was dual-agent SLND. With this technique the false-negative rate is low enough to be considered a suitable alternative to ALND in routine surgical practice.
The prospective Swedish Multicenter Cohort Study in 3354 patients from 26 hospitals treated by 131 surgeons demonstrated a negative SN in 2246 cases, and no further ALND was performed. Peritumoral, subcutaneous, or intracutaneous injection of radioisotope was followed with lymphoscintigraphy. SNs were identified intraoperatively with a handheld gamma detection probe. At 37 months, nodal failure was 1.2%. The overall survival was 91.6%, and the disease-free survival (DFS) was 92.1%.
Guidelines for successful SN identification require appropriate patient selection, defined tumor characteristics, and technical validation. A variety of factors may interfere with the accuracy of this procedure and decrease the chance of correctly identifying the SN ( Table 33.8 ). The technique can be used effectively in all age groups, in both males and females, with breast conservation surgery (BCS) or mastectomy, at the time of primary excision or reexcision, in bilateral breast cancer, after long-interval reduction mammoplasty, and with breast implants. American Society of Clinical Oncology (ASCO) guidelines (2014 and ASCO 2016 update, Lyman, 2017) and American Society of Breast Surgeons 2019 consensus guidelines are shown in Table 33.9 .
| Factor | Fraction Failed SN | Percent Failed SN | Chi-Square | P Value |
|---|---|---|---|---|
| Body Mass Index (kg/m 2 ) | 22.2687 | 0.0001 | ||
| <18.5 | 0/74 | 0 | ||
| 18.5–24.9 | 9/1881 | 0.5 | ||
| 25–29.9 | 25/1562 | 1.6 | ||
| 30–49.9 | 29/1406 | 2.1 | ||
| ≥50 | 2/41 | 4.9 | ||
| Age (year) | 20.5185 | 0.0004 | ||
| ≤39 | 1/330 | 0.3 | ||
| 40–49 | 8/1171 | 0.7 | ||
| 50–59 | 18/1668 | 1.1 | ||
| 60–69 | 21/1253 | 1.7 | ||
| ≥70 | 23/845 | 2.7 | ||
| Pathologic Tumor Stage | 1.6780 | 0.4322 | ||
| T 1 | 58/3947 | 1.5 | ||
| T 2 | 12/1063 | 1.1 | ||
| T 3 | 0/68 | 0 | ||
| Final Nodal Status | 2.6198 | 0.1055 | ||
| Negative | 59/3981 | 1.5 | ||
| Positive | 11/1251 | 0.9 | ||
| Biopsy Type | 0.3915 | 0.5315 | ||
| Excisional | 21/1606 | 1.3 | ||
| Nonexcisional | 45/2921 | 1.5 | ||
| Histology | 1.3555 | 0.5078 | ||
| Ductal | 60/4324 | 1.4 | ||
| Lobular | 7/435 | 1.6 | ||
| Other | 4/495 | 0.8 | ||
| Tumor Location | 3.8406 | 0.1466 | ||
| Medial | 21/1069 | 2 | ||
| Central | 15/1167 | 1.3 | ||
| Lateral | 35/3008 | 1.2 | ||
| Final Number of Positive Nodes | 4.5380 | 0.4748 | ||
| 0 | 56/4060 | 1.4 | ||
| 1 | 6/325 | 1.8 | ||
| 2 | 0/132 | 0 | ||
| 3 | 1/86 | 1.2 | ||
| 4 | 0/38 | 0 | ||
| ≥5 | 4/154 | 2.6 | ||
| Clinical Circumstance | Recommendation for Use of Sentinel Node Biopsy | Level of Evidence a |
|---|---|---|
| T1 or T2 tumors | Acceptable | Good |
| T3 or T4 tumors | Not recommended | Insufficient |
| Multicentric tumors | Acceptable | Intermediate |
| Inflammatory breast cancer | Not recommended | Insufficient |
| DCIS with mastectomy | Acceptable | Limited; informal consensus |
| DCIS without mastectomy | Not recommended except for large DCIS (>5 cm) on core biopsy or with suspected or proven microinvasion | Insufficient |
| Suspicious, palpable axillary nodes | Not recommended | Good |
| Older age | Acceptable | Intermediate |
| Obesity | Acceptable | Intermediate |
| Male breast cancer | Acceptable | Limited |
| Pregnancy | Not recommended | Insufficient |
| Evaluation of internal mammary lymph nodes | Acceptable | Limited |
| Prior diagnostic or excisional breast biopsy | Acceptable | Intermediate |
| Prior axillary surgery | Acceptable | Intermediate |
| Prior nononcologic breast surgery (reduction or augmentation mammoplasty, breast reconstruction) | Acceptable | Intermediate |
| After preoperative systemic therapy | Acceptable | Intermediate |
| Before preoperative systemic therapy | Acceptable | Intermediate |
Good: multiple studies of sentinel lymph node dissection (SLND) test performance based on findings on completion of axillary lymph node dissection (ALND).
Intermediate: moderate confidence that the available evidence reflects the true magnitude and direction of the net effect. Further research is unlikely to alter the direction of the net effect; however, it might alter the magnitude of the net effect.
Limited: few studies of SLND test performance based on findings on completion of ALND or multiple studies of mapping success without test performance assessed.
Insufficient: on studies of SLND test performance based on findings on completion of ALND and few if any studies of mapping success.
Informal consensus: the available evidence was deemed insufficient to inform a recommendation to guide clinical practice. The recommendation is considered the best current guidance for practice, based on informal consensus of the expert panel.
Sentinel lymphadenectomy has been used to stage the axilla in individuals spanning all decades. The identification of the SN, however, has been less successful in older patients. The failed identification rate is 0.3% for women 39 years of age or younger and 2.7% for those 70 years of age or older. The results from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial show a statistically significant, but not clinically relevant, difference in identification rates by 49 years of age or less versus 50 years of age or more, whereas a later study shows similar identification rates. A combination of vital dye and isotope improves identification in older patients.
SLND is an option for axillary staging of the older patient. The role of ALND for elderly patients has been questioned because it has significant morbidity. SLND provides prognostic information about axillary status with little or no morbidity and may change local and/or systemic treatment. In some studies, SN status was associated with significantly different rates of systemic therapy in women 70 years of age or older. Chagpar and colleagues evaluated 157,584 patients with clinically node-negative, hormone receptor–positive (HR+) breast cancer identified in the National Cancer Database (NCDB) over the age of 70. The authors found that 80% of patients had surgical axillary staging and 15% had positive nodes. Multivariable analysis identified SN status as an independent predictor of receipt of chemotherapy, hormonal therapy, and radiation therapy (RT). However, the NCDB does not capture information on patient comorbidities and other factors that may be important for patient selection.
In more recent years, genomic tumor assays combined with clinical data and patient life expectancy have become the main factors in determining adjuvant therapy recommendations in this age group. As a result, the decision regarding whether to perform a sentinel node biopsy (SNB) in an older patient with a low-risk breast cancer has become an area of controversy. There have been two randomized trials demonstrating no benefit for axillary surgical staging in patients over age 70. The Martelli study randomized 238 patients between ages 65 and 80 to ALND versus no ALND and demonstrated no difference in survival or axillary recurrence rates between the two arms. In the CALGB 9343 trial, 647 women with clinical T1N0, HR+ breast cancer over age 70 were randomized to radiation plus tamoxifen versus tamoxifen alone. Patients were encouraged to omit ALND, and 37% of patients underwent ALND. There was no difference in overall survival between the two arms and the rate of axillary recurrence was minimal with zero axillary recurrences in the radiation arm and only six axillary recurrences in the arm that had no radiation. National guidelines have been published with recommendations regarding SNB in this age group. The Society of Surgical Oncology Choosing Wisely Campaign and National Comprehensive Cancer Network (NCCN) guidelines state the SNB is not necessary in this patient population. As in any patient population, it is important to balance the risks and benefits of all aspects of breast cancer treatment, but achieving this balance is more difficult in women over 70 as life expectancy and patient comorbidity status present competing challenges.
Although male breast cancer accounts for less than 1% of breast cancer cases, the use of SLND has been studied in both male and female patients. Conventional surgical management of male breast cancer has been modified radical mastectomy, but more men are diagnosed with node-negative disease and can avoid ALND. Combined data from a number of series show a 96% identification rate, with 45% of patients having a positive node, and in 56% of those cases the SN is the only positive node. SLND is a less invasive staging procedure that is applicable to men with breast cancer who have a clinically negative axilla.
Failure to identify the SN is correlated with an increased mean body mass index (BMI). There is a progressive increase in failure rate as BMI increases above 26. The JWCI experience and Memorial Sloan-Kettering Cancer Center (MSKCC) experience find SN identification to be more challenging in the obese patient, and the combined vital dye and radioisotope technique may be considered for these patients.
The safety of SLND with radioisotope in pregnancy has been studied by Pandit-Taskar and colleagues at the MSKCC. Retrospective data from nonpregnant women with breast cancer and SN biopsy were used in a phantom model calculation of the radiation-absorbed dose of 99m Tc-sulfur colloid after a single intradermal dose of 0.1 mCi on the morning of surgery or 0.5 mCi on the afternoon before surgery. The highest estimated dose received by the fetus with the 2-day protocol, was measured at 0.014 mGy, which is less than the National Council on Radiation Protection and Measurements limit to the pregnant woman. Three other theoretical studies reached similar conclusions. Although radiolabeled technetium is probably safe in pregnancy, clinicians are reluctant to use it, and the ASCO guidelines do not recommend it. Use of isosulfan blue dye is contraindicated because there are no data in human pregnancy on whether fetal harm can result from intrauterine dye exposure, and it is classified by the US Food and Drug Administration as a category C drug in pregnancy. One small series of 25 pregnant patients reported use of methylene blue in 7 patients without any complications.
Previous breast reduction surgery, surgical implants, extensive injuries, burns, previous reconstructive surgery to the breast or axilla, surgery for hidradenitis, or congenital lymphatic problems may have an effect on the feasibility of the procedure with insufficient evidence to recommend it.
Application of the technique to recurrent disease, especially after SLND, has been shown to be feasible, although some question its value. Success of reoperative axillary surgery has been shown to be inversely related to the number of nodes removed initially and has been due in part to lymphoscintigraphy, which may identify sites of nonaxillary drainage. Preoperative lymphoscintigraphy shows variable drainage pathways and slower migration of radiocolloid but can be performed successfully in patients with ipsilateral breast tumor recurrence. Sato and colleagues found that patients with prior RT had more aberrant drainage patterns than those who did not have RT. Ugras and colleagues found that rates of axillary failure, nonaxillary failure, distant recurrence, and death did not differ between clinically node-negative patients with local recurrence who had axillary restaging (n = 47; 57%) versus those who did not (n = 36; 43%). The use of adjuvant therapy was similar between groups and the authors suggest that because randomized trials support the use of systemic therapy for all patients with invasive local recurrence, axillary staging may not be necessary in clinically node-negative patients.
The merit of repeat SN mapping after postmastectomy recurrences or ipsilateral breast tumor recurrences (IBTRs) is debated. The controversy rests on whether this procedure unmasks unsuspected disease, aids in maintaining locoregional control if positive nodes are discovered, or influences the choice of systemic therapy. Moreover, the proponents of repeat sentinel lymph node biopsy (SLNB) argue that decisions regarding the use of regional irradiation may be directed by these findings. Maaskant-Braat and colleagues conducted a meta-analysis of published studies reporting repeat lymphatic mapping and SLNB among 692 patients with a locoregional recurrence. SNs were successfully identified in 65.5% and 68.9% of patients who underwent lumpectomy and mastectomy, respectively. Aberrant drainage patterns, namely to the contralateral axilla, have been found in 17.4% of cases with a prior SLNB compared with 69.2% after ALND. Overall, the success rate of repeat SLNB after a previous SLNB for a primary cancer was 81%, compared with 52.2% ( P < 0.0001) if performed after a prior ALND. Contralateral axillary SNs can be resected when repeat mapping drains to that basin. Although controversial, if positive, completion ALND and/or regional nodal irradiation could be considered. However, long-term outcome data are unavailable with respect to local control and subsequent distant metastases.
Previous excision was considered a contraindication in some of the earlier SLND studies. It was hypothesized that when the breast lymphatics are transected, the drainage pattern would not be reliable. Haigh and colleagues demonstrated that there were no statistically significant differences for SN identification rate or accuracy by biopsy method (fine-needle aspiration, core biopsy, or excision), excision volume, time from initial biopsy to SLND, tumor size, and tumor location by univariate and multivariate analysis. In another study, 2206 patients were enrolled, and there was no statistically significant difference between SN identification rate or false-negative rate between those patients who had needle biopsy or excisional biopsy. Similar findings are observed in the European Institute of Oncology, where more than 50% of the patients present for definitive treatment after an excisional biopsy and have an SLND with 99% identification rate and axillary failure of 0.7%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here