Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neurologists often obtain cerebrospinal fluid (CSF) by performing a lumbar puncture (LP), one of the oldest neurologic tests still in use. Analysis of CSF can be very helpful, if not diagnostic, in many situations. When patients have at least two elements of the relatively common triad of headache, fever, and nuchal rigidity, neurologists usually perform an LP to check for meningitis, subarachnoid hemorrhage, or other inflammatory conditions affecting the central nervous system (CNS). They also perform an LP in cases of dementia that may be due to an infectious illness. In Creutzfeldt-Jakob disease (CJD), the CSF usually contains 14-3-3 protein and real-time quaking-induced conversion (RT-QuIC) assays are positive (see Chapter 7 ); in subacute sclerosing panencephalitis (SSPE), anti-measles antibodies; and in acquired immunodeficiency syndrome (AIDS), retrovirus markers. In many infectious illnesses of the CNS, tests on the CSF reveal the actual organism, a diagnostic antigen, or a DNA marker obtained by amplification through polymerase chain reaction (PCR): cryptococcal or tuberculous meningitis, herpes simplex encephalitis, and neurosyphilis. For patients with AIDS who have non-enhancing cerebral white matter lesions, neurologists test CSF for the JC virus. If present, the JC virus indicates a diagnosis of progressive multifocal leukoencephalopathy (PML) (see Fig. 15.11 ). Moreover, detecting JC virus obviates the need for a brain biopsy. Neurologists also test the CSF of patients suspected of having multiple sclerosis (MS) for oligoclonal bands and—during an exacerbation—myelin basic protein (see Chapter 15 ). In Alzheimer disease, the CSF contains increased levels of tau protein but decreased levels of β-amyloid and Aβ−42 peptide.
Diagnosing neurologic illnesses sometimes rests on abnormalities of the CSF profile, comprising its color, red and white blood cell (WBC) count, and concentrations of protein and glucose ( Table 20.1 ). For example, most infectious or inflammatory CNS illnesses cause a CSF pleocytosis (increase in the CSF WBC count). In these illnesses, a rise in protein concentration parallels CSF pleocytosis, and in their hallmark, glucose concentration falls to abnormally low levels. Bacterial meningitis accentuates that profile: CSF pleocytosis is markedly elevated, with a predominance of polymorphonuclear instead of lymphocytic cells, and the glucose concentration decreases markedly, sometimes to undetectable levels. Cultures of virus, fungus, and Mycobacterium may require 1 to 3 weeks to identify an organism, but sometimes antigen testing can immediately indicate bacterial and nonbacterial organisms. In an exception to the general observation that infectious and inflammatory conditions produce CSF pleocytosis, the CSF in Guillain-Barré syndrome contains elevated protein concentration but little or no increase in the WBC content (the “albumino-cytologic dissociation,” see Chapter 5 ).
| Protein | Glucose | ||||
|---|---|---|---|---|---|
| Color | WBC/mL | (mg/dL) | (mg/dL) | Miscellaneous | |
| Normal | Clear | 0–4 a | 30–45 | 60–100 | |
| Bacterial meningitis | Turbid | 100–500 b | 75–200 | 0–40 | Gram stain may reveal organisms |
| Viral meningitis | Turbid | 50–100 a,c | 50–100 | 0–60 | |
| tuberculosis (TB) and fungal meningitis d | Turbid | 100–500 a | 100–500 | 40–60 | Cryptococcus antigen testing should be ordered |
| Neurosyphilis | Clear | 5–200 a | 45–100 | 40–80 | venereal disease research laboratory (VDRL) positive e |
| Guillain-Barré syndrome | Clear | 5–20 a | 80–200 | 60–100 | |
| Subarachnoid hemorrhage | Bloody | f | 45–80 | 60–100 | Supernatant usually xanthochromic if not bloody |
b Mostly polymorphonuclear cells.
c In herpes simplex encephalitis, the CSF also contains red blood cells.
d In carcinomatous meningitis, the CSF profile is similar to fungal meningitis, but malignant cells may be detected on cytologic examination.
e Up to 40% of neurosyphilis cases have a false-negative VDRL CSF test (see Chapter 7 ).
f White and red cells are in same proportion as in blood (1:1000).
Despite the potential value of CSF examination, certain circumstances contraindicate an LP. For example, neurologists do not perform the procedure when patients have a sacral decubitus ulcer because the LP needle might drive bacteria into the spinal canal and infect the CSF. In addition, neurologists insert the LP needle only below the first lumbar vertebra, the lower boundary of the spinal cord, to prevent spinal cord injury.
An intracranial mass is another contraindication to an LP. This prohibition is based on the expectation a mass would raise intracranial pressure. An LP would suddenly reduce pressure in the spinal canal, allowing the unopposed force from above to cause the temporal lobe to herniate through the tentorial notch (i.e., transtentorial herniation) (see Fig. 19.3 ). Moreover, a CSF examination would not help in defining mass lesions because their CSF profiles are not distinctive. (However, neurologists perform LPs with impunity in patients with idiopathic intracranial hypertension [pseudotumor cerebri, see Chapter 9 ] for diagnosis and occasionally for treatment, even though intracranial pressure is elevated, because herniation is unlikely in the absence of a mass lesion.) Unless neurologists suspect acute bacterial meningitis or subarachnoid hemorrhage, where rapid diagnosis is essential, they usually do not perform an LP until imaging studies have excluded a mass.
Another potential problem during an LP occurs when trauma during the procedure allows blood to mix with CSF. In this situation, the contamination may falsely suggest a subarachnoid or other intracranial hemorrhage. To distinguish blood from a subarachnoid hemorrhage from blood induced by a traumatic LP, laboratories centrifuge bloody CSF. Xanthochromia (Greek, xanthos yellow + chroma color) in the supernatant of the CSF means bleeding into the CSF space occurred several hours before the LP because degraded heme from blood cells gave rise to yellow pigment. In contrast, a clear supernatant means a traumatic LP gave rise to the blood in the CSF.
Although computed tomography (CT) and magnetic resonance imaging (MRI) should not enslave physicians, they undeniably provide extraordinarily accurate diagnoses. Each technique has given clinical neurology a quantum leap forward. Drs. Allan M. Cormack and Godfrey N. Hounsfield garnered Nobel prizes in 1979 for development of CT, and Dr. Paul C. Lauterbur and Sir Peter Mansfield garnered Nobel prizes in 2003 for discoveries fundamental to MRI.
Conceding that in many situations these imaging studies surpass the reliability of neurologic examination, neurologists routinely order CT and MRI to evaluate patients’ dementia, aphasia, neuropsychologic deficits, seizures, and other conditions. Even in cases of apparent delirium, they often order it to exclude an underlying structural lesion. Neurologists also use imaging studies to follow the course of certain illnesses, such as brain tumors and MS, because their clinical manifestations often fail to reflect disease activity or extent as reliably as imaging studies.
On the other hand, neurologists do not routinely order imaging studies in evaluating patients with sleep disturbances, absence seizures, cluster and migraine headaches, Parkinson disease, tics, or essential tremor. They find that imaging studies do not help with patients’ diagnoses or management. For example, in autism, dyslexia and other learning disabilities, attention-deficit /yperactivity disorder (ADHD), Tourette disorder, and Rett syndrome, imaging studies usually show normal brains, small brains, or regional variations, such as cerebellar atrophy. Those findings are nonspecific and do not enlighten the physician or provide much benefit to the patient or family.
When neurologists consult on psychiatric patients, they often recommend imaging studies, among other tests, for patients who have a first episode of psychosis, atypical psychosis, major depression after age 50 years, episodic behavioral disturbances, and, in some cases, anorexia. They also suggest imaging studies for patients prior to electroconvulsive treatment (ECT) for several reasons. A CT or MRI might detect lesions, like those of neurocysticercosis or cerebral demyelination, which might explain psychiatric symptoms without producing overt physical deficits. Although helpful in many circumstances, imaging studies often fail to clarify the relationships, if any, between psychiatric symptoms and many common abnormalities that the studies reveal, such as cerebral atrophy, mild communicating hydrocephalus, small cerebral lesions, subcortical hyperintensities, and congenital abnormalities.
Using beams of ionizing radiation, which are essentially x-rays, CT generates images of the brain, other soft tissues, and the skull. CT displays structures increasingly more radiodense than the brain, such as tumors, blood, bone, calcifications, and surgical devices, in gradations increasingly closer to white than black. Similarly, CT shows structures increasingly less radiodense than the brain, particularly the CSF-filled ventricles, in gradations increasingly closer to black. Thus, it shows in dark to black gradations several common lesions characterized by the absence of acute blood, such as cerebral infarctions, chronic subdural hematomas, edema surrounding tumors, and the center of cystic lesions. As a corollary, CT shows in light to white gradations several common structures characterized by calcium or excessive blood, such as calcifications in the choroid plexus or meningiomas and hemorrhages. By manipulating the software, CT and MRI can display the brain from three major perspectives: axial (or horizontal, the conventional top-down view), coronal (front-to-back view), and sagittal (side view).
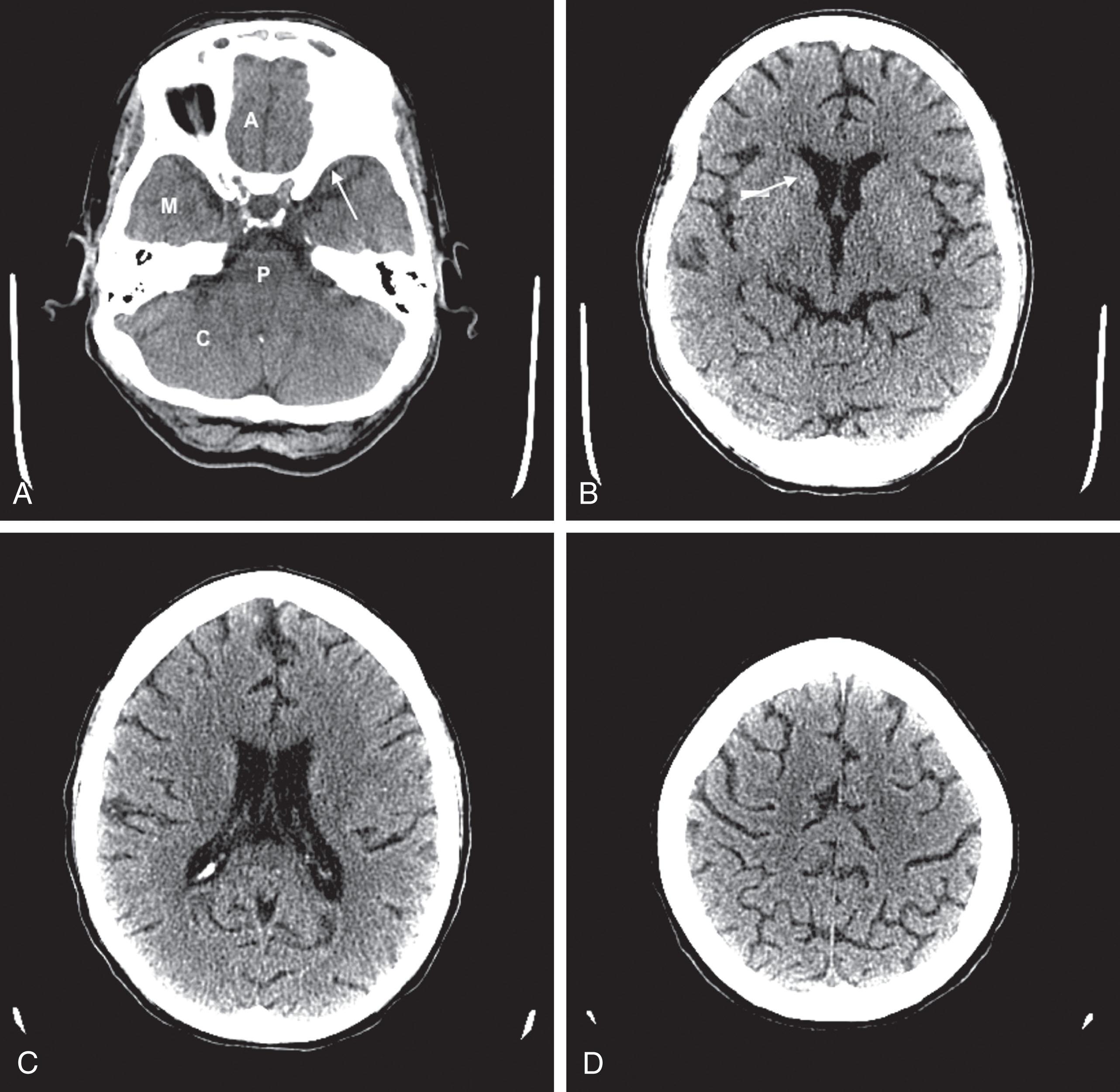
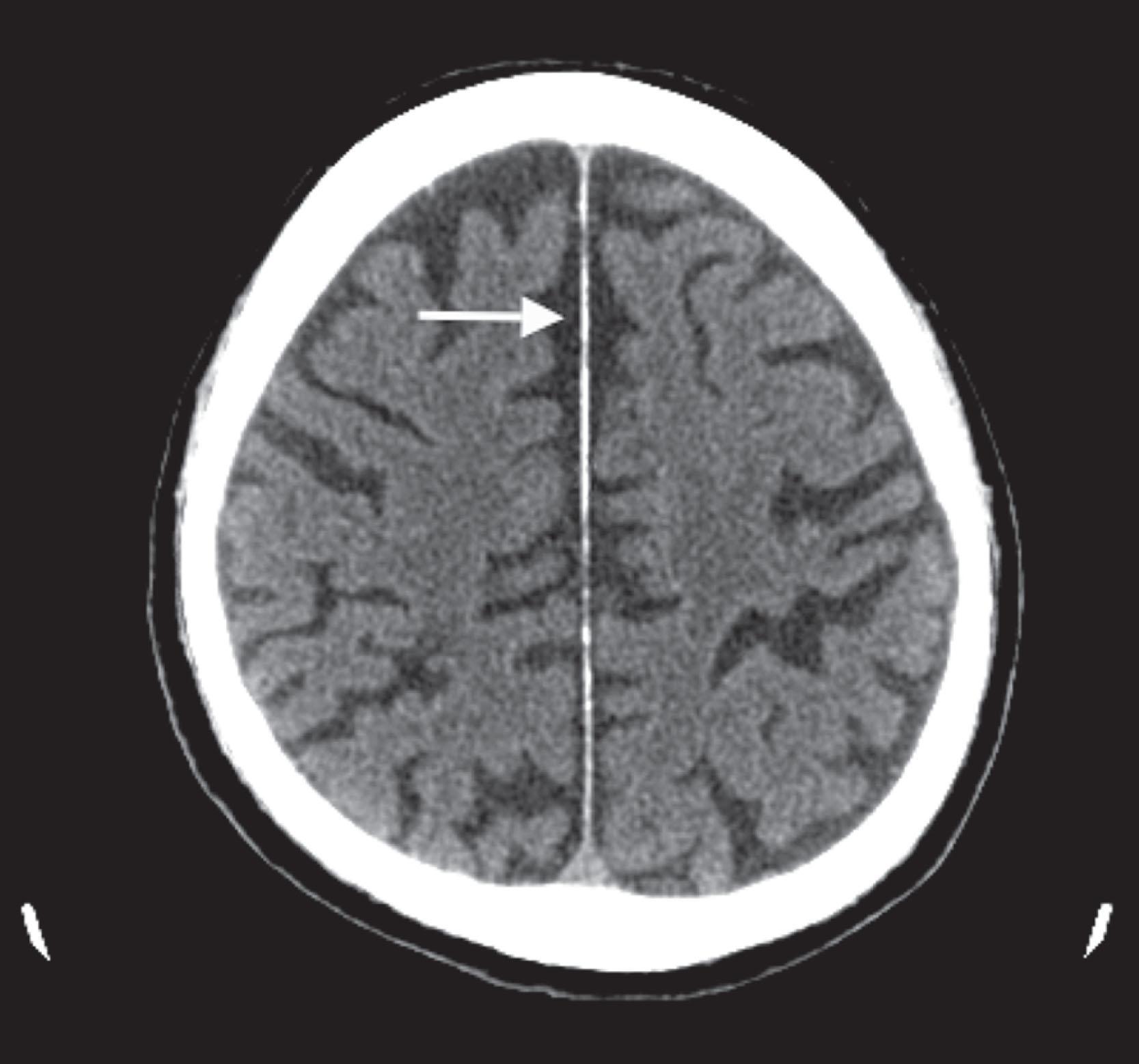
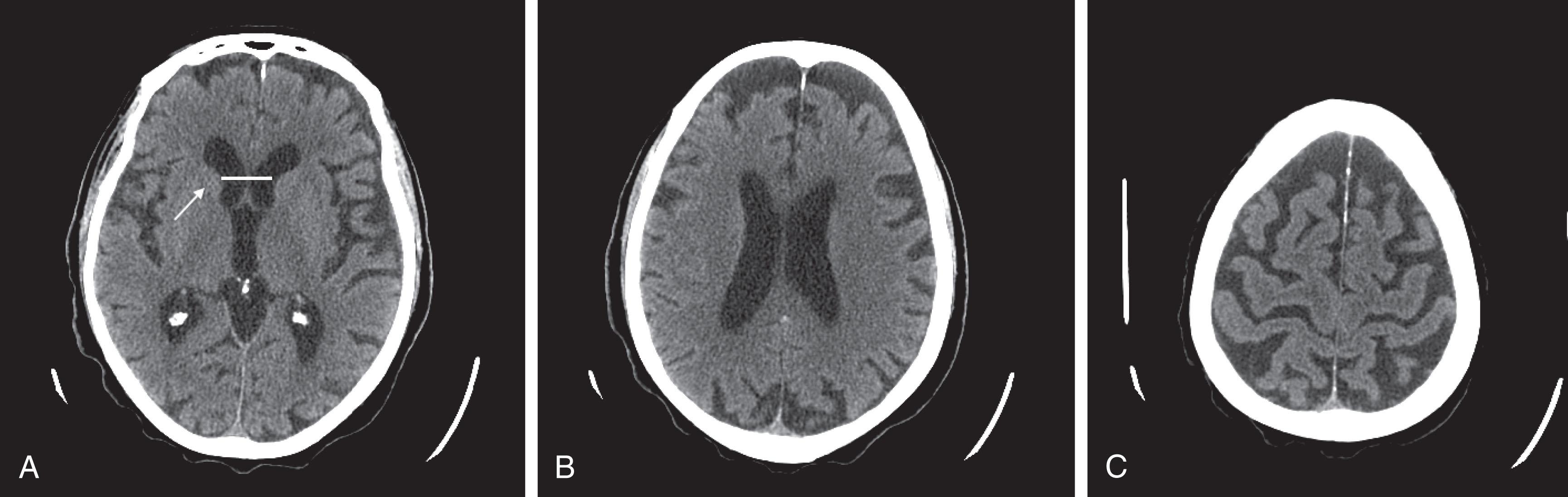
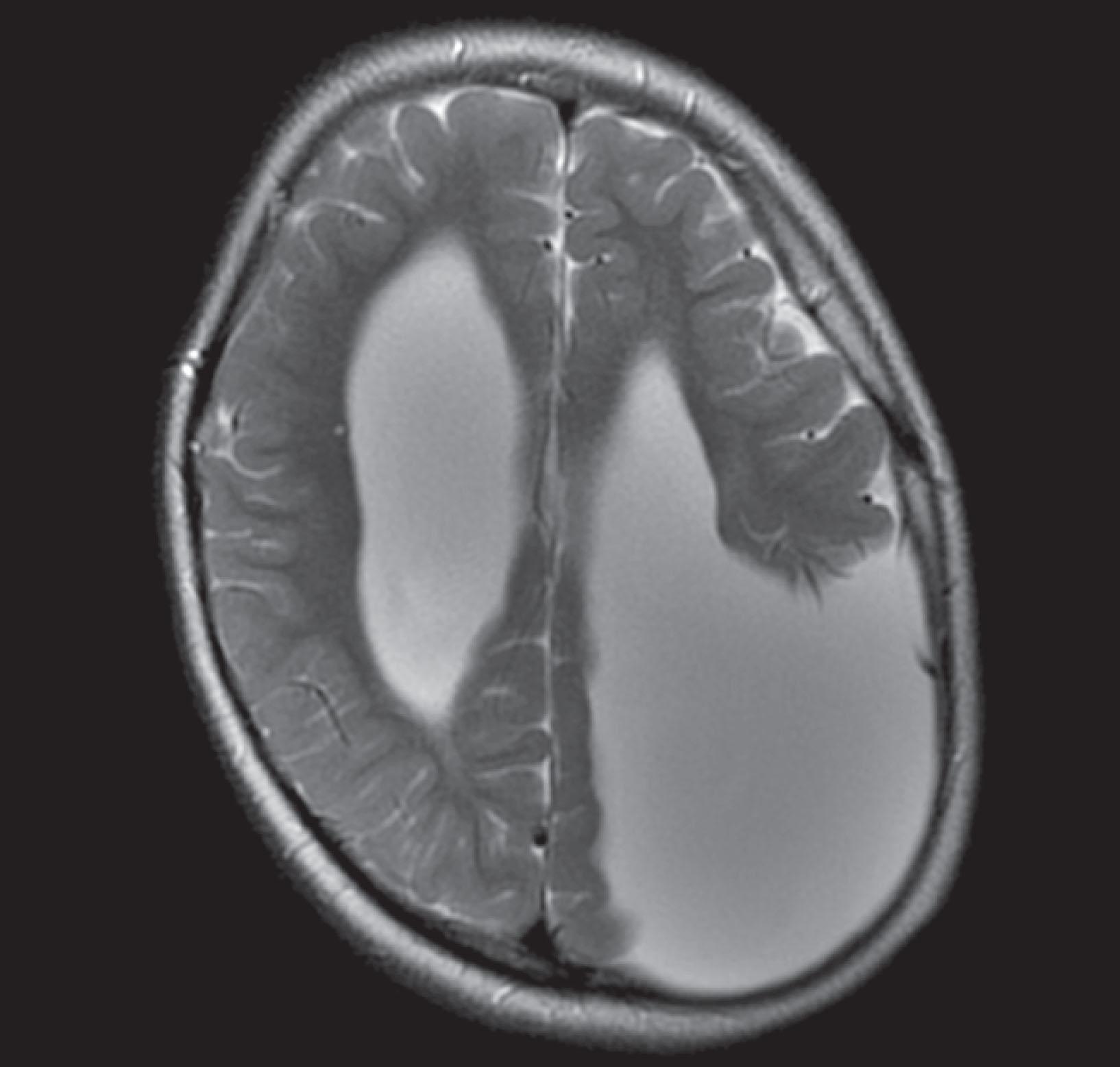
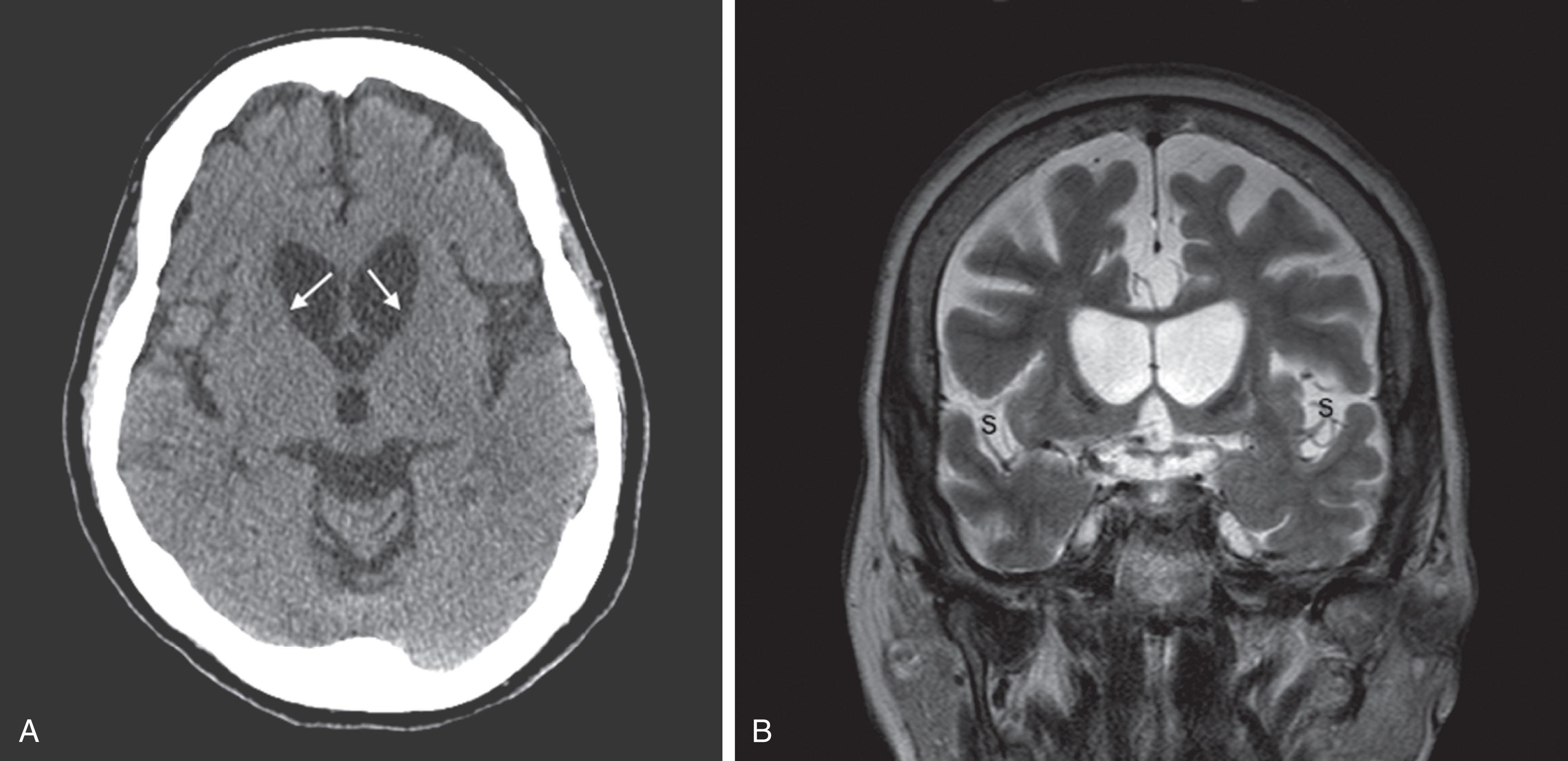
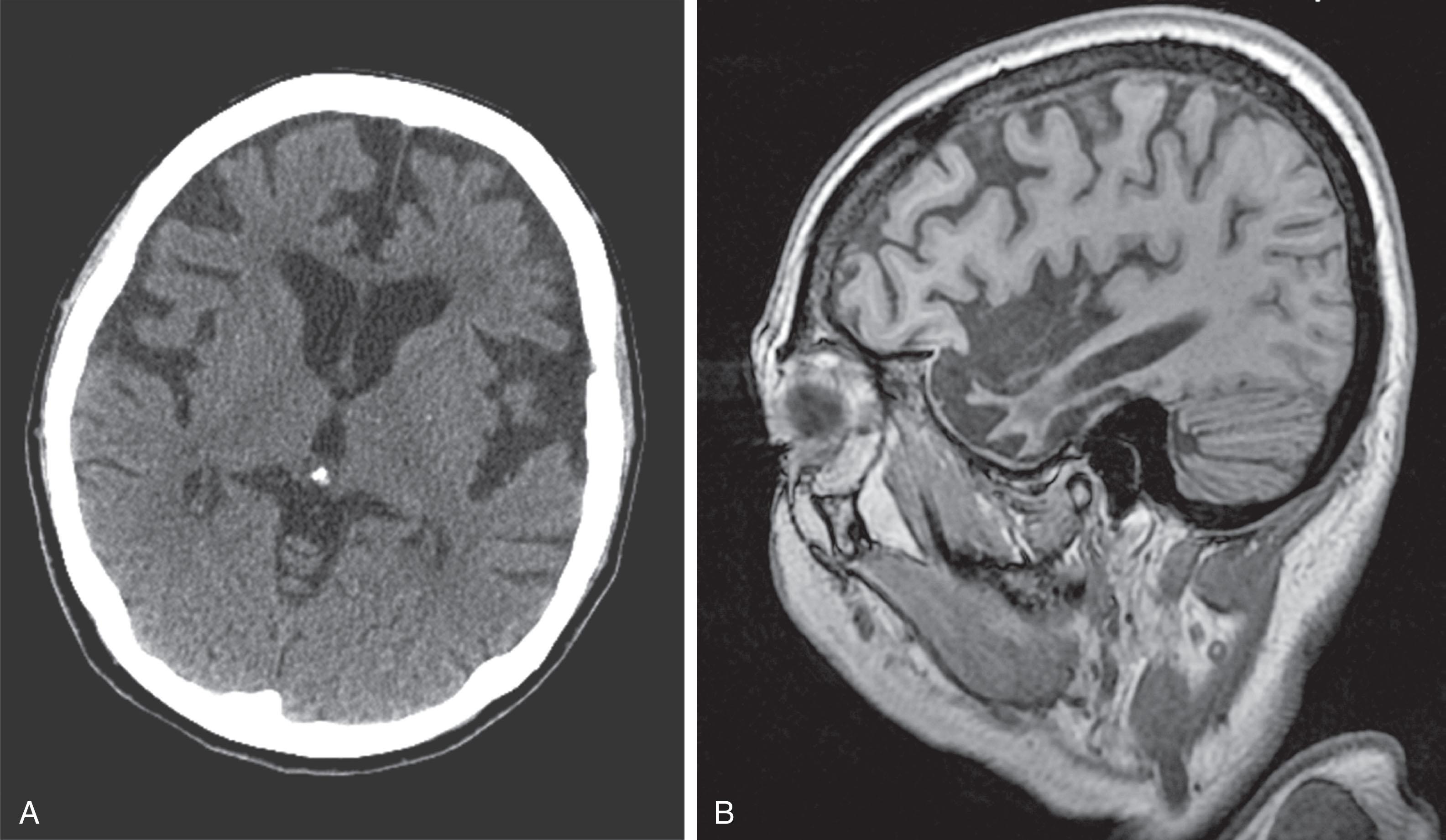
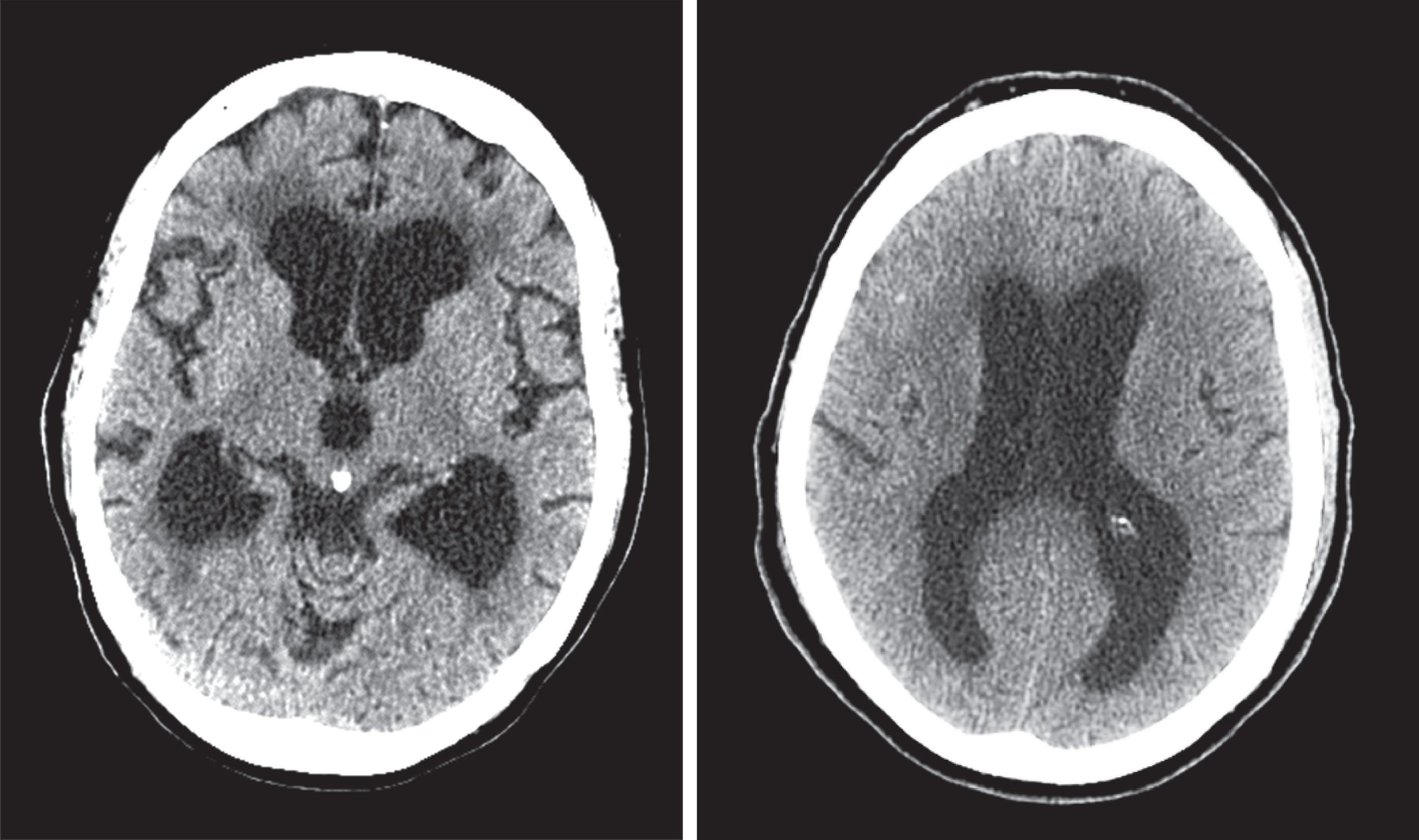
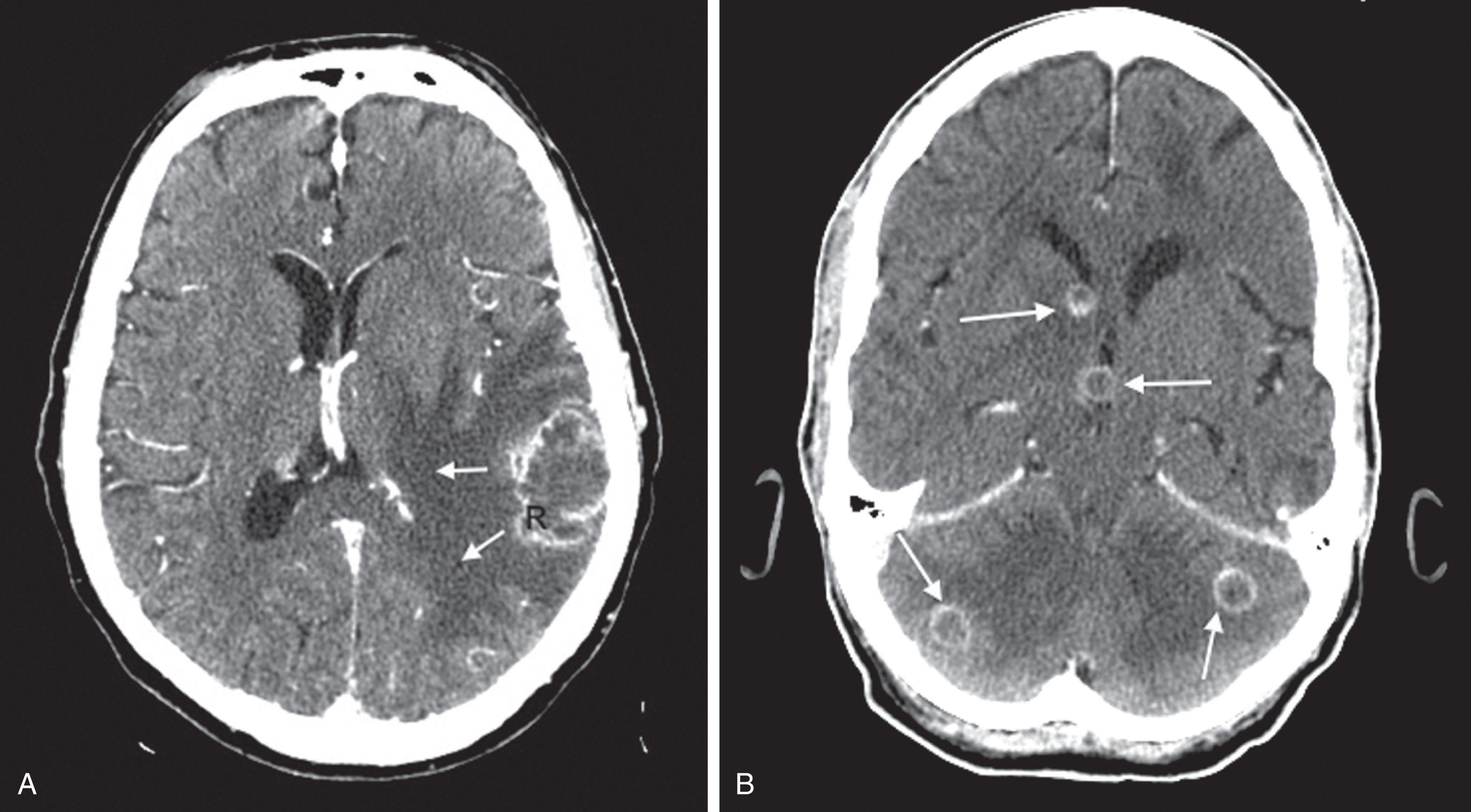
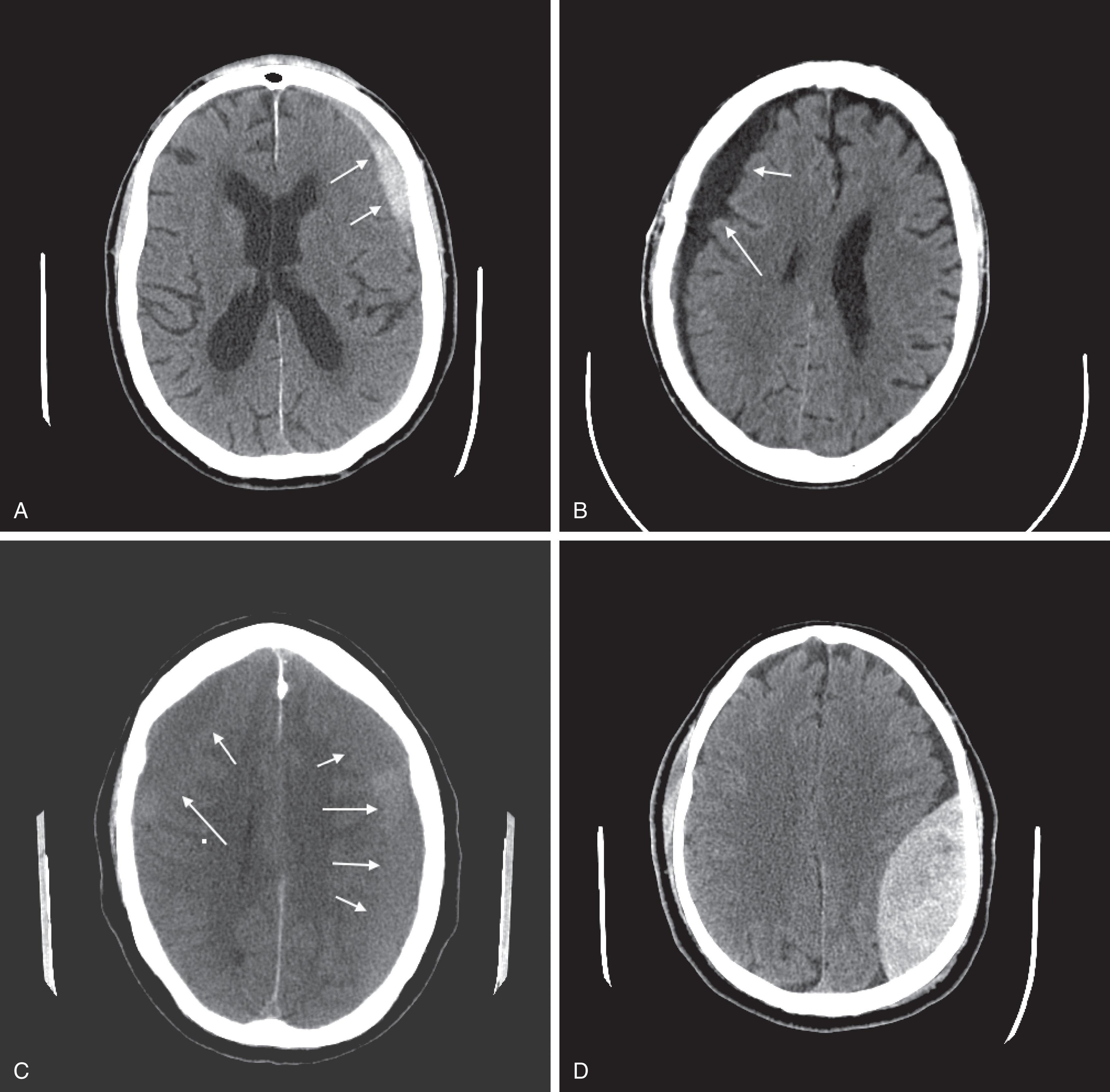
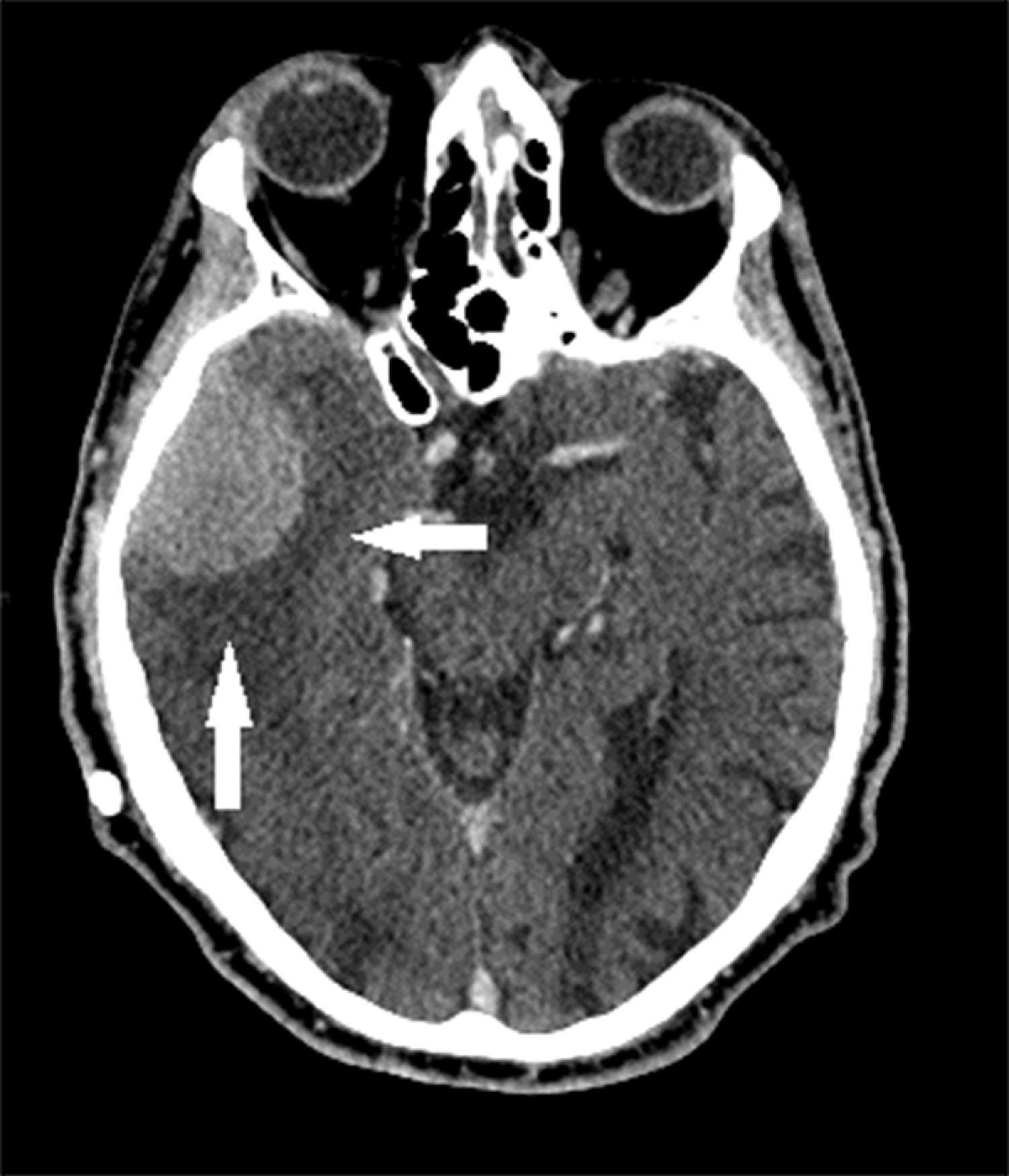
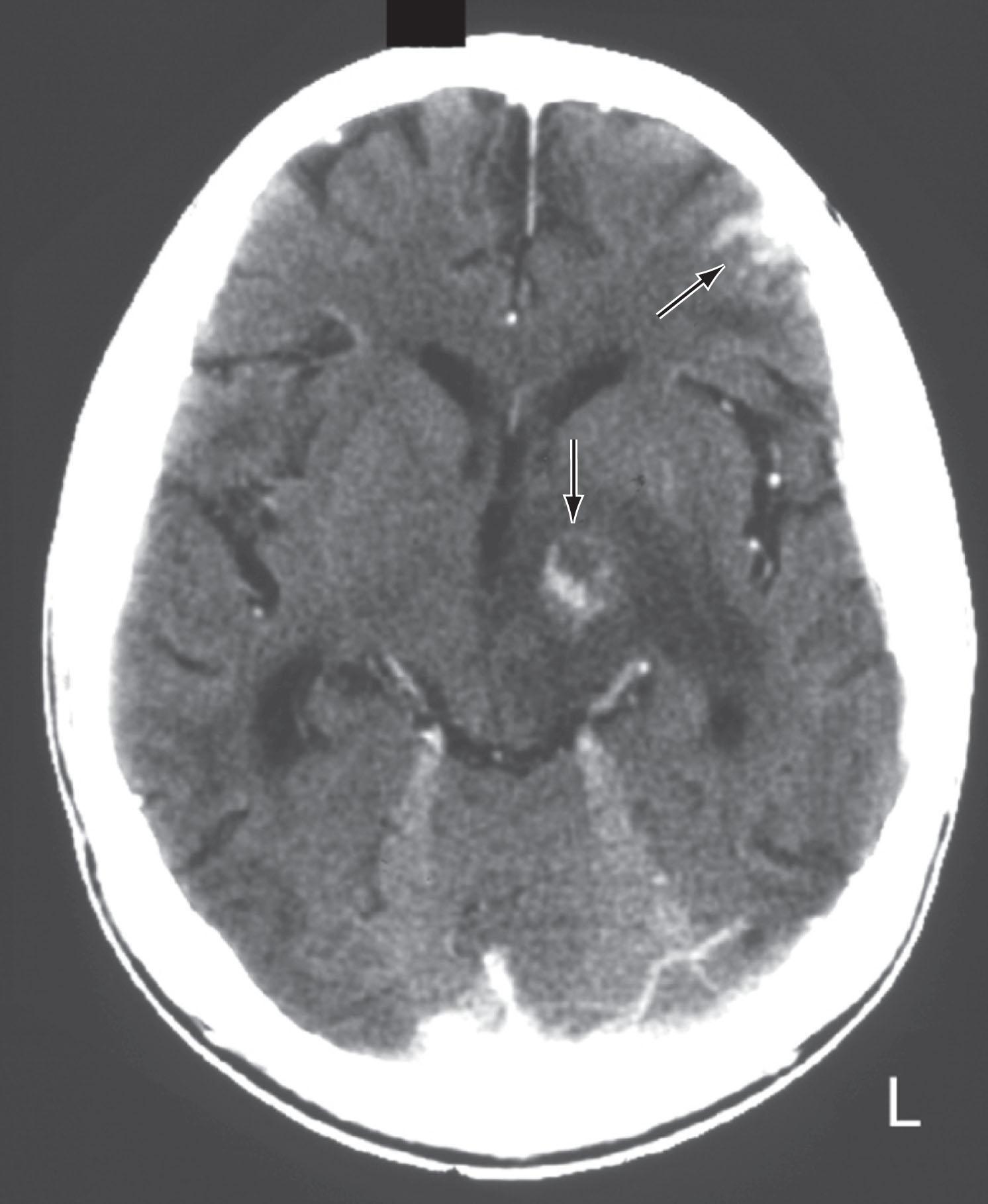
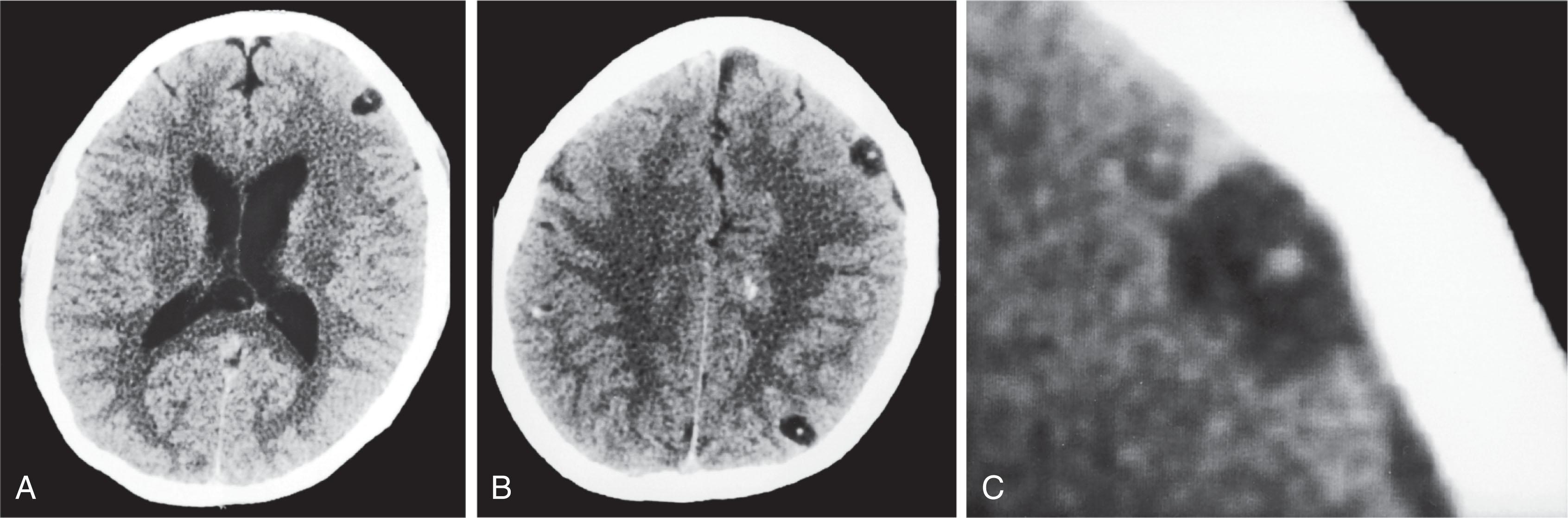
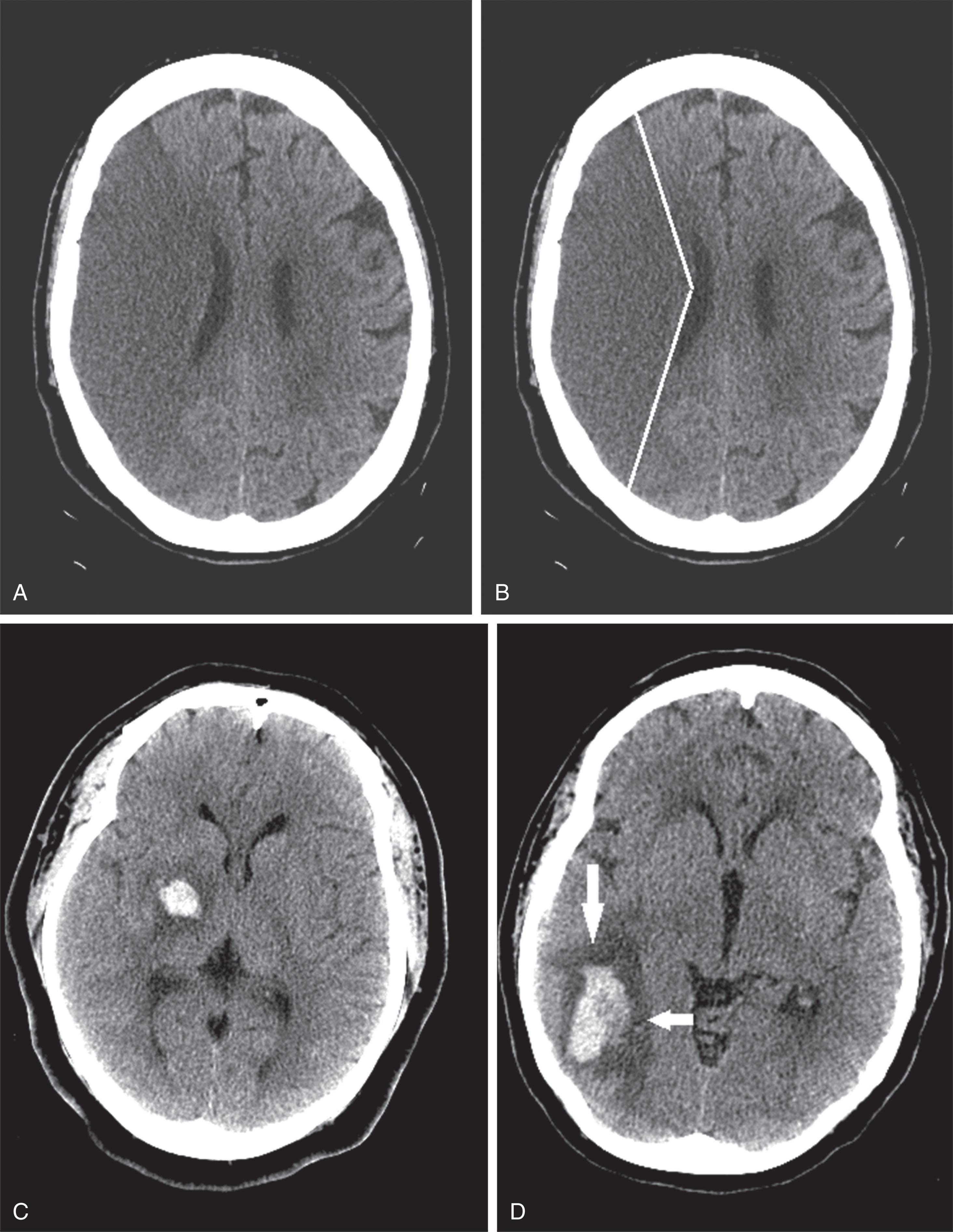
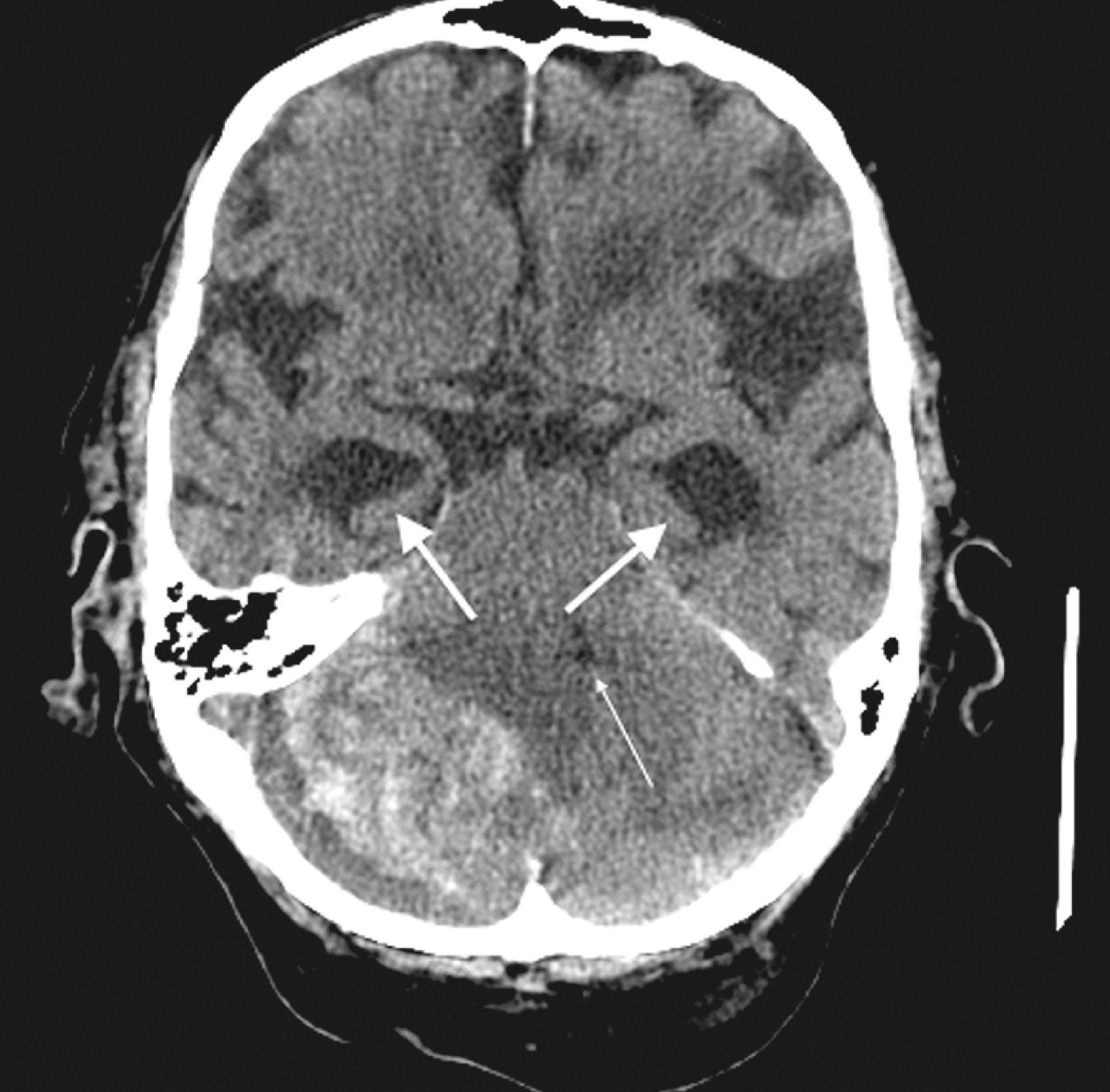
CT reveals major changes in brain structure ( Fig. 20.1 ). It shows generalized cerebral atrophy, as occurs in advanced age or Alzheimer disease ( Figs. 20.2 and 20.3 ), and atrophy of a particular region, as with porencephaly ( Fig. 20.4 ), Huntington disease ( Fig. 20.5 ), and frontotemporal dementia ( Fig. 20.6 ). Similarly, CT shows expansion of the ventricles—hydrocephalus—not only from generalized atrophy (hydrocephalus ex vacuo) (see Fig. 20.3 ) but also from normal-pressure hydrocephalus ( Fig. 20.7 ) and CSF obstructions (obstructive hydrocephalus) (see later). CT readily detects large lesions, like primary and metastatic tumors ( Fig. 20.8 ). CT will also reveal subdural hematomas, except perhaps for isodense ones ( Fig. 20.9 ). CT is even superior at finding dense, calcium-laden meningiomas ( Fig. 20.10 ). It can show numerous small lesions, such as in toxoplasmosis ( Fig. 20.11 ) and cysticercosis ( Fig. 20.12 ). (Cysticercosis, caused by the parasite Taenia solium , is the most common cerebral mass lesion in South and Central America.)
CT can also show established strokes and hemorrhagic ones ( Fig. 20.13 ); however, MRI can better locate small or acute strokes. CT is invaluable in detecting cerebellar hemorrhage, where rapid diagnosis is essential in preventing brainstem compression and obstructive hydrocephalus ( Fig. 20.14 ).
Despite the small risk of pediatric cerebral radiation exposure (see later), physicians routinely obtain head CT in suspected child abuse because 80% of cases include nonaccidental head injury (NAHI) . Moreover, up to 50% of cases of NAHI result in neurologic deficits and 30% in fatality. CTs and MRIs detect skull fractures and subdural hematomas. Bilateral subdural hematomas of different ages are a hallmark of NAHI.
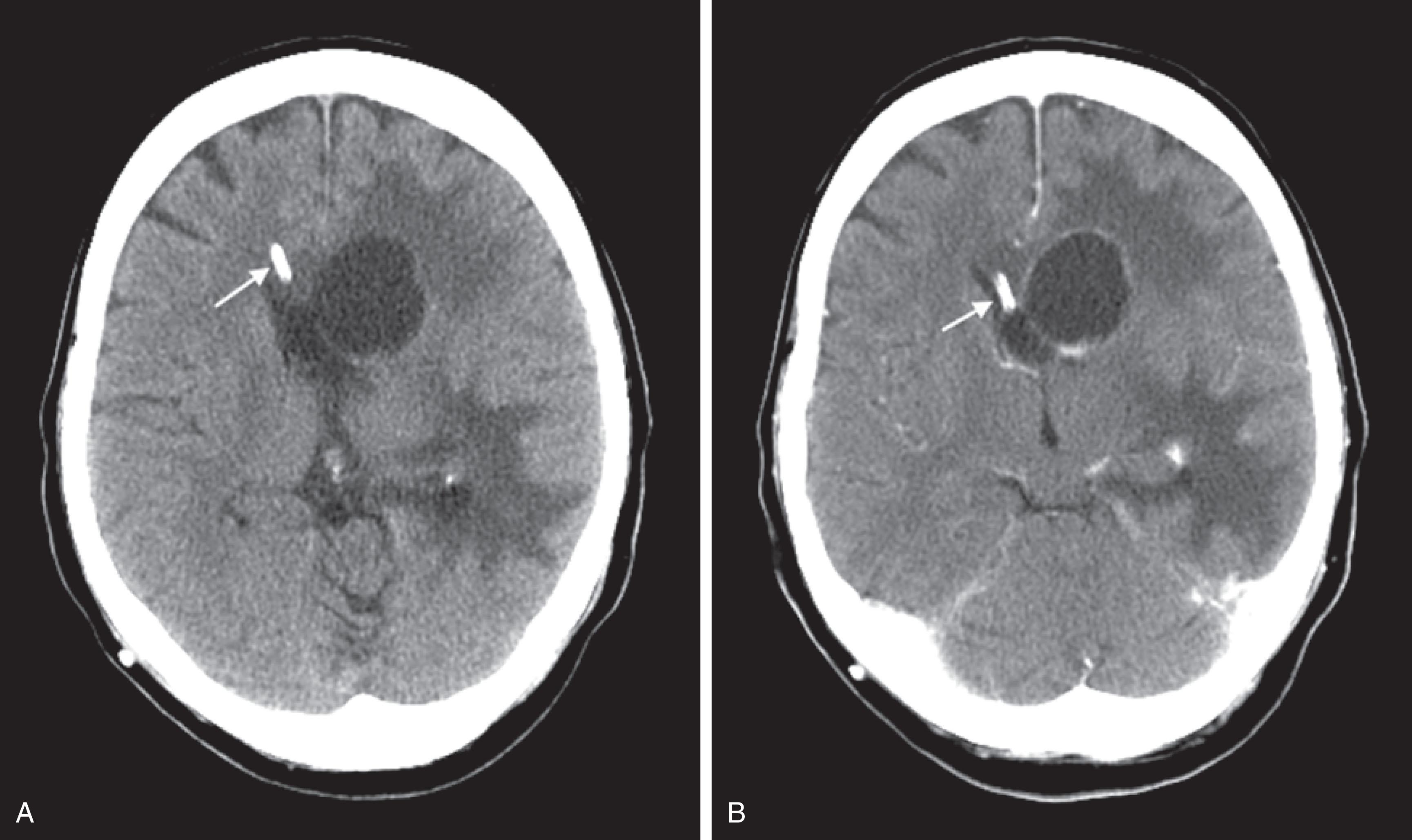
The administration of an intravenous contrast agent during CT (contrast enhancement) increases the density of blood-filled structures and whitens their image. It highlights vascular structures, such as arteriovenous malformations (AVMs), cysts, glioblastomas, and membranes surrounding chronic subdural hematomas ( Fig. 20.15 ).
Although MRI holds many advantages, CT remains less expensive than MRI, highly reliable, and available as movable compact machines. It is valuable during emergencies when speed is critical and gross anatomical pictures suffice. For example, the procedure, which takes less than five minutes, satisfactorily reveals lesions that require immediate attention, such as epidural and acute subdural hematomas, cerebellar hemorrhages, obstructive hydrocephalus, and subarachnoid hemorrhage. Also, patients with pacemakers, defibrillators, and other indwelling metallic devices, and those with claustrophobia can undergo CT but not most MRIs.
As for disadvantages, undergoing a CT exposes patients to ionizing radiation. This radiation exposure potentially endangers children and adolescents because their dosage is relatively greater than that of adults, and children’s skulls are thinner. Giving roughly the radiation of a skull x-ray series, a single head CT adds a small but measurable risk (0.07%) to the child’s lifetime cancer risk. Furthermore, dental x-rays and, more so, dental CTs add to their lifetime radiation exposure. Another consideration in children is that because ocular lenses are highly susceptible to radiation, CT adds to their risk of developing radiation-induced cataracts.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here