Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Amputation is thought to be one of the oldest surgical procedures. The earliest artificial limb dates from the Samnite wars of 300 bc . Until the time of Ambroise Paré (1510–1590), the techniques for amputation surgery and amputation level selection were extremely crude. Paré improved the surgical technique of amputation through the use of vascular ligatures and developed guidelines for the selection of appropriate levels of amputation. Because many of his original drawings and descriptions are not greatly different from today's surgical and prosthetic practices, he is considered the originator of the modern principles of amputation surgery. Paré's work was expanded by Dominique Jean Larrey (1776–1842) during the Napoleonic wars. Larrey advocated early amputation for traumatic limb injuries and was instrumental in the acceptance of both complete stump debridement and the modern surgical technique whereby bone is buried deep in the amputation stump, rather than the previous method of suturing skin tightly over the bone. Larrey was also involved in developing methods of early mobilization of war amputees.
The idea of immediate or rapid fitting of an artificial leg after lower extremity amputation is relatively recent. Credit for the concept of rapid fit is generally given to Berlemont, based on his work with patients who experienced delayed healing after lower extremity amputation; the concept of immediate postsurgical prosthetic fitting was developed by Weiss. In the late 1960s, Burgess and colleagues noted accelerated rehabilitation, increased acceptance of a prosthesis, and less psychological trauma associated with loss of a limb when a prosthesis was applied immediately after lower extremity amputation. Other advances in lower extremity amputation include the development of new techniques for amputation level selection, extension of the frontiers for limb salvage, and fabrication of prosthetic limbs that incorporate new designs and materials as well as energy storage.
Despite the long and colorful history of amputation surgery, most surgeons considered amputation a surgical defeat and an uninteresting and unrewarding surgical procedure. In many surgical institutions, amputation surgery was traditionally passed down to the youngest and least experienced member of the surgical team, who often operated without senior supervision. A report from the European surgical literature suggests that amputation failure is statistically linked to the lack of amputation experience of the surgeon.
There has been a resurgence of interest in amputation surgery and rehabilitation. It is my view that amputation surgery is not a failure; it is clearly a reconstructive surgical technique. In addition, rehabilitation must be provided to patients following amputation surgery.
Historically, amputations were performed by orthopedic rather than general surgeons. However, two thirds of all lower extremity amputations are necessitated by complications of peripheral vascular disease or diabetes mellitus, so it is not surprising that the majority of lower extremity amputations are now performed by general and vascular surgeons. Unfortunately, most general surgical training programs fail to provide education in prosthetics, prosthetic design, biomechanics, and rehabilitation. The qualifications of the surgeon performing the amputation are not nearly as important as his or her interest in providing postoperative rehabilitation for the newly amputated patient.
This chapter reviews important features of lower extremity amputation surgery and amputation rehabilitation and includes detailed subsections on patient evaluation and preparation for amputation; amputation level selection; indications, surgical techniques, and prosthetic requirements for each level of amputation; surgical morbidity and mortality; common principles of lower extremity prosthetics; techniques of postsurgical rehabilitation; and future trends.
A review of the literature suggests that the mortality rates for below-knee and above-knee amputation are 4% to 16% and 12% to 40%, respectively. It has been estimated that two thirds of patients undergoing lower extremity amputation have diabetes mellitus and that one half to two thirds have symptoms of cardiorespiratory disease. A review of the causes of late mortality following successful amputation surgery discloses that two thirds of patients die of cardiovascular disease, approximately half of whom die from myocardial infarction.
There are between 30,000 and 50,000 new lower extremity amputations performed in the United States each year. At present, it is unclear whether the increase in distal revascularization has decreased the number of amputations. It is clear that diabetes-related amputation rates exhibit high regional variation, even after adjustment for age, sex, and race. The indications for lower extremity amputation are listed in Table 64.1 . For patients with diabetes mellitus, it has been estimated that the risk of losing the second leg in the 5 years after amputation of the first leg ranges from 15% to 33% (3% to 7% per year) ; however, one third to one half of amputees with diabetes mellitus die of complications of diabetes or cardiorespiratory disease before undergoing amputation of the second extremity.
| Indication | Percentage (Range) |
|---|---|
| Complications of diabetes mellitus | 60–80 |
| Nondiabetic infection with ischemia | 15–25 |
| Ischemia without infection | 5–10 |
| Chronic osteomyelitis | 3–5 |
| Trauma | 2–5 |
| Miscellaneous (neuroma, frostbite, tumor, pain, nonhealing) | 5–10 |
In view of the mean age of patients undergoing lower extremity amputation (62 years), the incidence of associated diseases, and the morbid and nonmorbid complications associated with surgery, the importance of a careful preoperative physical examination cannot be overstated. The physical examination should include a search for physical signs and symptoms suggestive of cardiorespiratory disease. Documentation of all pulses, as well as a careful assessment of the presence or absence of physical findings suggestive of extremity ischemia (pain, paresthesias, elevation pallor, rubor, alteration of sensory or motor function), should be performed. The extent and depth of infection or gangrene should be noted. The presence of malnutrition or systemic diseases, such as diabetes mellitus, collagen-vascular diseases, and immunodeficiency syndromes, or the systemic administration of antiinflammatory drugs, such as steroids, should be noted because their presence may have a major influence on the preoperative preparation and timing of lower extremity amputation, as well as postsurgical recovery. All diabetic patients should be carefully screened for silent but significant coronary artery disease. A paper by Pinzur and colleagues reinforced the concept that multidisciplinary presurgical evaluation helps in amputation level selection and prosthetic limb fitting and improves patient rehabilitation.
The presence of lower extremity infection in a diabetic patient requires special mention. Historically, most studies have suggested that the primary organisms were Staphylococcus aureus and enteric gram-negative bacilli. More recent information, however, suggests that 60% of lower extremity infections in patients with diabetes mellitus involve both obligate and facultative anaerobic organisms. Fierer and associates also pointed out that patients with mixed infections required more operations than those with simple staphylococcal infections and that their surgical wounds tended to heal more slowly. I therefore recommend that antibiotic coverage for diabetics with lower extremity infections include drugs that provide broad-spectrum bactericidal aerobic and anaerobic coverage. A note of caution: there have been increasing reports of methicillin-resistant S. aureus infections, especially in patients with wound complications after vascular surgery.
In the absence of acute arterial embolization, diabetes mellitus, immunodeficiency syndromes, collagen-vascular diseases, or drugs that inhibit the immune system, it is unusual to see tissue loss with or without infection in patients without multilevel vascular occlusive disease. Chronic single-level arterial obstruction is not usually associated with limb loss. As a general guideline, chronic occlusion of the superficial femoral artery does not cause limb loss without outflow (tibial trifurcation) or inflow (iliofemoral or deep femoral) occlusive disease.
The aims of amputation are to remove gangrenous tissues, relieve pain, obtain primary healing of the most distal amputation possible, and obtain maximal rehabilitation after amputation. For purposes of further discussion, the indications and management of patients undergoing amputation are divided into three general categories: (1) acute ischemia, (2) progressive chronic ischemia, and (3) ischemia complicated by infection.
The choice of amputation for the management of acute ischemia in a patient whose arterial tree is considered unreconstructible or in a patient who presents late in the course of acute ischemia, such that arterial reconstruction may be contraindicated, is a decision that taxes the judgment of even the most experienced surgeon. In addition, urgent or emergent amputation following acute arterial occlusion is generally associated with the greatest risk of morbidity and mortality in the entire field of amputation surgery. The degree of urgency for amputation in the face of acute arterial ischemia is governed by multiple factors that include, but are not limited to, the extent of extremity ischemia, especially the muscle mass; the pain the patient is experiencing from the ischemic tissues; and the presence of signs of systemic toxicity resulting from products of necrotic muscle or bacteria reaching the general circulatory system. If the affected ischemic area is small (e.g., the toes or forefoot), the pain is relatively moderate, and there are no signs of supervening infection or systemic toxicity, amputation can and should be postponed as long as possible to allow maximum development of collateral circulation. Delay of amputation in such a case improves the likelihood that a more distal limited amputation will heal. Mild to moderate pain can be controlled with narcotics. Adjunctive therapy, including systemic heparin, low-molecular-weight dextran, or fibrinolytic agents (urokinase, tissue plasminogen activator, tenecteplase, or reteplase) may be valuable in preventing progression of thrombus within capillary beds during the period of reduced blood flow, thus maintaining the viability of marginally ischemic tissues while collateral channels are beginning to enlarge. The presence of severe pain, extensive muscle necrosis, or systemic toxicity may require more emergent amputation with less patient preparation preoperatively. If muscle necrosis is extensive, the need for urgent or emergent amputation is critical because of the risk of renal damage secondary to circulating myoglobin. In addition to renal dysfunction, necrotic muscle tissue may cause cardiovascular or respiratory compromise, adding to the need for emergent amputation. The presence of systemic toxicity, especially a necrotizing infection, often necessitates emergent amputation. For those patients with significant medical comorbidities or systemic toxicity, cryologic or physiologic amputation allows extended time for patient preparation for surgery and decreases mortality.
Several features of patient evaluation and clinical progression are helpful in this decision-making process. The first consideration is the extent of nonviable skin. Although discoloration or gangrene is readily apparent, hyposensitive areas due to severe skin ischemia do not allow adequate healing and may not be so easily identified. Except in patients with diabetic neuropathy, sensation to pinprick and light touch is a useful discriminant. If there is diminished sensation below the knee, for example, the chances of performing a successful below-knee amputation are small, and morbidity may be avoided by proceeding expeditiously to an above-knee amputation. The presence of significant calf muscle swelling or muscle rigidity is an ominous sign suggestive of myonecrosis. Careful monitoring of calf circumference, skin sensation, urine color and output, and signs of systemic toxicity such as lethargy, confusion, or hallucinations must be done on an hourly basis. The presence of systemic toxicity or significant changes in the physical examination indicate the need for immediate amputation or physiologic amputation. Similarly, the presence of myoglobinuria or cardiovascular instability represents an indication for immediate amputation. It is generally impossible to obtain rapid myoglobin determinations in serum or urine, but the presence of a urinary heme-positive dipstick in the absence of red blood cells under microscopic examination or pink serum (hemoglobin) is consistent with a diagnosis of myoglobinuria. In addition, the presence of markedly elevated creatine phosphokinase or lactate dehydrogenase isoenzymes in the serum is suggestive of myonecrosis in the absence of trauma, myocardial infarction, or recent surgery. If continued observation is elected, prophylaxis against renal damage from myoglobin pigment is recommended and includes maintenance of a diuresis in the range of 75 to 100 mL/hour by the administration of osmotic diuretics such as mannitol and fluids. In addition, the urine should be maintained at an alkaline pH, because myoglobin precipitates in the renal tubules at a pH less than 7. Usually, a mixture of mannitol and sodium bicarbonate in a balanced salt solution can be used to titrate urine output and pH. Finally, the presence of infection in the ischemic extremity is an ominous complication requiring careful consideration. The presence of such an infection in a diabetic patient is even more ominous because of the sometimes rapid progression of seemingly innocuous infections. Any evidence of systemic toxicity or progressive infection is an indication for immediate surgical debridement, which may take the form of a debriding amputation or a physiologic amputation followed by a formal surgical amputation later.
As long as there is continued improvement in collateral blood supply to an acutely ischemic extremity and there is no sign of systemic or renal toxicity, observation may be continued. As soon as circulatory improvement ceases or signs of toxicity develop, amputation should be performed promptly. An alternative to amputation in an unstable or high-risk patient is physiologic amputation, as previously discussed.
Evaluation for potential vascular reconstruction in a patient with a clear-cut demarcation of a nonviable area of a lower extremity after a period of observation for acute ischemia is not likely to be beneficial; however, if there is a question of marginal tissue viability above an area that is believed to be nonviable and the questionable tissue might permit a lower amputation level, evaluation for vascular reconstruction may be beneficial. For example, the presence of a nonviable forefoot and a marginal lower extremity between the knee and ankle may be an indication for evaluation for vascular reconstruction in an attempt to perform a below-knee rather than an above-knee amputation.
A patient with progressive chronic ischemia who ultimately presents for amputation has experienced one or more of the following problems: rest pain, nonhealing skin lesion or ulceration, gangrene, or gangrene with superimposed infection. Gangrene complicated by infection is a special problem and is discussed in a separate section.
From the patient's point of view, ischemic rest pain is one of the most compelling indications for amputation. Characteristically, the patient seeks relief by sitting up and allowing his or her legs to hang over the side of the bed or by ambulation. The dependent position provides relief of ischemic rest pain because gravity favors improved collateral blood flow, which augments arterial perfusion pressure. In its more severe forms, ischemic rest pain forms part of a vicious circle. As the patient more frequently uses dependency to achieve pain relief, the extremity becomes edematous and lymphatic return decreases, which eventually results in more ischemia. The end result of this vicious circle is a massively swollen, very painful ischemic extremity. Mild to moderate rest pain can often be successfully managed with narcotics. There have been reports that early mild rest pain may be relieved by sympathectomy. If there are no signs of systemic toxicity or supervening infection, and if mild ischemic rest pain can be controlled with medication, a patient can be followed and amputation postponed.
Another manifestation of chronic ischemia is the presence of ischemic skin ulcerations or nonhealing skin lesions on the lower extremity. Unfortunately, many of these skin ulcers occur in hospitalized patients as a result of abrasion of the foot or pressure necrosis (due to poor foot protection in the hospital bed). In a diabetic patient, ulceration usually occurs at pressure points, and the patient is often unaware of the problem because of diabetic neuropathy. Patients without peripheral neuropathy are very much aware of these ischemic ulcers because they are typically extremely painful. Besides obvious areas of pressure necrosis, such as the heel and the lateral and medial malleoli, other common points of ulceration include the bony prominences over the metatarsal heads and midphalangeal joints or between the toes.
Fortunately, most patients with chronic progressive ischemia come to medical attention before the presence of frank tissue loss. Even patients with tissue loss usually present early with involvement of one or more toes or, in more severe cases, the entire forefoot. Under these circumstances, there is usually ample opportunity to fully prepare and evaluate the patient for lower extremity amputation or arterial reconstruction if possible. Preoperative evaluation and management should include proper medical attention to all associated diseases and a careful evaluation for potential critical organ dysfunction, especially the heart, lungs, and kidneys.
All patients with chronic ischemia should undergo consideration for vascular reconstruction for limb salvage. Angiographic evaluation from the infrarenal abdominal aorta down to and including the pedal arches is mandatory. In general, the surgeon should ensure good inflow to the level of the deep femoral artery. Reconstruction distal to the level of the deep femoral artery should be done only if chances for success are reasonably high and if a successful procedure would obviate the need for a major amputation. There is continuing controversy over the effect of prior revascularization on amputation level. In my opinion, revascularization should always be attempted unless contraindicated by the patient's medical condition. If vascular reconstruction is brought below the knee, the incision required for approaching the distal popliteal artery and tibial trifurcation vessels should be planned along the lines of the posterior skin flap that might be required for a subsequent below-knee amputation. Techniques for selecting an amputation level are discussed later. However, one of the interesting spinoffs from studies of amputation level selection is the identification of patients who are unable to heal a low-level amputation and in whom the chance for rehabilitation at a high level of amputation is unlikely. When such objective information is available, attempts at proximal or extended distal extremity bypass to salvage a knee joint, for example, may be indicated to keep a patient ambulatory.
The presence of dry gangrene is not an emergent surgical problem or necessarily the hallmark of an ominous clinical situation. Dry gangrene, limited to the toes, for example, may be treated conservatively and requires little, if any, surgical or ancillary medical support; however, infection complicating dry gangrene (i.e., wet gangrene), especially in a diabetic patient, is a limb- and life-threatening emergency. Control of sepsis can sometimes be achieved with antibiotic therapy plus limited debridement and drainage, or it may require radical excision and debridement; antibiotic therapy alone is seldom adequate treatment for gangrene complicated by infection. Failure to institute prompt therapy, especially in diabetic patients, results in rapid ascension of the infectious process, the loss of potentially salvageable tissue, and a large increase in patient mortality. As noted earlier, in patients with systemic toxicity, cryoamputation before definitive surgical amputation helps decrease mortality.
The first step in the management of infected or wet gangrene is identification of the infecting organisms. Usually, the urgency of the clinical situation does not allow the identification of specific organisms by culture, although culture should be obtained and submitted for both anaerobic and aerobic organisms. It has been my practice to assume that gangrene complicated by infection includes a mixture of aerobic and anaerobic organisms; therefore broad-spectrum bactericidal antibiotic coverage is used.
Once antibiotic therapy has been started, the patient's status must be monitored carefully, including pulse rate, white blood cell count, blood pressure, temperature, extent of infection, severity of pain, and diffuse signs of systemic toxicity such as lethargy, hallucinations, and general mental status. In a diabetic patient, serum glucose and insulin requirements are additional useful monitoring parameters. If a prompt response to antibiotic therapy is noted, therapy should be continued for maximum effect and a definitive amputation then performed. If the patient does not respond promptly to antibiotic management or if undrained purulent material was initially evident, debridement of gangrenous tissue and establishment of drainage should be employed as an early adjunct to antibiotic therapy.
If the gangrenous infection extensively involves the foot and contaminates tendon and tissue spaces, especially in a diabetic patient, radical debridement should be performed. One of the better methods to obtain radical debridement and establish open drainage is a guillotine amputation of the foot carried out at the level of the malleoli ( Fig. 64.1 ). Guillotine amputation eliminates the septic focus and allows drainage of contaminated lymphatics and tissue spaces in the lower leg. The presence of cellulitis and lymphangitis at the level of ankle guillotine is not a contraindication to this type of debridement technique. After performance of a guillotine-type amputation for debridement, systemic antibiotics are continued until the definitive amputation is performed, usually 3 to 5 days later. Definitive amputation is performed after ascertaining that the infection has been controlled and the patient's preoperative status has been optimized.

The incidence of stump infection in patients who present with a septic foot is decreased if a preparatory ankle guillotine amputation is performed before definitive amputation. In my experience, such a two-stage surgical approach decreased the stump infection rate from 22% to 3% ( P = .01). This approach was reaffirmed in a prospective, randomized study by Fischer and colleagues, in which the incidence of stump infection was decreased from 21% (one stage) to 0% (two stage) ( P = .05).
Patients undergoing traumatic lower extremity amputation tend to be young and in good medical health, so patient evaluation and preparation for amputation are easier than that described for geriatric patients with peripheral vascular disease and diabetes. As a general principle, formal traumatic amputation is performed to achieve healing at the lowest amputation level possible, and amputation level selection is usually predetermined by the injury. Adequate debridement of dead tissue is mandatory. The presence of a large amount of marginally viable tissue or potentially infected tissue may be an indication for debridement and open amputation followed at a later date by formal amputation with skin closure. A special effort should be made to rule out proximal bony or ligamentous injuries. Careful attention must be paid to other potentially life- or limb-threatening injuries, and management of such injuries must be handled in an appropriate sequence to achieve the best overall result for the patient. The use of a proximal extremity tourniquet for control of bleeding is optional but should be discouraged in a dysvascular patient. The use of drains after traumatic amputation is controversial, although there is some evidence that drainage results in a more rapid resolution of postamputation stump edema. If drains are used, they should be the closed-system type (not open Penrose drains).
The objective of preoperative amputation level selection is to determine the most distal amputation site that will heal. The general requirements are as follows: (1) the amputation must remove all necrotic, painful, or infected tissue; (2) the amputation stump must be able to be fitted with a functional and easily applied prosthesis; and (3) the blood supply at the level of the proposed amputation must be sufficient to allow primary skin healing. Appropriate selection of the amputation level is critical. If the surgeon elects too proximal an amputation, such as a midthigh amputation, the patient may be deprived of the opportunity for ambulation and rehabilitation, although the amputation might heal without difficulty. If a distal amputation site is selected and the blood supply is inadequate for amputation healing, further surgery will be required for amputation at a higher level. This latter approach may result in increased morbidity and mortality and may ultimately result in rehabilitation failure.
The inherent advantages of a below-knee amputation as opposed to an above-knee amputation should be obvious. It is easier to ambulate on a below-knee prosthesis; this is extremely important, especially in geriatric patients. In general, a unilateral below-knee amputee requires a 10% to 40% increase in energy expenditure for ambulation compared with the energy required for walking with an intact extremity. In contrast, a unilateral above-knee amputee (using a prosthesis with a locked or unlocked knee) requires approximately a 50% to 70% increase in energy expenditure. Crutch walking without a lower extremity prosthesis uses approximately 60% more energy, whereas wheelchair use necessitates only a small increase in energy expenditure (9%). Patients with severe coronary artery disease or severe chronic obstructive pulmonary disease may be physically unable to provide the additional energy required for ambulation on an above-knee compared with a below-knee prosthesis.
It is usually possible for the surgeon to decide on an amputation level that will remove necrotic, painful, or infected tissue as well as to plan an amputation stump that can be fitted with a prosthesis. However, the decision regarding the adequacy of blood supply at the proposed amputation level is one of the most difficult problems facing the amputation surgeon.
The earliest attempts at amputation level selection used the presence of pulses in the affected extremity, skin temperature, correlation of arteriographic findings, and “clinical judgment.” It has been well documented that none of these selection techniques has a consistent enough correlation with amputation healing to provide a sound basis for clinical decision making. In a study by Robbs and Ray, the morbidity, mortality, and rates of healing of lower limb amputations in 214 patients, wherein the amputation level was determined by nonobjective criteria, were retrospectively analyzed. Six of 67 (8.9%) primary above-knee amputations and 37 of 147 (25%) primary below-knee amputations had to be revised to a higher level. The authors concluded “that flap viability could not be predicted by the extent of ischemic lesion in relation to the ankle joint, the popliteal pulse status, or lower limb angiography.” In a 1992 study comparing clinical parameters and skin perfusion pressure for amputation level selection, Dwars and coworkers noted that the presence of palpable pulses immediately above the selected level correlated well with primary healing. However, the absence of palpable pulses and angiographic patency scores were of no clinical value in amputation level selection. Golbranson and colleagues presented promising data on improved methods of skin temperature measurement that had a high degree of accuracy (90%) for selecting below- versus above-knee amputation levels. In addition, Spence and Walker demonstrated a clear correlation between three different temperature isotherms (1.8°C separation) and isotopically derived skin blood flow ( P < .001). Stoner and associates reported that when the ratio of temperatures at the posterior and anterior incision sites (Burgess posterior flap below-knee amputation) was greater than 0.98, healing was improved. In a study comparing several modalities for amputation level selection, Wagner and colleagues noted (for above- and below-knee amputations) that the average skin temperature at the amputation site was higher (34.3°C) in patients who healed primarily compared with those who required operative stump revision (33.3°C) ( P = .001). One physical finding that has some value in differentiating proposed amputation levels is the presence of dependent rubor. Skin that develops dependent rubor is clearly ischemic and thus, like gangrenous tissue, is an absolute contraindication to amputation at that level; however, the absence of dependent rubor does not necessarily ensure healing ability. Early workers in the field of amputation surgery solved the problem of level selection by performing above-knee amputations on almost all patients. In the 1960s, Lim and coworkers demonstrated that 83% of all patients requiring a lower extremity amputation would heal following a below-knee amputation. However, using empirical below-knee amputation selection may be detrimental to some patients who might have healed following a more distal amputation, such as a transmetatarsal or Syme amputation. In addition, identifying the 20% to 30% of patients in whom a below-knee amputation is doomed to failure would be advantageous so that either a knee disarticulation or an above-knee amputation could be performed primarily, saving the patient additional surgical procedures.
The need for more sensitive and objective methods for the preoperative selection of amputation level led to the development of numerous noninvasive techniques, including Doppler ankle and calf systolic blood pressure determinations, with or without pulse-volume recordings ; xenon 133 ( 133 Xe) skin blood flow studies ; digital or transmetatarsal photoplethysmographic pressures ; transcutaneous oxygen determination ; skin fluorescence after intravenous fluorescein dye ; laser Doppler skin blood flow measurements (F.A. Matsen, personal communication, 1978); pertechnetate skin blood pressure studies ; and photoelectrically measured skin color changes.
An overview of the various criteria for predicting the healing of digit and forefoot amputations is shown in Table 64.2 . Totals from Table 64.2 suggest that preoperative selection techniques correctly predicted primary healing in 174 of 189 toe and forefoot amputations (97%). Similar data for below-knee amputation levels are summarized in Table 64.3 . Excluding empirical below-knee selection, the tests listed in Table 64.3 correctly predicted primary healing of below-knee amputations in 560 of 603 elective amputations (93%). Clearly, objective amputation level selection can not only predict potential healing of a more distal level of amputation but also accurately assess the likelihood of healing of a below-knee compared with an above-knee amputation. It is my opinion that elective lower extremity amputation should not be performed without some type of preoperative testing to ensure primary healing of the most distal amputation possible.
| Criterion | Reference | Amputation Level | Number of Healing Patients (%) |
|---|---|---|---|
| Doppler Ankle Systolic Pressure | |||
| 70 mm Hg | Forefoot | 38/44 (86) | |
| 116 mm Hg | Digit/forefoot | 25/27 (93) | |
| 35 mm Hg | Digit | 44/46 (96) | |
| Fiberoptic fluorometry DFI >44 | Foot/forefoot | 18/20 (90) | |
| Photoplethysmographic digit or TMA pressure 20 mm Hg | Digit | 20/20 (100) | |
| Xenon skin clearance >2.6 mL/100 g tissue/min | Digit/forefoot | 25/28 (89) | |
| Transcutaneous P o 2 >20 mm Hg | Forefoot | 4/4 (100) | |
| Total | 174/189 (92) | ||
| Criterion | Reference | Number of Healing Patients (%) |
|---|---|---|
| Doppler systolic ankle pressure 30 mm Hg + calf pressure 65 mm Hg + pulsatile PVR | 27/27 (100) | |
| Doppler systolic calf pressure 70 mm Hg | 32/32 (100) | |
| Doppler systolic thigh pressure 80 mm Hg or calf pressure 50 mm Hg | 36/36 (100) | |
| Empirical below-knee | 38/46 (83) | |
| Fiber-optic fluorometry DFI >44 | 12/12 (100) | |
| Fluorescein dye | 24/30 (80) | |
| 99m Tc-pertechnetate skin blood pressure | 24/26 (92) | |
| Laser-Doppler velocimetry >20 mV | 25/26 (96) | |
| Photoelectric skin pressure 20–100 mm Hg | 60/71 (85) | |
| Transcutaneous P o 2 >10 mm Hg or >10 mm Hg increase on 100% O 2 | 76/80 (95) | |
| >35 mm Hg | 51/51 (100) | |
| >20 mm Hg | 16/16 (100) | |
| >0 to <40 mm Hg | 17/19 (89) | |
| 0 mm Hg | 0/3 (0) a | |
| Index >0.59 | 17/17 (100) | |
| Index >0.20 | 33/34 (97) | |
| Xenon skin clearance = 3.1 mL blood flow/100 g tissue/min | 23/26 (88) | |
| >2.6 mL blood flow/100 g tissue/min | 35/36 (97) | |
| Epicutaneous >0.9 mL/100 g tissue/min | 14/15 (93) | |
| Total | 560/603 (93) |
The techniques for the use of ankle, calf, and popliteal Doppler systolic blood pressure determinations have been well described and are not covered in this chapter. Similarly, use of the photoplethysmograph for determining digital and transmetatarsal blood pressures has been well described by Schwartz and coworkers and is not presented here. The potential advantages of both the Doppler instrument and the photoplethysmograph are that they are relatively simple, inexpensive, and totally noninvasive. The problem with these instruments is that the presence of a blood pressure less than a predetermined level does not necessarily guarantee failure of amputation healing at that level (negative predictive value). This problem was nicely summarized by Verta and colleagues, who noted that “for forefoot amputation a high Doppler ankle pressure did not guarantee successful healing and a low ankle pressure did not contraindicate primary healing.” In an effort to increase the accuracy of Doppler ankle pressures, both Gibbons and coworkers and Raines and associates suggested the ancillary use of pulse-volume recordings. Although Raines's group reported 100% successful healing in 27 below-knee amputations in which the Doppler systolic ankle pressure was greater than 30 mm Hg, calf pressure was greater than 65 mm Hg, and there was a pulsatile pulse-volume recording in the foot, Gibbons's group was unable to duplicate these results and concluded, “we find no consistent criteria which are more accurate and reliable than clinical judgment and no ankle pressure above which primary healing was guaranteed.” Gibbons and coworkers also noted decreased accuracy in amputation level prediction using pulse-volume recording and Doppler ankle systolic pressures in patients with diabetes mellitus. The problem with diabetic patients (falsely high systolic pressure measurements) is likely due to medical calcinosis of their vessels. Wagner and colleagues reported that Doppler pressures at the thigh, popliteal, midcalf, or ankle level were unreliable in predicting healing of a below-knee amputation.
Theoretically, the measurement of skin fluorescence with a Wood's ultraviolet lamp after intravenous injection of fluorescein dye (Funduscein) should be a reliable test for amputation level selection. Although this technique is somewhat more invasive than Doppler ankle systolic pressure measurements or pulse-volume recordings, it is less complicated and less invasive than 133 Xe skin blood flow or pertechnetate skin perfusion measurements. The commercial availability of two new types of fluorometers (Fiberoptic Perfusion Fluorometer, Diversatronics, Broomall, PA; Fluoroscan, V. Elings, PhD, University of California, Santa Barbara), which can provide objective numeric readings quickly and in the absence of a Wood's lamp, may further enhance the use of this technique. Development of a computerized video camera system to analyze skin perfusion after oral ingestion of fluorescein obviates the risk of intravenous injection and allows easy data manipulation for limb mapping. Such a system has been under study at Maricopa Medical Center in Phoenix, Arizona. McFarland and Lawrence reported an accuracy rate of 80% for skin fluorescence, compared with 47% for Doppler popliteal systolic blood pressure (50 mm Hg) for the prediction of healing of a below-knee amputation (see Table 64.3 ). In addition, when skin fluorescence and Doppler pressure did not agree on the level of amputation, fluorescein always predicted a more distal level. Silverman and associates, in 1985, reported their data on fiber-optic fluorometry for amputation level selection at the below-knee, below-ankle, and above-knee levels in dysvascular limbs. The overall success rate was 92% (36 of 39), and individual rates were 18 of 20 below-ankle (90%), 12 of 12 below-knee (100%), and 6 of 7 above-knee (86%) amputations. Discriminate analysis demonstrated an optimal reference point between healing and nonhealing amputations, and a dye fluorescence index of greater than 44 had 93% accuracy. Two later studies, however, did not demonstrate such promising results. In a blinded, prospective review of 56 patients undergoing below-knee amputation, objective measurement of fluorescein perfusion did not correlate with amputation healing. In a study comparing multiple methods of amputation level selection, Wagner and colleagues found that qualitative skin fluorescence was not as successful as cutaneous oxygen measurement.
Promising work with a modified Clark-type oxygen electrode (with a heating element and thermostat for temperature control; Transoxode, Hellige-Orager, FRG; US manufacturer, Litton Industries [Woodland Hills, CA]) for amputation level selection has been reported by several groups. Franzeck and colleagues reported that the mean transcutaneous partial pressure of oxygen (P o 2 ) values of patients who healed primarily compared with those who failed to heal were 36.5 ± 17.5 mm Hg and less than 0.3 mm Hg, respectively. However, in those patients with a transcutaneous P o 2 less than 10 mm Hg, six of nine failed to heal, and three of nine healed primarily. In a study on below-knee amputations, Burgess and coworkers found that 15 of 15 amputations healed primarily if the transcutaneous P o 2 was greater than 40 mm Hg, 17 of 19 healed if the transcutaneous P o 2 was greater than 0 mm Hg but less than 40 mm Hg, and none of the three amputations with a P o 2 of 0 mm Hg healed. Katsamouris and coworkers reported that 17 of 17 lower extremity amputations healed if the P o 2 was greater than 38 mm Hg or if the P o 2 index (chest wall control site) was greater than 0.59. Ratliff and colleagues noted that 18 below-knee amputations healed if the P o 2 was greater than 35 mm Hg, while 10 of 15 failed if the P o 2 was less than 35 mm Hg. Kram and associates noted success in 33 of 34 (97%) below-knee amputations with multisensor transcutaneous oxygen mapping when the critical P o 2 index was greater than 0.20. In addition, all six patients with an index less than 0.20 failed to heal. All investigators have reported some amputations that healed in patients with low P o 2 values. A partial explanation for this observation might be the nonlinear relationship between P o 2 and cutaneous blood flow. In a careful study, Matsen and coworkers reported that P o 2 measurements are most dependent on arteriovenous gradients and cutaneous vascular resistance. Techniques to improve the accuracy of transcutaneous P o 2 probes include local heating (to 44°C, which minimizes local vascular resistance and makes P o 2 more linear with respect to cutaneous blood flow), measurements before and after oxygen administration, oxygen isobar extremity mapping, and transcutaneous oxygen recovery half-time. Oishi and associates noted—in a study comparing skin temperature, Doppler pressure, and transcutaneous oxygen—that after the inhalation of oxygen, if the P o 2 increased 10 mm Hg or more, the P o 2 predicted amputation healing with a sensitivity of 98%. In another study, the authors prospectively compared the following tests for their accuracy in amputation level selection: transcutaneous oxygen, transcutaneous carbon dioxide, ratio of transcutaneous oxygen to transcutaneous carbon dioxide, foot-to-chest transcutaneous oxygen, intradermal 133 Xe, ankle-brachial index, and absolute popliteal artery pressure. All metabolic parameters had a high degree of statistical accuracy in predicting amputation healing, whereas none of the other tests had statistical reliability. All amputations—transmetatarsal, below-knee, and above-knee—healed primarily if the transcutaneous P o 2 level was greater than 20 mm Hg, and there was a 0% incidence of false-positive and false-negative studies. Most authors of transcutaneous oxygen testing studies suggest using a cutoff point of 35 to 40 mm Hg. I have used 20 mm Hg with excellent results. Recent data reconfirm the accuracy of a threshold of 20 mm Hg, especially in distal limb amputations. Also of importance is the observation that amputation site healing is not affected by the presence of diabetes mellitus, nor are the test results for any of the metabolic parameters. Similar data have been reported by Bacharach and colleagues, who stated that 51 of 52 limbs (98%) healed (primary and delayed) with a P o 2 greater than or equal to 40 mm Hg, whereas a P o 2 of less than 20 mm Hg was associated with universal failure. In that study, P o 2 measurements during limb elevation improved the predictability of outcome for patients with supine P o 2 values greater than 20 mm Hg but less than 40 mm Hg.
Theoretically, laser-Doppler velocimetry should be an ideal tool for skin blood flow determination; it is noninvasive and “measures” capillary blood flow (good correlation between laser-Doppler blood flow measurements using microspheres, electromagnetic flow probes, and 133 Xe clearance). However, data by Holloway and Burgess, Holloway and Watkins, Holloway, and Matsen (personal communication, 1978) suggest that although there is a linear relationship among techniques, there is a fair amount of variance. These groups noted that the use of local skin heating may enhance the accuracy of the laser-Doppler and make it a more valuable adjunct for amputation level selection. Holloway and Burgess reported their experience with laser-Doppler velocimetry in 20 lower extremity amputations at the foot, forefoot, below-knee, and above-knee levels, and the accuracy rates were as follows: foot and forefoot, two of six (33%); below-knee, eight of eight (100%); and above-knee, six of six (100%).
Malone and coworkers' and Moore's greatest postsurgical experience was with the use of 133 Xe skin clearance for amputation level selection. These techniques have been well described by Moore, Daly and Henry, and Malone and associates. One of the major difficulties with the application of 133 Xe skin clearance for amputation level selection is its reproducibility by other investigators. In an earlier publication, Holloway and Burgess were unable to document a clear-cut end point above which all amputations healed. In contrast, Silberstein and colleagues reported that 38 of 39 patients (11 above-knee amputations, 18 below-knee or transmetatarsal amputations, and 9 no amputation) healed when 133 Xe skin blood flow was greater than 2.4 mL/100 g tissue per minute; when flow was less than 2.4 mL/100 g tissue per minute, only four of seven patients healed. One significant advantage of 133 Xe clearance techniques that may offset both of these problems, if its ultimate reliability is demonstrated in other centers, is its potential ability to predict healing at all levels of lower extremity amputation.
A final problem with the intradermal use of 133 Xe for skin blood flow measurements is that the manufacturer no longer supplies 133 Xe. The product must be made by nuclear medicine departments. This limitation may further preclude widespread use of the intradermal 133 Xe technique. Finally, despite past publications and excellent results, I no longer use 133 Xe skin clearance for amputation level selection. In part, this change was made because of the enumerated difficulties; however, the major reason for this change was a study wherein 133 Xe was not found to be statistically reliable as a selection method for amputation level. (As noted previously, transcutaneous oxygen is very reliable.)
Using the disappearance of intradermal technetium 99m pertechnetate, 131 I-sodium, 131 I-antipyrine, or 133 Xe in the presence of external pressure, Holstein and Holstein and Lassen reported amputation level selection data comparable to data reported by Moore, Daly, Henry, Malone, and others. Because 133 Xe is trapped in subcutaneous fat, there are solid theoretical reasons to use an isotope other than 133 Xe. Holstein and associates found no significant difference among 131 I-sodium, 131 I-antipyrine, and 99m Tc-pertechnetate for the measurement of skin perfusion pressure.
Stockel and coworkers and Ovesen and Stockel reported preliminary data on the use of a photodetector and plethysmography (Medimatic, Copenhagen) for amputation level selection; these findings correlate well with the 133 Xe skin perfusion pressure techniques of Holstein and colleagues. This technique uses a blood pressure cuff placed over a photoelectric detector, which is connected to a plethysmograph, to measure the minimal external pressure required to prevent skin reddening after blanching. To date, 66 of 71 (93%) below-knee amputations healed with skin pressures between 20 and 100 mm Hg. In 1992, Dwars and associates reported that skin perfusion pressure measurements were of excellent predictive value for the healing of lower extremity amputations (positive predictive value, 89%; negative predictive value, 99%).
In summary, it is my opinion that elective lower extremity amputation should not be performed in the absence of objective testing to determine the most distal amputation that will heal primarily, yet allow the removal of infected, painful, or ischemic tissue. A variety of techniques are available, and the technique chosen depends on the available equipment, the amputation level under consideration, and the current accuracy rates for the reported techniques. However, in my opinion, the most reliable, easiest to use, and best overall technique for prospective amputation level selection is transcutaneous oxygen testing.
This section discusses only those amputation levels that are relevant to patients with peripheral vascular disease or diabetes mellitus. Amputation levels that are less desirable from the standpoint of healing or rehabilitation or those that present specific prosthetic fitting problems are omitted. In my experience and that of others, Chopart, Lisfranc, and Boyd forefoot amputations have been fraught with controversy because of healing problems, prosthetic fitting problems, and equinus deformities. Because these amputation levels are occasionally used by vascular surgeons, they are reviewed here only briefly.
Toe amputation is the most frequently performed peripheral amputation. It is especially common in patients with diabetes mellitus, who are prone to lesions (ulceration, osteomyelitis, gangrene) that necessitate amputation.
Patients who present with dry gangrene allow the surgeon a choice between direct surgical intervention and autoamputation. In the absence of supervening infection or pain, expectant management permits epithelialization to take place under the dry gangrenous eschar. As soon as epithelialization is complete, the toe will drop off, leaving a cleanly healed stump. Autoamputation is preferable to direct surgical intervention because it obviates the need for healing after amputation and probably results in a more distal site of healing than would be achieved with surgical intervention. However, this process often requires months before it is complete.
Gangrene, infection, neuropathic ulceration, or osteomyelitis should be confined to the midphalanx or distal phalanx. There must be no dependent rubor, and venous filling time should be less than 20 to 25 seconds. Sizer and Wheelock demonstrated that the presence of pedal pulses, even in patients with diabetes, is associated with a very high rate of healing after toe amputation (98%).
Cellulitis proximal to the area of proposed toe excision, the presence of dependent rubor, forefoot infection, and involvement of the metatarsophalangeal joint or (distal) metatarsal head all represent specific contraindications to toe amputation.
A single toe should never be amputated by disarticulation but should be transected through the proximal phalanx, leaving a small button of bone to protect the metatarsal head. Skin flaps can be of any design, as long as they obey basic surgical principles and have an adequate base for the length of flap. The flaps can be fish-mouth, plantar base, dorsal base, side to side, or any variation or combination; however, they must be long enough to close without tension. The most commonly used incision is circular ( Fig. 64.2 ). Amputation through the metatarsophalangeal joint or an interphalangeal joint should be avoided because of the avascular nature of cartilage and the likelihood of supervening infection or failure to heal.
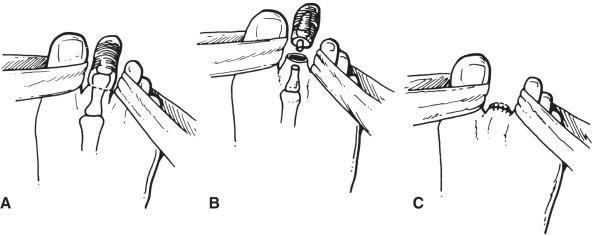
Careful atraumatic edge-to-edge skin closure without the use of forceps maximizes the chances of primary healing. Suture material that produces minimal reaction when left in place for long periods should be used, such as monofilament wire or plastic. A soft postoperative dressing that provides gentle wound compression should be applied.
Chronic osteomyelitis of the great toe without gangrene in a diabetic patient presents a difficult surgical problem. Because complete healing is not common, and total resection of the great toe results in some imbalance in walking (which can be accommodated with proper shoe orthotics), debridement and resection of the infected phalanges through a medial or lateral incision, leaving a soft tissue toe remnant in place, are probably best from a functional standpoint.
The primary advantage of toe amputation is the lack of requirement for prosthetic rehabilitation and the fact that minimal tissue is excised.
Except for the risk of nonhealing or secondary infection and stump breakdown, requiring a higher level of amputation, there are no disadvantages to this level of amputation.
Rehabilitation potential is 100%. However, the performance of a toe amputation in a patient with peripheral vascular disease, especially with concomitant diabetes, is an ominous sign with regard to long-term prognosis. Little and coworkers found that by 3.5 years after toe amputation, almost three fourths of their patients required a more proximal major amputation.
If the gangrenous skin or infectious process approaches the metatarsophalanged crease or includes the (distal) metatarsal head, this precludes a toe amputation. A conservative partial distal forefoot amputation can still be performed by extending the toe amputation to include the distal metatarsal shaft and head.
Gangrene, infection, cellulitis, and dependent rubor involving skin proximal to the metatarsophalangeal crease are contraindications to ray amputation. In addition, involvement of multiple toes is a relative contraindication, because a transmetatarsal amputation would be a more suitable surgical procedure. Ray amputation for gangrene or infection of the great toe also is a relative contraindication, because removal of the first metatarsal head leads to unstable weight bearing and difficulties with ambulation; however, with proper shoe orthotics, ray amputation of the first or great toe results in excellent foot salvage and provides patients with a stable gait pattern.
The incision begins vertically on the dorsum of the foot, bifurcates laterally and medially to encircle the toe, meets on the plantar aspect of the foot, and extends for a variable distance on the plantar aspect of the foot. The plantar incision is extended proximally as needed to allow removal of the toe and distal metatarsal head. Care should be taken not to injure the digital arteries or nerves adjacent to the metatarsal bone and not to enter into the deep tension or joint spaces of the medial and lateral toes. The distal metatarsal shaft is divided at its neck, and soft tissues are removed by sharp dissection. The surgical specimen consists of the toe, metatarsophalangeal joint, and distal portion of the metatarsal shaft and head. If possible, the surgical specimen should be removed in continuity. The metatarsal shaft must be transected in an area of normal bone. “Soft bone” suggests osteomyelitis, especially in diabetic patients, and mandates higher (i.e., more proximal) bone division.
I recommend that the surgical wound be generously irrigated with an antibiotic solution (the content of which is based on preoperative cultures, if available). Once again, attention is paid to meticulous hemostasis and atraumatic deep tissue and skin closure. Interrupted monofilament sutures that achieve edge-to-edge skin coaptation (without the use of forceps) should be placed ( Fig. 64.3 ). The postoperative dressing can be either a soft dressing with an outer elastic wrap (which allows compression of the forefoot and removes tension from the suture line) or a combination of a soft dressing with foot and lower leg plaster cast (which provides maximum skin and wound protection). In the event that adequate hemostasis cannot be obtained, the use of a drain is suggested. In the presence of infection in either the metatarsophalangeal joint or skin flaps, consideration should be given to leaving the wound open and doing a delayed primary closure or allowing secondary healing.
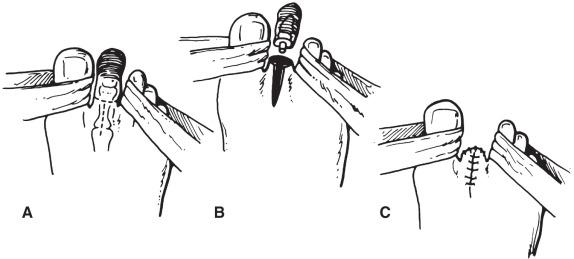
This relatively conservative amputation results in minimal cosmetic deformity and maximum (100%) rehabilitation potential. There are no prosthetics required; however, ray resection of the first metatarsal head causes some walking imbalance, and the foot should be fitted with a specially constructed shoe to minimize foot trauma and improve ambulatory balance.
There are no disadvantages, except for the risk of hematoma formation, nonhealing, secondary infection, or chronic osteomyelitis of the remaining metatarsal shaft.
The indication for transmetatarsal amputation is gangrene or infection involving several toes or the great toe (on the same foot). This amputation may also be used if the gangrenous or infectious process extends a small distance on the dorsal skin past the metatarsophalangeal crease (but not up to the distal third or midthird junction of the forefoot), provided that the plantar skin is uncompromised.
Deep forefoot infection, cellulitis, lymphangitis, or dependent rubor involving the dorsal forefoot proximal to the metatarsophalangeal crease all represent contraindications to amputation at this level. In addition, gangrenous changes on the plantar skin of the foot, even those extending only a small distance past the metatarsophalangeal crease, is a specific contraindication to amputation at this level. Foot pulses are not necessary for healing, and venous refill should probably be less than 25 seconds.
An excellent description of the technique for transmetatarsal amputation was presented by McKittrick and associates in 1949. A skin incision is designed that uses a total plantar flap. A slightly curved dorsal incision is carried from side to side of the foot at the level of the midmetatarsal shafts. The incision extends to the base of the toes medially and laterally in the midplane axis of the foot and then across the plantar surface at the metatarsophalangeal crease. It is important to place the dorsal skin incision slightly distal to the anticipated line of bone division. The dorsal skin incision is carried down to the metatarsal bones, and each metatarsal shaft is transected with an air-driven oscillating saw approximately 4 mm to 1 cm proximal to the skin incision ( Fig. 64.4 ).
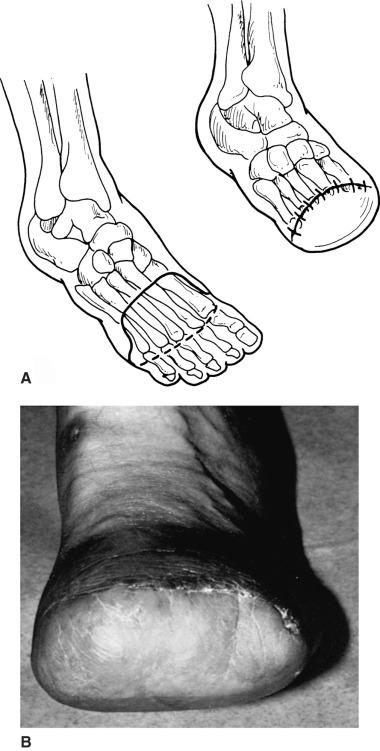
The plantar tissues in the distal forefoot are separated from the metatarsal shafts with a scalpel. The tissues of the plantar flap are thinned sharply, excising exposed tendons and leaving the underlying musculature attached to the skin flap. The plantar flap is then rotated dorsally for closure. Further tailoring or thinning of the plantar flap may be necessary to achieve good skin coaptation.
The importance of attention to absolute hemostasis cannot be overemphasized. A simple closure is performed, consisting of a deep layer of absorbable interrupted sutures and skin closure with a monofilament suture using a vertical mattress technique. Once again, careful approximation of skin edges is important, and I recommend not using forceps on the skin.
If adequate hemostasis cannot be readily achieved, use of a closed drainage system is suggested. Bone wax should not be used to control bleeding from the metatarsal shafts; the use of electrocautery to achieve hemostasis is preferable.
A well-padded short leg plaster cast is the best postoperative dressing because it controls edema and prevents stump trauma. I do not advise early ambulation after transmetatarsal amputation because of problems with flap necrosis and stump healing. If wound healing is satisfactory at the first cast change (7 to 10 days after surgery), a rubber heel may be incorporated into the second cast for ambulation. Subsequent casts are changed when they become loose, generally every 7 to 14 days, and a rigid dressing is used until the transmetatarsal flap is well healed, usually 3 to 4 weeks after surgery.
Transmetatarsal amputation provides an excellent result compared with more proximal foot or lower extremity amputation. Disability is minimal, and the prosthetic requirements are relatively simple.
The primary disadvantages of a transmetatarsal amputation are the risks of nonhealing, infection, and hematoma formation and the necessity for a secondary higher-level amputation.
To achieve maximum ambulation potential, some minor prosthetic modification should be considered. A shoe that incorporates a steel shank in the sole allows normal toe-off during ambulation. The spring steel shank reproduces the action of the longitudinal arch of the foot during ambulation. A custom-molded foam pad or lamb's wool can be used to fill the toe portion of the shoe. An alternative approach is to use a custom-molded shoe with a roller-shaped sole to provide toe-off motion during walking.
There are relatively few, if any, limitations in rehabilitation for a transmetatarsal amputation. With proper shoe modification, there should be no discernible physical disability for a transmetatarsal amputee during ambulation. It is important, however, that the shoe or other prosthetic device be properly constructed to avoid stump ulceration and breakdown. There are increased numbers of anecdotal reports combining guillotine forefoot amputation with secondary distal split-thickness skin graft to achieve successful healing at this amputation level. Although this latter technique allows the salvage of more proximal transmetatarsal amputations, I do not favor its use because of frequent problems with distal stump (skin graft) breakdown in active patients.
Some reports have called attention to foot-sparing amputations when a transmetatarsal amputation is precluded because of the extent of ischemia or infection. The Lisfranc amputation is a tarsometatarsal joint amputation, and the Chopart amputation is a midtarsal joint amputation. I agree with Chang and coworkers that both of these midforefoot amputations are easier to perform than a Syme amputation and may improve long-term ambulation.
Both Lisfranc and Chopart amputations result in the development of equinovarus deformity and require lengthening of the Achilles tendon to achieve maximum rehabilitation potential. In addition, Hirsch and colleagues documented force plate data showing that an abnormal pattern characterized by reduced stance duration and deficient forward propulsion on the amputated side was greater in a Chopart's prosthesis than in a transmetatarsal prosthesis. That study also documented stump problems as the principal difficulty with Chopart amputations over time.
Both Lisfranc (tarsometatarsal joint) and Chopart (midtarsal joint) amputations are well described in articles by Sanders and Chang and coworkers, to which interested readers are referred.
I agree with a modified version of the conclusion reached by Chang and coworkers that ischemic foot necrosis extending beyond the limits of conventional transmetatarsal amputation does not necessarily require a major limb amputation. With improvements in patient selection and surgical technique, Lisfranc and Chopart amputations are viable options when attempting to salvage mid- to hindfoot structures. From a prosthetic standpoint, fitting of these more distal and conservative amputation levels should emphasize unloading the distal part of the stump and smoothing out the impulsive force peak on the stump in late stance to minimize pain, decrease stump breakdown, and enhance ambulation capacity.
Syme first described this amputation in 1843. Then, as now, there were arguments over its merit. Harris (in Toronto) has championed Syme amputation and has written several excellent articles concerning its development and the surgical technique necessary for successful results. I believe that the Syme amputation is the most technically demanding lower extremity amputation, and attention to surgical detail is crucial for its success.
If the gangrenous or infectious process precludes transmetatarsal amputation, the next level to be considered is an ankle disarticulation, or Syme amputation.
If the gangrenous or infectious process involves the heel, if there are open lesions on the heel or about the ankle, if there is cellulitis or lymphangitis ascending up the distal leg, or if dependent rubor is present at the heel, Syme amputation is contraindicated. The presence of a neuropathic foot in a diabetic patient, when there is absence of heel sensation, is also a relative contraindication to Syme amputation. A high rate of primary healing demands the use of objective, noninvasive amputation level selection techniques before surgery and preservation of the posterior tibial artery (if patent).
The skin incision is placed to construct a posterior flap using the heel pad. The dorsal incision extends across the ankle from the tip of the medial malleolus to the tip of the lateral malleolus. The plantar incision begins at a 90-degree angle from the dorsal incision and progresses around the plantar aspect of the foot distal to the heel pad ( Fig. 64.5 ). The dorsal incision is deepened through subcutaneous tissues and carried down to bone without dissection in the tissue planes. The anterior tendons (tibialis anterior, extensor hallucis longus, and extensor digitorum longus) are pulled down into the wound, transected, and allowed to retract. The anterior tibial artery is identified, clamped, divided, and suture-ligated. The incision is then deepened, and the capsule of the tibial-talar joint is opened. The tibialis posterior tendon is divided, and the foot is forced into plantar flexion to provide increased visualization of the tibial-talar joint. Great care should be taken during medial dissection to preserve the posterior tibial artery. The joint is further dislocated by incising the posterior capsule. The peroneus brevis and tertius tendons are transected. The plantar aspect of the incision is deepened through all layers of the sole of the foot down to the neck of the calcaneus. The calcaneus is then carefully and sharply dissected from the heel pad. Dissection of the calcaneus is the most difficult part of the operation, and great care is needed to maintain the dissection on the bony surface of the calcaneus to prevent damage to the soft tissues of the heel, injury to the posterior tibial artery, and buttonholing of the posterior skin as the Achilles tendon is transected. Performance of Syme amputation by the one-stage and two-stage techniques is identical up to this point.
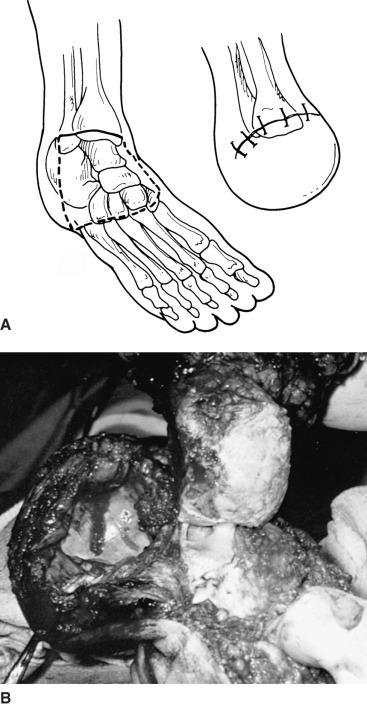
If the surgeon chooses the one-stage technique, the lateral and medial malleoli are transected flush with the articular surface of the tibial-talar joint with an air-driven reciprocating saw. Once again, the importance of hemostasis cannot be overemphasized. If adequate hemostasis cannot be achieved, a closed drainage system should be incorporated. Even in dry surgical wounds, the use of a drain is advocated by some authors. I prefer to irrigate the surgical wound with copious amounts of antibiotic solution before closure. The heel pad is rotated anteriorly and sutured to the proximal dorsal skin edge with a single layer of interrupted vertical mattress sutures. Once again, atraumatic placement of skin sutures is mandatory, and forceps should not be used on the skin edges.
If the two-stage technique is selected, the lateral and medial malleoli are not transected. A drain is placed, and the wound is closed as previously described. Approximately 6 weeks after performance of the first stage, the patient is returned to surgery for the second stage (which can be done under local anesthesia). Medial and lateral incisions are made over the dog ears on the amputation stump, and the incisions are carried down to bone with sharp dissection. The malleoli are removed flush with the ankle joint. The tibial articular cartilage is not disturbed. The distal tibia and fibula are exposed subperiosteally approximately 6 cm above the ankle joint, and the tibial and fibular flares are removed with an osteotome and a smooth rongeur. This last procedure produces a relatively square stump that simplifies postoperative prosthetic fitting and improves cosmesis. If the heel pad is loose after removal of the malleoli, it can be secured to the tibia and fibula through drill holes in the bones.
The postoperative dressing for a Syme amputation stump (for both one- and two-stage procedures) is extremely important; it is critical to maintain correct alignment of the heel pad over the end of the tibia and fibula during healing. Either a soft compression dressing or a rigid plaster cast can be used as a postoperative dressing; however, most authors prefer the application of a short leg plaster cast. If a cast is used, great care must be taken to avoid injury to the medial and lateral skin flaps (dog ears). Weight bearing should not occur during the early phases of healing of a Syme amputation because of the risks of nonhealing and flap necrosis. When the first cast is removed, usually 7 to 10 days after surgery, a second cast that incorporates a walking heel can be applied if healing is satisfactory. I prefer to keep Syme amputation patients nonambulatory for 3 weeks after amputation to allow good heel pad fixation and healing. After ambulation begins, the patients are kept in a short leg walking cast for an additional 3 to 4 weeks before construction of a temporary removable prosthesis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here