Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital long QT syndrome (LQTS), a leading cause of sudden death in the young, is characterized by a prolongation of the QT interval in the standard electrocardiogram (ECG) and by syncopal episodes, which often result in cardiac arrest (CA) and sudden death and usually occur under physical or emotional stress in otherwise healthy young individuals. The availability of very effective therapies for this otherwise highly lethal disorder among symptomatic and untreated patients makes the existence of symptomatic and undiagnosed patients unacceptable. LQTS represents a unique paradigm for genetically mediated sudden cardiac death (SCD) because it allows a tight correlation between genotype and phenotype and provides a direct bridge between molecular biology and clinical cardiology, with a direct impact on patient management. There are few cardiac diseases in which the progress made, both in its understanding and in its management, has surpassed that realized during the last 50 years for LQTS.
So far, 17 genes have been associated with LQTS ( Table 96.1 ), but the first three identified in 1995 and 1996 ( KCNQ1, KCNH2, and SCN5A ) remain responsible for the vast majority of genotype-positive cases. , Of note, the so-called long QT 4 (LQT4), LQT7 (Andersen-Tawil syndrome [ATS]; see Chapter 97 ), and LQT17 (triadin knockout syndrome) are complex clinical disorders in which a modest prolongation of the QT interval is only a secondary epiphenomenon and should not be regarded as part of LQTS. For other variants (LQT9 through LQT13), there are only preliminary descriptions. These 17 genes can be classified into three major groups: those reducing potassium outward currents, those increasing the sodium inward current, and those increasing the calcium inward current. The consistent outcome, however, is a prolongation of the action potential (AP) duration at the cellular level and of the QT interval on the standard ECG with an increased risk for life-threatening arrhythmias. Recently, an evidence-based reappraisal of these 17 genes proved that only a few had definite or strong evidence for their association with the various forms of LQTS (see Table 96.1 ).
| Gene | Syndrome | Frequency | Locus | Protein (Functional Effect) | Evidence for Association With LQTS |
|---|---|---|---|---|---|
| KCNQ1 (LQT1) | RWS, JLN | 40–55 | 11p15.5 | Ik (↓) | Definitive |
| KCNH2 (LQT2) | RWS | 30–45 | 7q35–36 | Ik (↓) | Definitive |
| SCN5A (LQT3) | RWS | 5–10 | 3p21-p24 | Na V 1.5 (↑) | Definitive |
| ANKB (LQT4) | RWS | <1% | 4q25-q27 | Na V 1.5 (↑) | Disputed |
| KCNE1 (LQT5) | RWS, JLN, aLQTS | <1% | 21q22.1 | Ik (↓) | Strong in JLN and aLQtS, limited in RWS |
| KCNE2 (LQT6) | RWS, aLQTS | <1% | 21q22.1 | Ik (↓) | Strong in aLQTS, disputed in RWS |
| KCNJ2 (LQT7) | AS | <1% | 17q23 | Kir2.1 (↓) | Definitive in AS, limited in RWS |
| CACNA1C (LQT8) | TS | <1% | 12p13.3 | L-type calcium channel (↑) | Definitive in TS, moderate in RWS |
| CAV3 (LQT9) | RWS | <1% | 3p25 | Na V 1.5 (↑) | Limited |
| SCN4B (LQT10) | RWS | <1% | 11q23.3 | Na V 1.5 (↑) | Disputed |
| AKAP9 (LQT11) | RWS | <1% | 7q21-q22 | Ik (↓) | Disputed |
| SNTA1 (LQT12) | RWS | <1% | 20q11.2 | Na V 1.5 (↑) | Disputed |
| KCNJ5 (LQT13) | RWS | <1% | 11q24 | Kir3.4 (↓) | Disputed |
| CALM1 (LQT14) | RWS | ? | 14q32.11 | L-type calcium channel (↑) | Definitive |
| CALM2 (LQT15) | RWS | ? | 2p21 | L-type calcium channel (↑) | Definitive |
| CALM3 (LQT16) | RWS | ? | 19q13.32 | L-type calcium channel (↑) | Definitive |
| TRDN (LQT17) | Recessive LQTS | ? | 6q22.31 | L-type calcium channel (↑) | Strong |
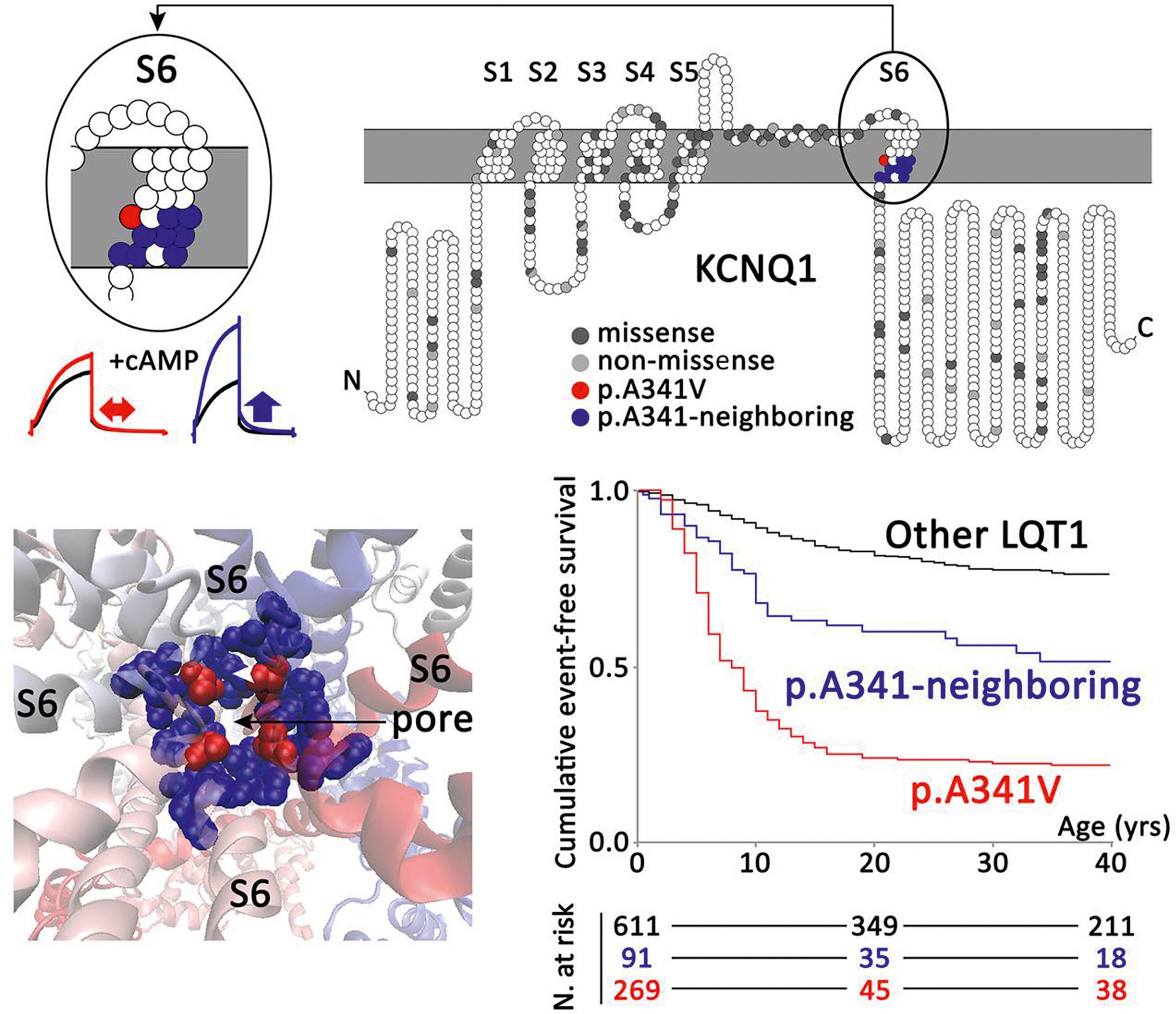
The delayed rectifier potassium current (I K ) is a major determinant of phase 3 of the cardiac AP. It includes two independent components: one rapid (I Kr ) and one slow (I Ks ). The KCNQ1 and KCNE1 genes encode, respectively, the α-KvLQT1 and β-MinK subunits of the potassium channel conducting the I Ks current. KCNQ1 mutations produce the LQT1 variant of LQTS, which is also the most prevalent.
Homozygous or compound heterozygous mutations in KCNQ1 and KCNE1 cause the recessive Jervell and Lange-Nielsen (JLN) variants of LQTS (JLN1 and JLN2, respectively). LQT5 is an uncommon variant (2%–3%) caused by mutations in the KCNE1 gene usually associated with low penetrance and a mild phenotype.
The KCNH2 and KCNE2 genes encode, respectively, the α-(hERG) and β-(MIRP) subunits of the potassium channel conducting the I Kr current and are responsible for the LQT2 and LQT6 variants of LQTS, respectively. LQT2 is the second most prevalent variant of LQTS, accounting for 35% to 40% of LQTS genotype-positive patients.
Mutations in these genes causing LQTS provoke a loss of I Ks or I Kr current through different mechanisms. Defective proteins may coassemble with wild-type protein and exert a dominant-negative effect. Other mutations lead to defective proteins that do not assemble with wild-type peptides, resulting in a loss of function that reduces the I Ks current by 50% or less (haploinsufficiency). Finally, defective peptides may not even reach the membrane of the cardiac cell because the mutations interfere with intracellular protein trafficking, this being the predominant mechanism in LQT2 mutations. Specifically, mutations in KCNH2 have been recently classified into four classes according to the biophysical property impaired: class 1, which involves disrupting the synthesis of K V 11.1 α-subunits; class 2, which involves causing a trafficking defect; and classes 3 and 4, which involve affecting K V 11.1 channel gating and permeation.
The I Ks current is augmented by sympathetic activation; heart rate increases and is essential for QT adaptation. If I Ks is defective, the QT interval will fail to shorten appropriately during tachycardia, thus creating a highly arrhythmogenic condition.
Other very rare genetic subtypes caused by mutations inducing a reduced potassium outward current are LQT11 (because of a mutation in the AKAP9 gene encoding for yotiao, a protein modulating I Ks current), and LQT13 (because of a mutation in the KCNJ5 gene, causing a reduced acetylcholine-dependent inward rectifier current [I KACh ]; see Table 96.1 ). , Andersen-Tawil (see Chapter 97 ) syndrome, caused by mutations in the KCNJ2 gene, was initially known as LQT7. Nevertheless, ATS should not be considered part of LQTS. It is a syndromic form characterized by periodic paralysis and distinctive dysmorphic facial or skeletal features, with prominent U waves but usually without a real QT prolongation. The arrhythmias of ATS range from premature ventricular beats (PVBs) to polymorphic/bidirectional ventricular tachycardia (VT); SCD could be somewhat more common than previously reported.
The SCN5A gene encodes the protein of the cardiac sodium channel. In vitro expression studies have shown that LQTS- SCN5A mutations produce the LQTS phenotype by inducing a gain of function, leading to an increase in the sodium inward current, which prolongs AP duration. The prevalence of LQT3 among genotype-positive LQTS patients is 10% to 15%. Other, less common genetic subtypes of LQTS are related to proteins that are part of the so-called sodium channel macromolecular complex. Specifically, mutations in ANK4 (LQT4), CAV3 (LQT9), SCN4B (LQT10), and SNTA1 (LQT12) have been associated with the disease, and they act by directly or indirectly increasing the sodium current (see Table 96.1 ). Mutations in SCN5A are also associated with overlapping phenotypes (LQTS, Brugada syndrome [see Chapter 95 ] and cardiac conduction defects [see Chapter 106 ]). The most common and best studied mutations associated with overlapping phenotypes are SCN5A -E1784K, 1795insD, ΔKPQ, and ΔK1500; it would appear that a negative shift of steady-state sodium channel inactivation and enhanced tonic block by class IC drugs represent the common biophysical mechanisms underlying the phenotypic overlap of LQT3 and Brugada syndrome.
Another peculiarity of sodium-related LQTS is that, despite being less frequent than potassium-related LQTS in adulthood, it constitutes the most common genetic form associated with LQTS-related perinatal mortality, including life-threatening arrhythmias in the first year of life, sudden infant death syndrome (SIDS), and sudden unexplained intrauterine fetal demise (IUFD; Fig. 96.1 ). , This opposite pattern between perinatal life and adulthood is likely because of a negative selection for SCN5A carriers in the perinatal and early infancy period.
The genetic variants identified on SCN5A in SIDS, IUFD, and neonatal LQT3 have different topologic distributions. Indeed, mutations located in the transmembrane and linker regions are significantly more often present in neonatal LQT3 cases than in SIDS/IUFD (86% vs. 22%; P = .002), whereas the variants located in the N-terminal and interdomain linkers (the regions where variants present in controls are more frequently observed) are found in 9.5% of neonatal LQT3 versus 44.4% of SIDS/IUFD ( P = .01). Furthermore, although none of the mutations identified in severe neonatal LQT3 cases is present in the publicly available control databases, 47% of functional variants associated with SIDS/IUFD are also observed in the general population. These considerations support the concept that severe neonatal LQT3 cases are caused by mutations able, per se, to cause a phenotype unlikely to be compatible with survival to adulthood. Indeed, the mutations identified in this subgroup of patients are mainly de novo. By contrast, the genetic variants identified in SIDS and IUFD cases have a wider range of clinical severity, and some of them, identified also in the general population, are probably not able to cause SCD, per se, although they can act as favoring factors. In further support of this view, we have observed that the SCN5A rare variants with a functional effect identified in SIDS and IUFD, also present in the publicly available databases, are significantly more prevalent in the SIDS/IUFD cohorts than in the general population (12 of 634 [1.9%] vs. 45 of 6261 [0.7%]; P = .0019).
In the heart, prolonged calcium current delays cardiomyocyte repolarization and increases the risk for arrhythmia. Five major genetic forms of LQTS linked to this mechanism have been described.
LQT8 is a rare variant characterized by marked QT interval prolongation, often presenting with 2:1 functional atrioventricular block, macroscopic T wave alternans, and syndactyly. LQT8 is highly malignant, and 10 (59%) of the 17 children reported by Splawski et al. died at a mean age of 2.5 years. Some children with Timothy syndrome (see Chapter 98 ) also had congenital heart diseases, immune deficiency, intermittent hypoglycemia, cognitive abnormalities, and autism.
Molecular screening identified a missense mutation (G406R) in the voltage-gated calcium channel gene ( CACNA1c ) in all probands analyzed. G406R produces sustained inward calcium currents by causing nearly complete loss of voltage-dependent inactivation. In an independent cohort of Timothy syndrome patients the G406R mutation was identified in 9 of the 17 patients, whereas the remaining carried the G402S, G402R, S405R, K1211E, or G1021R. Also, in this cohort the carriers had mainly the de novo mutation or paternal/maternal mosaicism. In a 5-year follow-up, 77% of patients were alive, mainly with a combination of antiarrhythmic agents, left cardiac sympathetic denervation (LCSD), and device therapy. In addition, classic LQTS cases have been associated with CACNA1C gain-of-function mutations (A28T, R860G, I1166T, I1166V, I1475M, and E1496K). Furthermore, the R518C and the R518H manifested with a complex phenotype including LQTS, hypertrophic cardiomyopathy (HCM), and congenital heart defects.
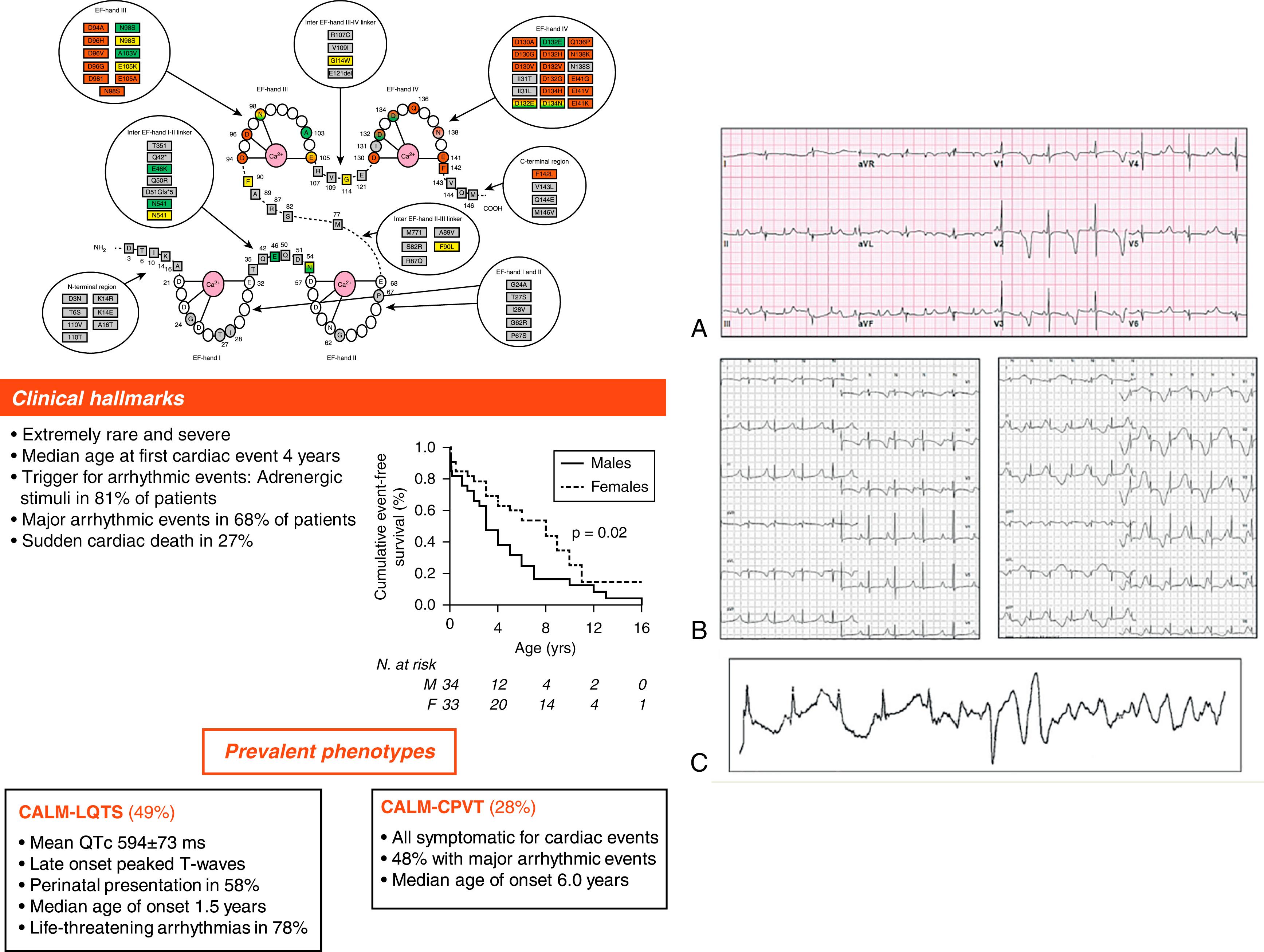
The small calcium-binding protein calmodulin (CaM) is an important modulator of different ion channels, such as the L-type calcium channel, the sodium channel, potassium channels, and ryanodine receptor. The evolutionary importance of this protein is highlighted by the presence of three different genes located in three different chromosomes (14q32.11, 2p21, and 19q13.32) encoding the same protein, a unique example in the human genome. Mutations in CaM genes can cause severe cases of LQTS, catecholaminergic polymorphic ventricular tachycardia (CPVT; see Chapter 91 ), or idiopathic ventricular fibrillation (IVF). In 2013 we reported on four infants and very young children presenting with major QT prolongation, recurrent CA, and poor response to therapy. Using exome sequencing in parents-child trios, we identified de novo mutations in either CALM1 or CALM2, two of the three human genes encoding CaM. Mutations in CALM3 were also identified. All mutation carriers were infants with recurrent VF usually triggered by sympathetic activation and major QT prolongation. The mutated proteins exhibit major reductions in calcium binding affinity, and their overexpression in heterologous systems shows that the LQTS phenotype is mainly because of a reduction in the calcium-dependent inactivation of L-type calcium channels. , Because this overexpression does not take into account the native allelic balance of CaM, in which six alleles are expressed, we generated patient-specific induced pluripotent stem cells (iPS) from one mutation carriers ( CALM1 -F142L) and differentiated them into cardiomyocytes. Our results confirm that the derangement of L-type Ca 2+ current (I CaL ) inactivation is the dominant mutation consequence and suggest that the resulting repolarization delay is the most likely arrhythmogenic effect. The causes underlying this dominant negative mechanism have been recently discussed.
To better understand the natural history, clinical features, and response to therapy, we established an international calmodulinopathy registry that enrolled 74 subjects with a mutation in one of the CALM genes. The LQTS phenotype was the most prevalent and severe one. Indeed, life-threatening arrhythmias occurred in 78% of the cases with a median age of onset of 1.5 years, mean QTc was 594 ± 73 ms and a perinatal presentation occurred in 58%. Adrenergic stimulation was the major trigger for cardiac events and in the cases in which the arrhythmia onset was recorded, it was always an abrupt onset of VF. The ECG pattern was similar to those observed in LQT3 and Timothy syndrome, with late-onset peaked T waves. Perinatal presentation was often associated with an extremely long QTc (628 ± 62 ms), 2:1 AV block, and T wave alternans. The mutations identified were mainly de novo with a few cases of mosaicism identified ; therefore no true familial cases were so far detected. β-Blocker monotherapy was insufficient to protect these patients and a combination of β-blockers, mexiletine, and LCSD was the best combination to improve protection. Nonetheless, most patients needed an ICD to prevent SCD ( Fig. 96.2 ). CALM-LQTS might not always be a pure electrical disorder because some patients had neurologic features unrelated to cardiac arrest, and others had cardiac structural abnormalities.
Cardiac triadin is a protein critical to the structure and functional regulation of cardiac muscle calcium release units and excitation-contraction coupling. Through whole-exome sequencing (WES), a homozygous frameshift mutation (D18fs13) was identified in a 10-year-old girl with LQTS, with a QTc of 500 ms and life-threatening arrhythmias occurring since the age of 1 year. Subsequent targeted screening of the TRDN gene allowed for the identification of either homozygous or compound heterozygous frameshift mutations in four of an additional 33 unrelated LQTS cases. All these cases had exercise-induced CA in early childhood and T wave inversion in leads V 1 to V 4 (but their ages ranged from 2–6 years) together with QTc prolongation. Despite the lack of functional data, the role of triadin is supported by sympathetically mediated arrhythmias and cardiomyocyte calcium overload because of slower calcium-dependent inactivation of the L-type calcium channel in TRDN null mice. Recently, the international triadin knockout syndrome registry reported data on 21 patients from 16 families. All patients had either homozygous (57%) or compound heterozygous triadin null variant. Cardiac arrest was the first clinical manifestation in 71% of patients, and transient QT prolongation was often observed, sometimes with T wave inversion in V 1 to V 3 /V 4 . An exercise stress test induced ventricular arrhythmias in most patients. One-third of the patients exhibit mild muscle weakness. Conventional therapy does not adequately protect these patients. Recently, a functional characterization of a missense homozygous mutation ( TRDN -L56P) suggested that the mutated protein may affect its ability to be stably assembled within calcium release complex and therefore can trigger arrhythmias by altering calcium homeostasis. Given all these clinical characteristics, triadin knockout syndrome should not be considered to be a LQTS subtype, but a distinct clinical entity, such as ATS.
A genome-wide association study (GWAS) was recently conducted in 1656 unrelated LQTS patients of European or Japanese ancestry and in 9890 controls to evaluate the influence of single nucleotide variants (SNVs) in disease susceptibility. Most cases (89%) were genotype positive (800 LQT1, 661 LQT2, and 123 LQT3), whereas 197 were genotype negative. Three loci reached the threshold for genome-wide statistical significance (rs12143842 on NOS1AP, rs179405 on KCNQ1 , and rs179405 on KLF12 ) in both the European and Japanese populations; each of these three SNVs had been previously associated with QT interval duration in the general population. Furthermore, KCNE1 -D85N showed an association at the suggestive threshold of P > 10 -6 . Subsequently, the aggregate effect of the 68 QT-SNVs that had shown association with the QT interval in the general population (i.e., the weighted QT polygenic risk score [PRS QT ]), was evaluated and showed a significant association with LQTS diagnosis in both populations. Interestingly, the association with PRS QT was greater in genotype negative patients compared with genotype positive patients, suggesting a polygenic architecture in genotype negative LQTS cases.
At variance with all other cardiac diseases of genetic origin for which only estimates exist, the prevalence of LQTS has been established on the basis of hard data provided by the largest prospective study of neonatal electrocardiography ever performed, which involved 44,596 infants aged 3 to 4 weeks and was complemented by genetic screening. Among these infants, 1.4% had a QTc between 440 and 469 ms and 0.7 of 1000 had a QTc of at least 470 ms, regarded as markedly prolonged by the European Task Force on Neonatal Electrocardiography. In the latter group ( n = 31), more than 90% of infants underwent molecular screening, and LQTS disease-causing mutations were found in 13 of 28 (46%). Because almost 50% of the infants with a QTc of at least 470 ms (0.7 per 1000) are affected by LQTS, and because some of those infants with a QTc between 440 and 469 ms are also affected (unpublished data), it follows that the prevalence of LQTS must be close to 1 in 2000 live births. This does not include the silent mutation carriers (QTc < 440 ms), a group ranging between 10% and 36% according to genotype that escapes ECG diagnosis and is usually identified using cascade screening.
Over the years, cardiologists have become progressively more aware of LQTS and now tend to suspect it more often. This has contributed to the realization that a relatively large number of LQTS patients may remain asymptomatic even off therapy. The typical clinical presentation of LQTS years ago, when only the more serious cases were diagnosed, was the occurrence of syncope or CA, precipitated by emotional or physical stress, in a young individual with a prolonged QT interval on ECG. If these symptomatic patients were left untreated, the syncopal episodes would recur and eventually prove fatal in most cases. Nowadays, the majority of patients are identified before symptoms occur, largely because of ECG screening either before practicing sports or for other reasons, and it is clear that many affected patients have a very benign course.
The clinical history of repeated episodes of loss of consciousness under emotional or physical stress in a subject with a prolonged QT interval is so typical as to be almost unmistakable, provided that the physician is aware of LQTS. Nevertheless, the clinical presentation is not always so clear.
The syncopal episodes are caused by torsades de pointes (TdP) VT often degenerating into VF. Most patients develop their symptoms under stress, but these life-threatening cardiac events can also occur at rest. These different patterns remained puzzling until molecular biology allowed for the differentiation among different genotypes. In 670 LQTS patients of known genotype who had all experienced symptoms (syncope, CA, or sudden death), we examined possible relations between the three major genotypes and the conditions (triggers) associated with the events. As predicted by the impairment of the I Ks current in LQT1 patients (essential for QT shortening during increases in heart rate), most of their events occurred during exercise or stress. Conversely, most of the events in LQT2 patients occurred during emotional stress, such as auditory stimuli (sudden noises and telephone ringing, especially occurring at rest), and most of the events among LQT3 patients occurred when they were asleep or at rest. LQT2 and LQT3 are at low risk during exercise because they have a well-preserved I Ks current and are therefore able to shorten their QT interval whenever heart rate increases. The practical implication is that LQT2 and LQT3 children should be allowed to play (no competitive sports) rather freely, with significant psychological benefits.
Even in the postpartum period, genotype is important because the risk is higher for LQT2 than for LQT1 patients. The higher risk for LQT2 women is probably partly related to sleep disruption, but hormonal changes could play a role as well. Indeed, LQT2 women have an increased risk for cardiac events during the menopause transition and postmenopausal periods (hazard ratio [HR] of 3.38 and 8.10, respectively), whereas for LQT1 women, the onset of menopause is associated with a reduction in the risk for cardiac events (HR, 0.19).
A recent study on 103 LQTS families has shown that stillbirths are eight times more frequent among LQTS mothers than in the general population and that the likelihood of fetal death is significantly greater with maternal versus paternal LQTS (24% vs. 3%).
The bizarre ECG of many LQTS patients should be easily recognized. Besides QT prolongation, the T wave has several morphologic patterns easily recognizable with clinical experience, which are difficult to quantify but useful for diagnosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here