Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver is the largest and most complex organ in the body. The anatomy of the liver is intricate, and its function is dependent on the close interaction of resident cell lineages; the arterial, venous, and portal vasculature; and the biliary system. The liver plays a central role in numerous biochemical processes, executing metabolic and catabolic functions that are vital for homeostasis and health. Biochemical tests can be used to determine the cause and prognosis and to monitor liver diseases.
This chapter reviews the anatomy and physiology of the liver, the major causes of acute and chronic liver diseases, and the patterns of biochemical test results associated with these disorders.
The chapter describes how tests can be used to investigate the liver through the measurement of the enzymes and proteins it produces and the processes that it regulates. The chapter also explains how clinical chemistry can provide powerful insights into the health of the liver, the likely etiology of disease, and prognosis.
In chronic liver disease, inflammation initiates liver fibrosis, which can progress to cirrhosis and liver cancer. This chapter describes how biochemical markers of liver fibrosis can be used to determine the severity and prognosis of liver disease and monitor disease course.
This chapter covers the use of biochemical tests to help determine the etiology, chronicity, severity, and prognosis, as well as monitoring the course of a wide range of liver pathologies.
The liver has a central and critical biochemical role in the metabolism, digestion, detoxification, and elimination of substances from the body. All blood from the intestinal tract initially passes through the liver, where products derived from digestion of food except lipids are processed, transformed, and (in some cases) stored. These include amino acids, carbohydrates, vitamins, (apart from fat-soluble vitamins D, A, K, and E) and minerals (see Chapters 31 , 35 , and 39 , respectively). Most major plasma proteins (with the exception of immunoglobulins [Igs] and the von Willebrand factor) are mainly or exclusively synthesized in the liver. The liver responds to multiple hormonal and neural stimuli to regulate blood glucose concentrations. Not only does it extract glucose from blood for use in generating energy, but it also stores dietary glucose as glycogen for later use. The liver is also the major site for gluconeogenesis, which is critical for maintaining blood glucose concentration in the fasting state. The liver plays a major role in lipid metabolism; it is the principal site of cholesterol, triglyceride, and lipoprotein synthesis; it extracts and processes fatty acids that are generated through lipolysis in adipose tissue. It also removes cholesterol from the circulation by endocytosis of remnants of chylomicrons and very-low density lipoproteins (VLDLs) and low-density lipoproteins (LDLs) and by selective uptake from high-density lipoproteins (HDLs). Cholesterol and bile acids synthesized by the liver from cholesterol are secreted into the bile, which facilitates the absorption of dietary fat and fat-soluble vitamins. The liver is also the primary site of metabolism of both endogenous substances and exogenous compounds (e.g., drugs and toxins). This process, known as biotransformation, converts lipophilic substances to hydrophilic ones for subsequent elimination. The liver is a major site of catabolism of hormones, and thus participates in regulation of plasma hormone concentrations. The liver is also involved in hormone synthesis, producing such hormones as insulin-like growth factor 1, angiotensinogen, hepcidin, thrombopoietin, erythropoietin, and the prohormone 25-OH vitamin D. Many of these hepatic functions can be assessed by laboratory procedures to gain insight into the integrity of the liver.
As a large organ, the liver shares with many other organs the ability to perform its functions with extensive reserve capacity. In many cases, individuals with liver disease maintain normal function despite extensive liver damage. In such cases, liver disease may be recognized only by using tests that detect injury. Most commonly, this is accomplished by measuring plasma activities of enzymes found within liver cells, which are released in somewhat specific patterns with different forms of injury. Chronic liver injury often involves fibrosis in the liver; markers of the fibrotic process can indicate the degree of injury. Chronic damage is often due to chronic inflammation; cytokines alter the pattern of liver protein production, which allows detection of inflammation (although not necessarily that involving the liver). Some proteins are produced in increased amounts with liver regeneration and neoplasia; such markers may be useful in detecting liver cell proliferation.
The chapter begins by describing the anatomy and biochemical functions of the liver. Various disease states that involve the liver are then discussed. The chapter concludes with a discussion of the use of laboratory test results in recognizing and characterizing patterns of liver injury.
The adult liver weighs approximately 1.2 to 1.5 kg. It is located beneath the diaphragm in the right upper quadrant of the abdomen and is protected by the ribs and held in place by ligamentous attachments.
The liver is divided into left and right anatomic lobes by the falciform ligament, an anterior extension of the peritoneal folds that connects the liver to the diaphragm and the anterior abdominal wall ( Fig. 51.1 ). Two smaller lobes are found between the left and right lobes: the caudate lobe, situated on the posterior-superior surface of the right lobe, receiving blood supply from the left and right hepatic arteries and the portal vein, and the quadrate lobe that sits on the under surface of the medial segment of the left lobe. Riedel’s lobe, an anatomic extension of the right lobe of the liver, consists of a projection that may feel like a mobile tumor in the right abdomen.

The liver has a dual blood supply. The portal vein, which carries blood from the spleen and nutrient-enriched blood from the gastrointestinal (GI) tract, supplies approximately 70% of the blood supply; the hepatic artery, a branch of the celiac axis, provides oxygen-enriched arterial blood. Each supplies approximately half of the oxygen reaching the liver, making it highly resistant to infarction. Ultimately, these two blood supplies merge and flow into the sinusoids that course between individual hepatocytes. Venous drainage from the liver ultimately converges into the right and left hepatic veins, which exit on the posterior surface of the liver and join the inferior vena cava near its entry into the right atrium. The caudate lobe drains directly into the inferior vena cava and so may continue to function if the hepatic venous outflow is occluded (Budd-Chiari syndrome).
The liver is covered by an anterior reflection of the peritoneum known as Glisson capsule. Other extensions of the peritoneum form ligaments that hold the liver in place. Internal extensions of the capsule provide an internal supporting framework that divides the liver into lobules and ultimately surrounds blood vessels and nerves. One of the ligaments, the ligamentum teres, is the vestigial remnant of the umbilical vein; it connects the umbilicus to the inferior border of the liver. When portal hypertension occurs, the umbilical veins may reopen, leading to venous dilatation around the umbilicus (termed caput medusae).
The nerve supply to the liver comes from the vagus and phrenic nerves, and the sympathetic ganglia originating from cell bodies in the spinal cord that are located between the seventh and tenth thoracic vertebrae. These merge to accompany the hepatic arteries and bile ducts throughout the liver.
Biliary drainage originates at the bile canaliculi; these grooves between adjacent hepatocytes form ductules that merge to create the intrahepatic bile ducts, which ultimately join to form the right and left hepatic bile ducts, which exit from the liver at the porta hepatis and combine to form the common hepatic duct. The hepatic duct is joined by the cystic duct that drains the gallbladder to form the common bile duct (see Fig. 51.1 ). The common bile duct then enters the duodenum (usually with the pancreatic duct) at the ampulla of Vater. The duodenal portion of the common bile duct is surrounded by longitudinal and circular muscle fibers that form the sphincter of Oddi. This musculature relaxes when the gallbladder contracts, allowing bile to enter the duodenum; in its normally contracted state, the sphincter prevents reflux of acidic duodenal contents into the bile duct. The gallbladder, which is located on the undersurface of the right lobe of the liver, is the site for storage and concentration of bile, a complex mixture of bile salts and waste products. In the adult, it averages approximately 10 cm in length and has a capacity of 30 to 50 mL of bile. Hormonal stimuli initiated by food ingestion cause contraction of the muscular wall of the gallbladder, releasing bile salts into the intestine to facilitate digestion of fat.
The functional anatomical unit of the liver is the acinus, which is adjacent to the portal triad (which consists of a branch of each of the portal vein, hepatic artery, and bile duct). Each acinus is a diamond-shaped mass of liver parenchyma that is supplied by a terminal branch of the portal vein and of the hepatic artery and is drained by a terminal branch of the bile duct. The blood vessels radiate toward the periphery, forming sinusoids, which perfuse the liver and ultimately drain into the central (terminal) hepatic vein ( Fig. 51.2 ). The sinusoids are lined by fenestrated endothelial cells (which allows free filtration of blood) and phagocytic Kupffer cells (see Fig. 51.1 ). The Kupffer cells are derived from blood monocytes. They contain lysosomes with hydrolytic enzymes that break down phagocytized foreign particles (e.g., bacteria). They also have Ig and complement receptors and are the main site for clearance of antigen–antibody complexes from blood. Kupffer cells secrete interleukins (ILs), tumor necrosis factor (TNF), collagenase, prostaglandins, and other factors involved in inflammatory responses.

Hepatocytes are the major functioning cells in the liver and are responsible for approximately 70% of liver mass. They perform most of the metabolic and synthetic functions of the liver. Two other cell types are found in small numbers within the liver. The stellate cells (sometimes referred to as Ito cells) are located between the endothelial lining of the sinusoids, and the hepatocytes are within a small cleft referred to as the space of Disse. In their normal, quiescent state, stellate cells serve as a site of storage for fat-soluble vitamins, particularly vitamin A. When stimulated, stellate cells are morphologically and functionally transformed. They synthesize collagen and are the cells responsible for fibrosis, and eventually, cirrhosis. They also synthesize nitric oxide, which helps to regulate intrahepatic blood flow. Oval cells, found near the portal areas around small bile passages, are believed to be liver stem cells involved in regeneration of hepatocytes and bile ducts after liver injury.
The blood supply to each acinus consists of three zones ( Fig. 51.3 ). Hepatocytes in zone 1, the area immediately adjacent to the portal tract, are enriched with lysosomes and mitochondria. Zone 1 appears to be involved in protecting the liver from external injury and providing a base for hepatic regeneration. Zone 2 predominantly contains hepatocytes that perform the major metabolic functions of the liver. The periphery of the acinus, zone 3, contains hepatocytes that are enriched with endoplasmic reticulum, are metabolically active, and have relatively low oxygen tension. This area is most susceptible to injury.

Hepatocytes contain a well-developed organelle substructure ( Fig. 51.4 ). Mitochondria, which constitute approximately 18% of hepatocyte volume, are the sites of oxidative phosphorylation and energy production. They contain enzymes involved in the citric acid cycle and in β-oxidation of fatty acids. The rough endoplasmic reticulum is the site of synthesis of many proteins, including albumin, coagulation factors, enzymes (e.g., glucose 6-phosphatase), and triglycerides. The smooth endoplasmic reticulum contains microsomes that are involved in bilirubin conjugation, detoxification (cytochrome P 450 –dependent isoenzymes), steroid synthesis, cholesterol synthesis, and bile acid synthesis. Several microsomal enzymes, including γ-glutamyltransferase, are induced by many drugs and inhibited by others. γ-Glutamyl transpeptidase (GGT) catalyzes the transfer of the γ-glutamyl moiety from γ-glutamyl peptides to other peptides such as glutathione and to L-amino acids. GGT is present in the cell membranes of many tissues, with greatest activity in biliary epithelial cells, pancreatic acinar cells, renal tubular epithelial cells, and also in mammary glands in some species. It is believed that it is involved in amino acid transport across membranes as part of the γ-glutamyl cycle, although hydrolysis of glutathione may be a more important function. This is the site of most drug metabolism and many important drug interactions.

Peroxisomes are found near the smooth endoplasmic reticulum and contain oxidases that use molecular oxygen to modify a variety of substrates, leading to the production of hydrogen peroxide. They also contain catalase, which decomposes hydrogen peroxide. Peroxisomes catalyze the β-oxidation of fatty acids that have 7 to 18 chain lengths. Approximately 5 to 20% of the metabolism of ethanol also occurs in the peroxisomes. Lysosomes are dense organelles that contain hydrolytic enzymes that act as scavengers. Deposition of iron, lipofuscin, bile pigments, and copper occurs in the lysosomes. The Golgi apparatus lies near the canaliculus and is involved in the secretion of various substances, including bile acids and albumin.
The human intestine is home to a variety of microbes (bacteria, archaea, fungi, and viruses). The gut contains approximately the same number of bacterial cells as there are human cells in the body, but the human microbiome contains over 3 million genes, compared with only around 23,000 in the human genome. ,
The gut microbiota plays crucial roles in: the maturation and continued stimulation of the host immune response ; the maintenance of the intestinal barrier integrity, which limits pathogen perpetuation in the gut; modulation of host-cell proliferation and vascularisation ; and regulation of intestinal, neurologic, and endocrine functions, and bone density. The human gut microbiota provides a source of energy ; helps in the synthesis of vitamins and neurotransmitters; metabolizes bile salts ; reacts to or modifies specific drugs; and eliminates exogenous toxins. In a healthy colon, the gut microbiota maintains a symbiotic relationship with the host and rapidly adapts to maintain eubiosis after an acute insult.
The gut-liver axis refers to the bidirectional relationship between the gut and its microbiota, and the liver. There is a constant interchange generated by dietary, genetic, and environmental factors established via the portal vein that carries gut-derived products to the liver, and the liver feedback of bile and antibody secretion from the liver into the intestine via the biliary tree. The control of the microbial communities is critical to maintain the homeostasis of the gut-liver axis, and as a part of this two-way communication, the liver shapes intestinal microbial communities.
An altered microbiota or “dysbiosis,” an impaired intestinal barrier, and endotoxemia are well-recognized features of advanced alcohol-related liver disease (ArLD). Disturbances of the intestinal barrier can result in an increased portal influx of bacteria or their products to the liver where they can cause or worsen hepatic diseases and inflammation and potentiate disease. In alcoholic liver disease, the toxic effect of alcohol on hepatocytes, abnormal microbiota, and loss of intestinal function all contribute to the pathogenesis of the disease. Alcohol causes bacterial overgrowth and reduced bacterial diversity and a reduction in the diversity of the mycobiome particularly influencing yeast and fungi and a reduced mycobiome (yeast and fungi). These changes lead to alterations in bile acid homeostasis increasing intestinal deconjugation of bile acids and exposure of hepatocytes to more toxic bile acids. Alcohol disrupts the intestinal microbiome, alters the intestinal barrier, and might affect various other intestinal functions such as mucosal immunity. Although the role of the bacterial component of the gut microbiota in ArLD has been the major focus of research, the contribution of the mycobiota in this disease is of particular interest, given the increased risk of fungal infections in patients with ArLD. The mycobiota is also known to be altered in other diseases, including hepatitis B and inflammatory bowel disease.
In nonalcoholic fatty liver disease (NAFLD), bacterial overgrowth and changes in the microbiota population occurs and these changes correlate with increased intestinal permeability and features of the metabolic syndrome. ,
In cirrhosis, there is marked impairment of the gut barrier that worsens as the disease advances. The gut microbiota in cirrhosis is characterized by reduced diversity, increased overgrowth of potentially pathogenic bacteria, and decreased abundance of beneficial bacteria. , Infections are the most common cause of death in these patients and with advanced liver cirrhosis which is associated with a profound dysbiosis. It is suggested that decompensation of liver events in cirrhosis and typical complications of advanced liver disease such as hepatic encephalopathy and bacterial peritonitis are substantially driven by the microbiota.
Alteration of gut microbiota may play an important role in the development and progression of NAFLD. It was shown more than 20 years ago in animal models that changing the microbiota composition by using prebiotics such as inulin-type fructans reduces hepatic steatosis and de novo lipogenesis. Feeding of prebiota was found to inhibit all lipogenic enzymes and thereby VLDL production so that plasma triglyceride concentrations are decreased. The fermentation of prebiotics by gut microbes increases the abundance of short-chain fatty acids in the caecum and also in the portal veinous blood, where the concentration of both acetate and propionate is doubled leading to a reduction in hepatic lipogenesis. In this way, the microbiota contributes to the regulation of de novo hepatic lipogenesis. Further, specific nutrients such as fat and alcohol change the composition of the microbiota in a harmful manner, whereas prebiotics may counteract these effects. Hepatic lipid metabolism is influenced by the innate immune system and xenobiotic metabolism control liver lipid metabolism via mechanisms involving bacterial components and metabolites. Thus hepatic innate immunity can influence the liver’s production of bioactive lipids and contribute to switch from NAFLD to non-alcoholic steatohepatitis.
Emerging work suggests that altered gut microbiota may contribute to the pathology of other liver diseases. There is a specific microbiome signature found in primary sclerosing cholangitis (PSC) with an increased population of Veillonella as is the situation with other human chronic inflammatory disorders. This microbiome signature is different than the one observed in patients with ulcerative colitis without liver disease.
The liver is involved in various excretory, synthetic, and metabolic functions. Clinical laboratories perform numerous tests that are useful in the biochemical assessment of these functions.
Organic compounds of both endogenous and exogenous origin are extracted from the sinusoidal blood, biotransformed, and excreted into the bile or urine. Assessment of this excretory function provides valuable clinical information. The most frequently used tests involve the measurement of plasma concentrations of endogenously produced compounds, such as bilirubin and bile acids. In specialist centers, these tests may be augmented by determination of the rate of clearance of exogenous compounds, such as aminopyrine, lidocaine, and caffeine.
Bilirubin is the orange-yellow pigment derived from heme, which is mainly a product of red blood cell (RBC) turnover. It is extracted and biotransformed in the liver and excreted in bile and urine.
Bilirubin was discovered by Virchow in 1849 in blood extravasates; he called the yellow pigment “hematoidin.” The term bilirubin was coined by Stadeler in 1864, and in 1874, Tarchanoff demonstrated the direct association of bile pigments with Hb. In 1942, Fisher and Plieninger synthesized bilirubin IXα and proposed the structure shown in Fig. 51.5 A. This linear tetrapyrrolic structure of the bilirubin molecule was accepted for longer than 30 years. However, important chemical properties of the bilirubin molecule are its insolubility in water and its solubility in a variety of nonpolar solvents. The solubility of bilirubin in nonpolar, lipid solvents is not predicted from this linear tetrapyrrole structure because the two propionic acid side chains would be expected to make the bilirubin molecule highly polar and, therefore water soluble. The overall chemical structure of bilirubin was established by x-ray crystallography. According to this work, bilirubin assumes a ridge-tiled configuration stabilized by six intramolecular hydrogen bonds. Two additional important structural features have also been noted: (1) a so-called Z-Z (trans) conformation for the double bonds between carbons 4 and 5 and 15 and 16, and (2) an involuted hydrogen-bonded structure in which the propionic acid–carboxylic acid groups are hydrogen-bonded to the nitrogen atoms of the pyrrole rings (see Fig. 51.5 B). These bonds stabilize the Z-Z configuration of bilirubin and prevent its interaction with polar groups in aqueous media. When exposed to light, the Z-Z configuration is converted to the E-E (cis) conformation and to other combinations, namely, 4 E -15 Z and 4 Z -15 E . The E-E conformation and other E -containing isomers do not permit the degree of internal hydrogen bonding that occurs in the Z-Z conformation and, therefore are more water soluble than in the Z-Z conformation. Thus light-exposed forms of bilirubin are more water soluble and are readily excreted in the bile. This is the rationale for irradiating jaundiced newborns with 450-nm light.
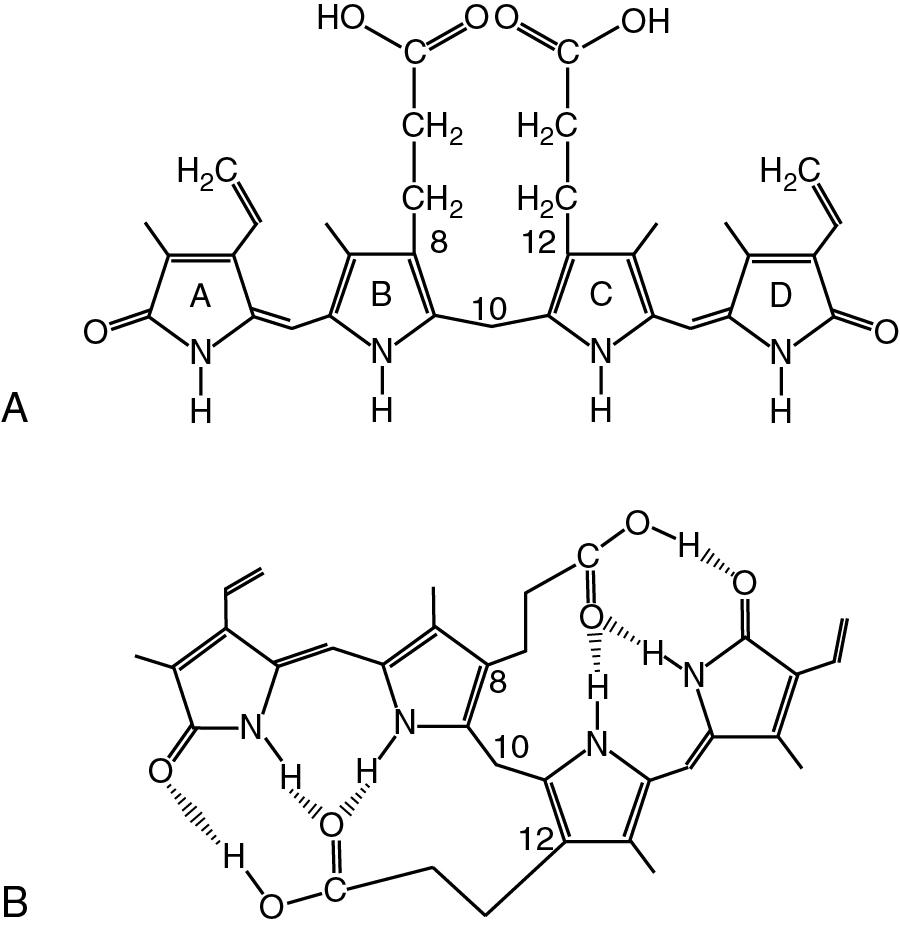
The bilirubin molecule in the crystalline state takes, as mentioned earlier, the form of a ridge tile rather than a linear tetrapyrrole, with the ridge being along the line C8-C10-C12. In this configuration, rings A and B lie in one plane and rings C and D in another, with a 98° angle between the two rings. The preferred conformation of bilirubin in aqueous solution at pH 7.4 is not known, but the occurrence of a hydrogen-bonded structure in aqueous solution would explain some of the unique chemical properties of bilirubin IXα. For example, the addition of hydrogen bond–breaking chemicals, such as caffeine, methanol, ethanol, urea, or surface active agents, is required for unconjugated bilirubin to react with diazo reagent. These reagents likely act by breaking the internal hydrogen bonds of the bilirubin molecule, allowing it to react with diazotized sulfanilic acid or other diazo compounds. In contrast, bilirubin IXα monoglucuronide and diglucuronide are soluble in water and react readily with diazo reagents. The bulky glucuronic acid moiety precludes conjugated bilirubin from undergoing internal hydrogen bond formation. Bilirubin glucuronides, which are water soluble, are readily excreted in the bile and urine, whereas unconjugated bilirubin is not.
Bilirubin derived from natural sources consists almost entirely (99%) of the isomer IXα. Bilirubins IXβ and IXδ, which arise from cleavage of the β- and δ-methene bridges, consist of less than 0.5% of bilirubin isolated from bile. However, bilirubin reference materials available from commercial sources and from the National Institute of Standards and Technology (Standard Reference Material 916a) contain variable quantities of IIIα and XIIIα isomers. The two isomers are formed by cleavage of bilirubin IXα at the central methylene bridge; subsequent recombination of the two different dipyrrole units gives a mixture of the three isomers. This isomerization of bilirubin occurs in aqueous solution at acidic or neutral pH, but not when bilirubin is bound to albumin.
Bilirubin IXα is produced from the catabolism of protoporphyrin IX by a microsomal heme oxygenase. The tetrapyrrolic product of the ring opening at the α-methene bridge is the green pigment biliverdin, which is subsequently reduced to bilirubin by the reduced form of nicotinamide adenine dinucleotide phosphate–dependent cytosolic enzyme biliverdin reductase ( Fig. 51.6 ). For each mole of heme catabolized by this pathway, one mole each of carbon monoxide, bilirubin, and ferric iron is produced. Daily bilirubin production from all sources in humans averages from 250 to 300 mg. Approximately 85% of the total bilirubin produced is derived from the heme moiety of Hb released from senescent erythrocytes that are destroyed in the reticuloendothelial cells of the liver, spleen, and bone marrow. The remaining 15% is produced from RBC precursors destroyed in the bone marrow (so-called ineffective erythropoiesis) and from the catabolism of other heme-containing proteins, such as myoglobin, cytochromes, and peroxidases.

In blood, bilirubin is bound to albumin ( K d ≈ 10 −8 mol/L) and is transported to the liver. Bilirubin then dissociates from albumin by an unknown process at the sinusoidal membrane of the hepatocyte. It is transported across the membrane ( Fig. 51.7 ).

It is theorized that the organic anion transport proteins (OATPs) 1A1 (OMIM*604843) and 1B3 (OMIM*605495), which are encoded on the solute carrier organic anion transporter (SLCO) superfamily of genes, are responsible for the uptake of bilirubin into the hepatocyte. Once inside the liver cells, bilirubin is reversibly bound to soluble proteins known as ligandins or protein Y . Ligandins are cytosolic proteins of the glutathione- S -transferase gene family and include approximately 5% of the total protein of human liver cytosol. , Ligandin also binds a variety of other compounds, such as steroids, bromsulphthalein (BSP), indocyanine green, and some carcinogens. Ligandin likely plays an important role in the processing of these compounds; it may increase the net efficiency of uptake by retarding the reflux of these substances back to plasma.
Inside the hepatocytes, bilirubin is rapidly conjugated with glucuronic acid to produce bilirubin monoglucuronide and diglucuronide, which then are excreted into bile (see Fig. 51.7 ). The enzyme bilirubin uridine diphosphate (UDP)–glucuronyltransferase 1A1 (OMIM*191740) is a tetramer that catalyzes the formation of bilirubin monoglucuronide and diglucuronide. This is a transmembrane protein primarily localized to the smooth endoplasmic reticulum. A specific binding site exists for bilirubin and glucuronic acid. It is speculated that the monomer catalyzes monoglucuronide formation and the tetramer is required for the diglucuronide conjugate at the luminal surface of the endoplasmic reticulum. The bilirubin diglucuronide returns to the cytosol, likely through a transporter, and binds to ligandin where it will diffuse to either the canalicular pole for secretion into bile or the sinusoidal pole for secretion back into plasma. The process is mediated by an adenosine triphosphate–binding cassette transporter ABCC2, which was previously named multidrug-related protein 2 (MRP2), at the canalicular pole. Although other transporters exist for this function, such as ABCG2, most are removed by MRP2/ABCC2. At the sinusoidal pole, an ABCC3 transporter returns bilirubin into plasma where reuptake is possible by the OATP1B1 and OATP1B3 transporters.
In the presence of bilirubin monoglucuronide, albumin (and other proteins) can be postsynthetically modified by covalent attachment to lysine residues. In the case of albumin, this produces a protein-bound form termed biliprotein or δ-bilirubin. Increases in conjugated bilirubin or δ-bilirubin are highly specific markers of hepatic dysfunction (except in the presence of rare inherited disorders that impair excretion of conjugated bilirubin, such as Dubin-Johnson syndrome).
In adults, virtually all bilirubin excreted in bile is in the form of glycosidic conjugates; glucuronides account for approximately 95% of them, and glucosides and xylosides constitute the remainder. Of the glucuronides, diglucuronide is the major fraction (≈90%), and monoglucuronide is the minor fraction (≈10%).
Bilirubin glucuronides are not substantially reabsorbed in the intestine. Rather, they are hydrolyzed by the catalytic action of β-glucuronidase from the liver, intestinal epithelial cells, and bacteria. This unconjugated bilirubin is then reduced by anaerobic intestinal microbial flora to form a group of three colorless tetrapyrroles collectively called urobilinogens . In each of these three bilirubin reduction products, all bridge carbons are in the saturated (methylene) form. The urobilinogens differ from one another in the degree of hydrogenation of the vinyl sidechains and in the two end pyrrole rings. Urobilinogens contain 6, 8, or 12 more hydrogen atoms than does bilirubin and are named stercobilinogen, mesobilinogen, or urobilinogen , respectively. Up to 20% of the urobilinogen produced daily is reabsorbed from the intestine and enters the enterohepatic circulation. Most of the reabsorbed urobilinogen is taken up by the liver and is reexcreted in the bile; a small fraction (2 to 5%) enters the general circulation and appears in urine. In the lower intestinal tract, the three urobilinogens are spontaneously oxidized at the middle methylene bridge to produce the corresponding bile pigments stercobilin, mesobilin, and urobilin, which are orange-brown and the major pigments of stool. Approximately 50% of the conjugated bilirubin excreted in bile is metabolized to products other than the urobilinogens. The detailed structure of these metabolites has not been characterized.
Increased plasma bilirubin typically is classified as primarily indirect (an approximation of unconjugated bilirubin) or direct (an approximation of the sum of conjugated bilirubin and biliprotein). Increased indirect bilirubin indicates overproduction of bilirubin, which is usually caused by hemolysis, or decreased metabolism by the liver, which is primarily caused by congenital defects involving uridine 5-phosphate-glucuronyl transferase. The physiologic jaundice observed in neonates is due to increased indirect bilirubin caused by the delayed maturation of the conjugation process to remove it. With severe liver injury, which occurs with fulminant hepatic failure and end-stage cirrhosis, liver disease may cause primarily unconjugated hyperbilirubinemia. Increased urine urobilinogen occurs when bilirubin delivery to the intestinal tract is increased (as with hemolysis, or after recovery from hepatitis or obstruction) or when liver clearance is decreased, which occurs in portal hypertension.
Increased direct bilirubin generally results from functional or mechanical impairment in bilirubin excretion from the hepatocyte. Increased conjugated bilirubin is found in most cases of acute hepatitis and cholestasis (stoppage or suppression of the flow of bile); the percentage of direct bilirubin is similar in both types of liver disease. Urine bilirubin reflects increased plasma concentrations of conjugated bilirubin. With resolution of liver disease, conjugated bilirubin is rapidly cleared, and biliprotein may become the only form present; urine bilirubin is typically absent in such circumstances. Increased conjugated bilirubin is rarely seen with congenital defects in bilirubin excretion, such as Dubin-Johnson syndrome, and with impaired bilirubin excretion, which occurs in sepsis or other acute illness.
Bilirubin is known as a strong antioxidant and mild or moderately increased serum bilirubin seems to be beneficial ; the protective effects of bilirubin on atherogenesis and cancerogenesis have been demonstrated in both in vitro and in vivo studies. , However, high concentrations of unconjugated hyperbilirubinemia are toxic and cause bilirubin encephalopathy (kernicterus) which is due to inhibition of DNA synthesis and direct neurotoxicity causing mass destruction of neurons through apoptosis and necrosis. Bilirubin may also uncouple oxidative phosphorylation and inhibit adenosine triphosphatase (ATPase) activity of brain mitochondria. Bilirubin mediated inhibition of various enzyme systems, RNA synthesis and protein synthesis in the brain and liver, and/or alteration of carbohydrate metabolism in the brain can also contribute to its toxicity. The accumulation of bilirubin in plasma and tissues results in characteristic yellow discoloration of tissues known as icterus or jaundice.
Jaundice is a condition characterized by hyperbilirubinemia and deposition of bile pigment in the skin, mucous membranes, and sclera, with a resulting yellow appearance of the patient; it is also called icterus . Defects in bilirubin metabolism resulting in jaundice can occur at each step of the metabolic pathway (see Fig. 51.7 ). The disorders are usually classified as inherited disorders of bilirubin metabolism and jaundice of the newborn. All of these disorders are characterized by increases in conjugated or unconjugated bilirubin in the absence of other abnormal liver tests. Bilirubin fractionation is clinically useful only for these disorders.
Patients are occasionally seen with isolated increases in bilirubin concentration. In most cases, this is due to inherited disorders of bilirubin metabolism, familial hyperbilirubinemia, or hemolysis. It is not difficult to establish hemolysis as the cause of hyperbilirubinemia because the patient with severe hemolysis will have many other disease manifestations. An algorithm for differentiating familial causes of hyperbilirubinemia is presented in Fig. 51.8 .

Several analytical techniques are used to measure bilirubin and metabolites in serum, urine, and feces. Measurement of bilirubin in amniotic fluid is discussed in Chapter 45 on Body Fluids.
The reaction of bilirubin with diazotized sulfanilic acid, known as the diazo reaction, discovered by Ehrlich in 1883 and applied to the measurement of bilirubin in serum and bile by van den Bergh and Muller in 1916, is the basis of the most widely used methods for measuring bilirubin. These researchers observed, in sera from jaundiced infants, the reaction was slow and required an accelerator to proceed, and that it was rapid in bile and in adult sera without addition of ethanol, which led to the terms indirect and direct bilirubin, respectively. The chemical nature of direct and indirect bilirubins was elucidated by Billing and colleagues in the mid-1950s. By using open-column, reversed-phase chromatography on siliconized kieselguhr (cellite or diatomaceous earth), investigators isolated three bilirubin fractions—unconjugated bilirubin (indirect reacting fraction) and bilirubin monoglucuronide and diglucuronide (direct reacting fractions). Kuenzle and colleagues were the first to successfully use an open-column chromatography technique that did not involve a deproteinization step. They obtained four bilirubin fractions—unconjugated bilirubin (α-bilirubin), monoconjugated bilirubin (β-bilirubin), diconjugated bilirubin (γ-bilirubin), and a fraction bound strongly to protein (δ-bilirubin). The last fraction was clearly distinct from the albumin-bilirubin complex that exists in serum.
The most widely used chemical methods for bilirubin measurement are those based on the coupling of bilirubin with a diazo compound. In this reaction ( Fig. 51.9 ), diazotized sulfanilic acid (the diazo reagent) reacts with bilirubin to produce two azodipyrroles (azopigments), which are reddish-purple at neutral pH and blue at low or high pH values. Van den Bergh and Muller applied this reaction to the quantitation of bilirubin in serum. They described the fraction of bilirubin that reacted with the diazo reagent in the absence of alcohol as the direct bilirubin fraction and used the term indirect bilirubin for the difference between total bilirubin (found after the addition of alcohol to the reaction mixture) and the direct bilirubin fraction. Numerous variations of the van den Bergh and Muller method have been developed. All use one of a variety of “accelerators,” which, like alcohol, facilitate the reaction of unconjugated (indirect) bilirubin with the diazo reagent; the most commonly used accelerators are caffeine, dyphylline, and several surface active agents. The diazo method of Malloy and Evelyn, which uses methanol as an accelerator, has substantial matrix effects, negative interference by Hb, turbidity due to protein precipitation by methanol, and a long reaction time. This method, which has been virtually abandoned, is mentioned here for historical reasons only.

The diazo method described by Jendrassik and Grof in 1938 and later modified by Doumas and colleagues gives results for serum total bilirubin that are reproducible and reliable. In this procedure, an aqueous solution of caffeine and sodium benzoate serve as the accelerators. Studies on the mechanism by which the caffeine-benzoate solution facilitates the reaction of unconjugated bilirubin with the diazo reagent have provided strong, albeit indirect, evidence that caffeine, and perhaps benzoate, displaces unconjugated bilirubin from its association sites on albumin. This occurs by (1) formation of hydrogen bonds between bilirubin and caffeine, , thus making bilirubin water soluble, or (2) complex formation and disruption of the bilirubin internal hydrogen bonds. With the use of samples prepared by addition of unconjugated bilirubin and authentic human diconjugated (with glucuronic acid) bilirubin to low-bilirubin pooled sera—and a nuclear magnetic resonance technique—Lo and Wu have shown that the modified Jendrassik-Grof total bilirubin assay detects unconjugated and diconjugated bilirubin quantitatively (as unconjugated bilirubin equivalents). This method has acceptable transferability among laboratories , , and is currently the method of choice.
Other methods for determining bilirubin include direct spectrophotometric measurement of total bilirubin in serum using analysis of a two-component system by measuring absorbance at two wavelengths and solving a system of two simultaneous equations. This approach is applicable to sera from healthy neonates because only unconjugated bilirubin is present in such sera. Correction for oxyhemoglobin is necessary because it is invariably present in sera from neonates.
A number of instrument manufacturers use bovine serum, instead of human serum, as the protein base for preparing fluids for calibrating methods for total and direct bilirubin; the protein base is enriched with unconjugated bilirubin or ditaurobilirubin or both. Unconjugated bilirubin in human serum reacts completely with the reference method and with diazo methods available in commonly used clinical analyzers; however, its reaction in bovine serum from commercial sources is incomplete and unpredictable. That makes the assignment of accurate bilirubin values to calibrators virtually impossible, the protein base of which is commercial bovine serum. In human serum, ditaurobilirubin was underestimated by two of seven clinical analyzers tested; the calibrators of these two analyzers were made in bovine serum. Ditaurobilirubin in commercial bovine serum was underestimated by all analyzers and by the reference method; in human serum, it was underestimated by two analyzers only. The practice of using bilirubin calibrators in bovine sera should be abandoned because it compromises the accuracy of bilirubin measurements in jaundiced neonates. However, fresh bovine serum (obtained from a slaughterhouse) has only a small effect on the measurement of unconjugated bilirubin or ditaurobilirubin.
High-performance liquid chromatography (HPLC) methods have been developed for relatively rapid separation and quantification of the four bilirubin fractions. HPLC has been helpful in separating and detecting the various bilirubin photoisomers produced during phototherapy in newborns and thus in elucidating the mechanism by which phototherapy lowers the concentration of bilirubin in newborn blood. , Several HPLC methods are available for analysis of bilirubin fractions. In the method of Blanckaert, bilirubin conjugates, but not unconjugated bilirubin, are converted to the corresponding bilirubin methyl esters by base-catalyzed transesterification in methanol followed by extraction with chloroform. With this procedure, the α-, β-, and γ-bilirubin fractions are recoverable, but the δ-fraction (δ-bilirubin) remains in the denatured protein pellet that is produced by the chloroform extraction. In the HPLC method of Lauff and coworkers, all four bilirubin fractions remain in solution after a step that involves salting out globulins with sodium sulfate. Both methods require the use of dim incandescent or yellow light to minimize photodegradation of the various bilirubin species. A simple and fast HPLC method has been published by Adachi and associates ; this method uses a Micronex RP-30 column (Sekisui Chemical Co., Mount Laurel, NJ), which does not require salting out of globulins or chemical transformation of bilirubin conjugates. This method separates serum bilirubin into five fractions; the fifth fraction eluted between the monoglucuronide and the unconjugated bilirubin is the Z,E or the E,Z photoisomer. The elution sequence is the same as in the procedure of Lauff and colleagues. Osawa and associates have successfully developed an isocratic mobile phase. The elution buffer includes 0.8% sodium ascorbate to maintain the stability of the bilirubin species. Isolation was improved with the addition of 1% Brij 35. This method strongly correlates with the HPLC method by Adachi and colleagues. Using the method by Osawa and colleagues, molar absorptivities for unconjugated bilirubin, bilirubin monoglucuronide, bilirubin diglucuronide, and δ-bilirubin were calculated at 450 nm, giving this method the potential for evaluating the accuracy of bilirubin assays.
Additional studies have indicated that the δ-bilirubin fraction consists of one or more bilirubin species that are covalently bound to albumin. Existence of covalent linkage is supported by the fact that the associated bilirubin species are not released from the albumin fraction by treatment with strong acid or base, or a variety of strong denaturing agents, by hydrolysis with proteolytic enzymes, or by boiling in methanol. Delta-bilirubin reacts directly (without a promoter) with diazotized sulfanilic acid. The discovery of δ-bilirubin has solved the mystery of persistent high bilirubin concentrations that mostly direct react in patients with intrahepatic or obstructing jaundice long after hepatitis has subsided or obstruction has been relieved. It is the slowest fraction to clear from serum because it follows the catabolism of albumin, which has a half-life of approximately 17 to 19 days.
HPLC has been helpful in elucidating the nature of the bilirubin species that occur naturally in blood or are formed during phototherapy. Clinically, it offers little, if any, aid to the physician in the differential diagnosis of jaundice, because knowing the percentage of each of the bilirubin fractions in blood is of no diagnostic value. It cannot be considered as a reference method for measuring total bilirubin in blood because its accuracy and precision are inadequate. The method is calibrated with unconjugated bilirubin with the untested assumption that the other three bilirubin fractions have molar absorptivities identical to that of the calibrator, when in fact this is not known. Furthermore, errors in measurement of the four species may be cumulative and may result in a large total error; also, the method is insensitive at total bilirubin concentrations of less than 1 mg/dL (17 μmol/L) and is too laborious for routine clinical analysis. Some of the δ-bilirubin may be lost during pretreatment of samples.
A capillary electrophoresis method for measuring the different types of bilirubin has been developed by Wu and his associates.
Enzymatic methods for total and direct bilirubin and for bilirubin conjugates with glucuronic acid are based on the oxidation of bilirubin with bilirubin oxidase to biliverdin with molecular oxygen. At a pH near 8, and in the presence of sodium cholate and sodium dodecylsulfate, all four bilirubin fractions are oxidized to biliverdin, which is further oxidized to purple and finally colorless products. The decrease in absorbance at 425 or 460 nm is proportional to the concentration of total bilirubin. Results obtained by the bilirubin oxidase method were in good agreement with those obtained by the Jendrassik-Grof procedure. Direct bilirubin is measured at pH 3.7 to 4.5; at this pH range, the enzyme oxidizes bilirubin conjugates and δ-bilirubin, but not unconjugated bilirubin. , At pH 10, the enzyme selectively oxidizes the two glucuronides. , Delta-bilirubin is not oxidized at all, and only 5% of unconjugated bilirubin is measured as conjugates.
A noninvasive approach for measuring bilirubin was introduced in 1980 by Yamanouchi and colleagues. The first bilirubinometer (icterometer) was a reflectance photometer, which used two filters to correct for the color of Hb and required measurements at eight body sites. Efforts to improve the accuracy of such measurements have been successful and led to the development of devices of acceptable performance. Reports indicate that at least one of these devices (Bili Check SpectR x Inc., Norcross, GA) provides results that are within ±2 mg/dL (34 μmol/L) of those obtained using a serum diazo procedure. , Another study found that the Bili Check underestimated serum bilirubin when its concentration was greater than 10 mg/dL (170 μmol/L).
Although transcutaneous bilirubin measurements may not substitute for laboratory quantitative determinations, they provide instantaneous information, reduce the necessity for serum bilirubin determinations, spare infants the trauma of heelsticks, and save money. Furthermore, they are useful in determining whether it is necessary to draw blood in a jaundiced infant before initiating treatment, such as phototherapy or exchange transfusion (currently, this is extremely rare). Another application is predicting those babies who require follow-up according to the “hour-specific” serum bilirubin nomogram developed by Bhutani and coworkers.
Because only conjugated bilirubin is excreted in urine, its presence indicates conjugated hyperbilirubinemia. The most commonly used method for detecting bilirubin in urine involves the use of a dipstick impregnated with a diazo reagent. Dipstick methods are capable of detecting bilirubin concentrations as low as 0.5 mg/dL (9 μmol/L).
A fresh urine specimen is required because bilirubin is unstable when exposed to light and room temperature, and it may be oxidized to biliverdin (which is diazo negative) at the normally acidic pH of the urine. If the test is delayed, the sample must be protected from light and stored at 2 to 8 °C for no longer than 24 hours. The reagent strip (Chemstrip, Roche Diagnostics, Indianapolis, IL; Multistix, Siemens Healthcare Diagnostics, Deerfield, IL) is immersed in the urine specimen for no longer than 1 second and is read 60 seconds later. During this time, bilirubin reacts with a diazo reagent, yielding a pink to red-violet color, the intensity of which is proportional to the bilirubin concentration. The reaction mechanism for urinary conjugated bilirubin is the same as that described in Fig. 51.9 , except that 2,6-dichlorobenzene-diazonium-tetrafluoroborate is substituted for diazotized sulfanilic acid in the Chemstrip, and 2,4-dichloroaniline diazonium salt in the Multistix. Another commonly used test, more sensitive than the Multistix, is the Ictotest reagent tablet (Siemens Healthcare Diagnostics); in this semiquantitative procedure, the diazo reagent is p- nitrobenzenediazonium- p- toluenesulfonate.
Chemstrip and Multistix strips for bilirubin in urine are highly specific tests and have a low incidence of false-positive results. However, medications that color the urine red or that give a red color in an acid medium, such as phenazopyridine, can produce a false-positive reading. Large quantities of ascorbic acid or of nitrite also worsen the detection limit of the test. In practice, bilirubin is rarely measured in urine.
The most common method for measuring serum total bilirubin uses a diazo reagent.
Calibrators for bilirubin measurement use unconjugated bilirubin and ditaurobilirubin as a conjugated bilirubin surrogate.
The HPLC method is helpful in identifying different bilirubin species that naturally occur in blood; however, it is of limited use clinically.
While transcutaneous measurement of bilirubin has its advantages, it is not a substitute for quantitative bilirubin determinations.
The measurement of urobilinogen in urine is of no diagnostic value in the assessment of liver disease. The same applies to the measurement of urobilinogen in fecal 72- or 96-hour specimens. Both tests are obsolete and are not presented here.
Regulation of bile acid metabolism is a major function of the liver. Alterations in bile acid metabolism are usually a reflection of liver dysfunction. Cholesterol homeostasis is in large part maintained by the conversion of cholesterol to bile acids and subsequent regulation of bile acid metabolism. Bile acids themselves provide surface-active detergent molecules that facilitate both hepatic excretion of cholesterol and solubilization of lipids for intestinal absorption. Bile acid homeostasis requires normal terminal ileum function to absorb bile acids for recirculation (enterohepatic circulation). Alterations in hepatic bile acid synthesis, intracellular metabolism, excretion, intestinal absorption, or plasma extraction are reflected in derangements of bile acid metabolism.
Four major bile acids are known. Cholic acid and chenodeoxycholic acid, the primary bile acids, are synthesized in the liver. The sequence of reactions involved in the synthesis of cholic acid from cholesterol is shown in Fig. 51.10 . To date, nine inborn errors of bile acid synthesis have been identified; these can present with neonatal hepatitis, fat malabsorption, or neurologic defects that can progress to chronic liver disease or liver failure and death. The primary bile acids are metabolized (by bacterial 7α-dehydroxylase) in the intestinal lumen to the secondary bile acids—deoxycholic acid and lithocholic acid. Bile acids (through their carboxylate groups) are conjugated in the liver with the amino acid glycine or taurine. This decreases passive absorption in the biliary tree and proximal small intestine but permits conservation through active transport in the terminal ileum. Approximately 0.1 to 0.6 g of bile acids is lost in the feces daily.

Because they possess both polar and nonpolar regions, molecules of bile acids are able to solubilize biliary lipids. Such molecules align at water–lipid interfaces and reduce surface tension, acting as detergents. In an aqueous solution, bile acids aggregate to form small polymolecular aggregates approximately 5 nm in diameter called micelles, which are capable of incorporating cholesterol and phospholipids to form mixed micelles. Micellar solubilization of these water-insoluble constituents maintains cholesterol in solution. In the intestinal lumen, dietary cholesterol and the products of triglyceride digestion (predominantly free fatty acids and monoglycerides) are incorporated into mixed micelles. Micelles deliver lipolytic products to the mucosal surface. To carry out these functions, a critical micellar bile acid concentration of approximately 2 mmol/L is necessary. Bile acids are thus important for ensuring the solubility of cholesterol (a major component of most gallstones) in bile and dietary lipids (including fat-soluble vitamins) in the intestinal lumen.
In view of the multiple processes involved in bile acid synthesis, conjugation, and excretion, and in its hepatic and intestinal uptake, several potential sites for primary or secondary disturbances have been identified ( Box 51.1 ). With hepatocyte dysfunction (which occurs in many liver disorders), decreased bile acid synthesis results in low primary bile acid concentrations and a decreased ratio of primary to secondary bile acids in plasma; in addition, decreased extraction from plasma often leads to increased concentrations of bile acids, particularly in the nonfasting state. With cholestatic disorders, decreased delivery of primary bile acids to the intestine with resulting decreased secondary bile acid production causes an increased ratio of primary to secondary bile acids, as well as increased total bile acid concentrations. With intestinal disease (including bypass operations that may be performed to treat obesity), increased fecal loss of bile acids leads to decreased concentrations of both primary and secondary bile acids and often a decrease in plasma cholesterol concentration caused by an increased need for bile acid synthesis. Although plasma bile acid concentrations are abnormal in many situations, their measurement adds little to standard tests of liver function, and they are rarely used in clinical medicine except in the investigation of unexplained pruritus. This is because of the wide biological variation due to changes in prandial state and due to diurnal rhythm which is independent of food intake.
Defective bile acid synthesis
Inherited defects in bile acid synthesis
Acquired defects in bile acid synthesis secondary to liver disease
Extrahepatic bile duct obstruction
Bile acid malabsorption
Effective uptake or altered intracellular metabolism
The value of measuring total bile acid in serum or plasma for diagnosing intrahepatic cholestasis in pregnancy has been recently highlighted and endorsed by a number of scientific societies. In particular, the guidelines of the UK Royal College of Obstetricians and Gynaecologists state that abnormal values of aminotransferases, GGT, and total bile acids are sufficient to support the diagnosis of obstetric cholestasis, and also provide valuable therapeutic information for preventing fetal complications attributable to the toxic effects of bile salts. The reference range of total bile acids in the serum of pregnant women has been recently established at 0.3 to 10 μmol/L, whereas serum or plasma values greater than 40 μmol/L are diagnostic of severe obstetric cholestasis and were found to be strongly associated with impaired fetal outcome.
Analytical techniques used to quantify total or individual bile acids in biological fluids include gas-liquid chromatography, HPLC, enzymatic assay, radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and high-resolution tandem mass spectrometry.
The liver has extensive synthetic capacity and plays a major role in the regulation of protein, carbohydrate, and lipid metabolism (see Chapters 31 , 35 , and 36 ). A bidirectional flux of precursors and products, such as glucose, amino acids, free fatty acids, and other nutrients, occurs across the hepatocyte membrane. Normal blood glucose concentrations are maintained during short fasts by the breakdown of hepatic glycogen and during prolonged fasts by hepatic gluconeogenesis. The primary sources of carbon atoms for gluconeogenesis are amino acids derived from muscle proteins. To a lesser extent, lactate (produced in skeletal muscle and erythrocytes) and glycerol (obtained from hydrolysis of triglycerides) also serve as substrates for gluconeogenesis. In humans, the oxidation of odd-numbered fatty acids yields propionyl-coenzyme A (CoA), which can be converted to glucose. However, the formation of glucose in this manner is not quantitatively significant. Protein, triglyceride, fatty acid, cholesterol, and bile acid synthesis also occur within the liver.
The liver is the primary site of the synthesis of most plasma proteins (see Chapter 31 ). Synthesis occurs in the rough endoplasmic reticulum of hepatocytes, followed by release into the hepatic sinusoids. Although disturbances of protein synthesis occur as a consequence of impaired hepatic function, a variety of other factors may affect plasma protein concentrations. These include decreased availability of amino acids (malnutrition, maldigestion, and malabsorption), catabolic states (hyperthyroidism, Cushing syndrome, burns, postsurgery recovery), protein-losing states (nephrotic syndrome and protein-losing enteropathy), actions of cytokines (decrease in transport proteins, such as albumin, transferrin, and lipoproteins, but an increase in inflammatory response modifiers such as α 1 -antitrypsin [AAT], ceruloplasmin, and α 2 -macroglobulin), action of hormones (such as growth hormone [GH], cortisol, estrogen, androgens, and thyroid hormones) to increase or decrease production of specific proteins, and congenital deficiency states (Wilson disease and AAT deficiency). In addition, the liver has a significant reserve capacity that prevents protein concentrations from decreasing unless liver damage is extensive. In addition, many liver proteins have relatively long half-lives, such as albumin, which lasts approximately 3 weeks. For this reason, the sensitivity and specificity of protein concentrations for diagnosis of liver disease are far from ideal.
The patterns of plasma protein alterations seen in liver disease depend on the type, severity, and duration of liver injury. For example, in acute hepatic dysfunction, there is usually little change in the plasma protein profile or the total plasma protein concentration; with fulminant hepatic failure or severe liver injury, concentrations of short-lived hepatic proteins (such as transthyretin and prothrombin) fall quickly and become abnormal, whereas those of proteins with longer half-lives are normal or minimally changed. In cirrhosis, concentrations of liver-synthesized plasma proteins and Igs decrease and increase, respectively. Serial determination of plasma proteins provides prognostic information; for example, worsening of prothrombin time (PT) during acute hepatitis suggests a poor prognosis.
Albumin, the most commonly measured plasma protein, is synthesized exclusively by the liver. The rate of synthesis varies, depending on the hormonal environment, nutritional status, age, and other local factors. In inflammatory conditions, IL-6 inhibits albumin synthesis but induces synthesis of acute-phase response proteins (see Chapter 31 ). With liver disease, hypoalbuminemia is noted primarily in cirrhosis, autoimmune hepatitis (AIH), and alcoholic hepatitis. The mechanism is multifactorial. In cirrhosis, hepatic synthesis of albumin may be decreased, normal, or increased. Loss of albumin into ascitic fluid seems to be responsible for the decrease in albumin in many cases. There is a strong correlation between serum albumin and mortality risk in a wide range of diseases.
One important consideration in measurement of albumin is the inaccuracy of dye-binding methods in patients with liver disease. Although bromocresol green measurements tend to overestimate albumin concentration at low concentrations, bromocresol purple methods give falsely low values in patients with jaundice because of the interference of bilirubin at the site of binding.
This protein has a short half-life of 24 to 48 hours, making it a sensitive indicator of current synthetic ability. Transthyretin is typically decreased in cirrhosis (among other conditions) as a result of decreased synthesis. It is more commonly used as a measurement of nutritional status.
Plasma Ig concentrations are commonly increased in cirrhosis, AIH, and primary biliary cirrhosis (PBC), but they are normal in most other types of liver disease. IgG is increased in AIH and cirrhosis; IgM is increased in PBC. IgA tends to be increased in all types of cirrhosis. None of these findings are specific, and they are seldom used in the diagnosis of liver disease, but IgG concentrations can be used to track response to treatment and disease activity in AIH.
The concentration of this protein is decreased in Wilson disease, cirrhosis, and many causes of chronic hepatitis, but it may be increased by inflammation, cholestasis, hemochromatosis, pregnancy, and estrogen therapy. It is discussed in greater detail in the section on Wilson disease.
Concentrations of this protein, which is the major serine protease inhibitor (serpin) in plasma, is decreased in homozygous deficiency and cirrhosis, and is increased by acute inflammation. It is discussed in greater detail later in the section on Alpha 1 -Antitrypsin Deficiency.
The concentration of this protein, a normal component of fetal blood, falls to adult values by 1 year of age. Mild increases are seen in patients with acute and chronic hepatitis and indicate hepatocellular regeneration. It is present at higher concentrations in hepatocellular carcinoma (HCC), and is discussed in greater detail later and in Chapter 33 .
The coagulation proteins that are synthesized in the liver are listed in Table 51.1 . These proteins interact to produce a fibrin clot (see Chapter 79 ). Inhibitors of the coagulation system, including antithrombin, protein C, and protein S, are also synthesized in the liver. Some of the coagulation factors (II, VII, IX, and X) require vitamin K for post-translational carboxylation within the hepatocyte. Proteins C and S are also carboxylated by a vitamin K–dependent enzyme. Activated protein C in plasma inhibits coagulation by inactivating factors V and VIII. Parenchymal liver disease of sufficient severity to impair protein synthesis or obstructive liver disease sufficient to impair intestinal absorption of vitamin K is, therefore a potential cause of bleeding disorders. Because of the great functional reserve of the liver, failure of hemostasis usually does not occur except in severe or long-standing liver disease.
| Number or Abbreviation | Name |
|---|---|
| I | Fibrinogen a |
| II | Prothrombin a , b |
| III | Tissue factor |
| IV | Calcium (Ca 2 + ) |
| V | Proaccelerin a |
| VI | — |
| VII | Proconvertin a , b |
| VIII | Antihemophilic factor |
| IX | Christmas factor a , b |
| X | Stuart-Prower factor b |
| XI | Plasma thromboplastin antecedent a |
| XII | Hageman factor a |
| XIII | Fibrin-stabilizing factor a (Laki-Lorand factor) |
| PK | Prekallikrein (Fletcher factor) a |
| HMWK | High-molecular-weight kininogen a |
PT depends on the activity of fibrinogen (factor I), prothrombin (factor II), and factors V, VII, and X. Because all of these factors are made in the liver, and several are vitamin K–dependent, a prolonged PT often indicates the presence of significant liver disease. In cholestasis, vitamin K deficiency may cause an increase in PT. In this case, the coagulation abnormality is corrected in 1 to 2 days by parenteral injection of 10 mg of vitamin K. In contrast, if PT is prolonged because of hepatocellular disease, factor synthesis is decreased, and administration of vitamin K does not typically correct the problem. PT is also prolonged in some patients with liver disease because of the presence of dysfibrinogenemia, an abnormal form of fibrinogen that does not clot normally, which may predispose patients to thrombosis.
The method for reporting PT in liver disease remains controversial. PT measures the time for plasma to clot after exposure to tissue factor. Reagents differ in the amount of tissue factor present; in patients on warfarin, clotting times are more greatly prolonged when lower amounts of tissue factor and other reagents that stimulate clotting are in the reagents. This makes a reagent more sensitive to clotting factor abnormalities but makes standardization of results among laboratories difficult. The international normalized ratio (INR) was developed by the World Health Organization (WHO) and the International Committee on Thrombosis and Hemostasis for reporting the results of blood coagulation (clotting) tests. All results are standardized using the international sensitivity index (ISI) for the particular thromboplastin reagent and instrument combination used to perform the test. In practice, it requires determination of the ISI based on the slope of the relationship between PT using the reagent and that using a reference method in patients on warfarin. The INR is then calculated as follows:
INR has been found to standardize interpretation of PT measurements among laboratories for those taking warfarin. Unfortunately, INR does not have the same relationship with impairment of clotting in individuals with liver disease. The liver is responsible for the synthesis of nearly all clotting factors and their inhibitors. As a result, patients with chronic liver disease and cirrhosis experience a rebalancing of their hemostatic variables. Patients with acute liver failure (ALF) likely experience minimal effects on their in vivo coagulation profiles as assessed with thromboelastography (TEG) despite mean INR values greater than 3. Furthermore, these patients have significant rates of hypercoagulable (35%) and hypocoagulable (20%) states. To further complicate matters, the presence of a hypercoagulable state does not exclude the presence of a tendency toward increased bleeding risk, and conversely, increased bleeding risk does not rule out the development of a new thrombus. The apparent explanation lies in the mechanism of clotting factor deficiency in liver disease and warfarin administration. Although liver disease inhibits synthesis of clotting factors, warfarin impairs vitamin K–dependent carboxylation, which impairs the ability of the factors to bind calcium. These noncarboxylated clotting factors (termed proteins induced by vitamin K absence [PIVKAs]) appear to act as inhibitors of coagulation; thus when lower amounts of tissue factor are present, clotting times are more prolonged. In contrast, in liver disease, factor deficiency is due to impaired factor synthesis, and no PIVKAs are present (except in HCC, as discussed later in this chapter). This leads to lesser increases in PT in individuals with liver disease and an underestimation of the degree of clotting impairment when reagents with a low ISI are used. Studies have shown that calculation of a different ISI using plasma samples from patients with liver disease can standardize PT results with different reagents, , but to date, such liver ISI information is not readily available to laboratories.
The liver plays a key role in the metabolism of lipids and lipoproteins (see Chapter 36 ). On a daily basis, approximately 33% of the fatty acids originating from adipose tissue enter the liver, where they undergo esterification into triglycerides or are oxidized. Oxidation is favored in the fasting state and esterification is favored in the nonfasting state. Excessive esterification results in fatty liver, a disorder in which excess triglycerides are deposited in large vacuoles that displace other cellular components. Most cholesterol is synthesized endogenously, in the liver. The endogenously synthesized cholesterol and that of dietary origin enter the hepatic pool, where they are converted to bile acids, incorporated into lipoproteins, or used in the synthesis of liver cell membranes. The relative rates of secretion of bile acids, cholesterol, and lecithin are important factors in the pathogenesis of cholesterol gallstones.
Patients with end-stage liver disease may have low concentrations of urea in plasma (see Chapters 34 and 49 ). The rate of urea excretion in urine is lower in these patients than that in healthy individuals. In addition, plasma concentrations of urea precursors—ammonia and amino acids—are increased. In nonalcoholic steatohepatitis, the function of urea cycle enzymes may be affected, resulting in hyperammonemia and the risk of disease progression. In nonalcoholic steatohepatitis (NASH) animals, gene and protein expression of ornithine transcarbamylase (OTC) and carbamoylphosphate synthetase were reversibly reduced. Hypermethylation of urea cycle enzymes is a potential underlying mechanism. The functional changes of urea synthesis in NASH are associated with hyperammonemia. Hyperammonemia can cause progression of liver injury and fibrosis.
Hypermethylation of Otc gene promoters has been observed. Additionally, in animal models of NASH and in patients with NAFLD, OTC concentration and activity were reduced and ammonia concentrations were increased, which was further exacerbated in those with NASH. In primary hepatocytes, induction of steatosis was associated with Otc promoter hypermethylation, a reduction in the gene expression of Otc and Cps1 , and an increase in ammonia concentration. These findings suggest that patients with liver disease have an impaired ability to metabolize protein nitrogen and to synthesize urea. The rate of hepatic urea synthesis also depends on exogenous intake of nitrogen and on endogenous protein catabolism.
A recurring theme is the central importance of the liver in metabolic and regulatory pathways. The functional expression of the complex, integrated organelle structure includes the metabolism of drugs (activation and detoxification) and the disposal of exogenous and endogenous substances, such as galactose and ammonia. In addition, metabolic abnormalities due to specific inherited enzyme deficiencies can affect the liver. A classic example is galactosemia. In this condition, congenital absence of galactose 1-phosphate uridyltransferase allows accumulation of the toxic metabolite galactose 1-phosphate, which causes injury to the liver, brain, and kidneys.
Ammonia is a by-product of nitrogen metabolism, and its formation in the body is predominantly a consequence of the action of the enzyme glutaminase, located within enterocytes of the small intestine and colon, as well as the action of the vast number of urease-producing bacteria located in the gut. Plasma ammonia concentration in the hepatic portal vein is typically fivefold to tenfold higher than that in the systemic circulation. Under normal circumstances, most of the portal vein ammonia load is metabolized to urea in hepatocytes through the Krebs-Henseleit (urea) cycle during the first pass through the liver; this process includes intramitochondrial and cytosolic enzyme-catalyzed steps ( Fig. 51.11 ).

Ammonia enters the tissue of the central nervous system by passive diffusion. The rate of entry increases in proportion to the plasma concentration and is dependent on pH. Ammonia crosses the blood–brain barrier more readily than the ammonium ion. As pH increases, the rate of entry of ammonia into the central nervous system tissue increases as the result of an increase in ammonia relative to ammonium. Because the acid dissociation constant (pK a ) of ammonia is 9.1 at 37 °C, approximately 3% of total blood ammonia is ammonia at the normal physiological pH of 7.4. An increase in pH to 7.6 produces an increase in ammonia to approximately 5% of total blood ammonia, which is a 67% increase in concentration.
Animal and human studies have shown that an increased concentration of ammonia (hyperammonemia) exerts toxic effects on the central nervous system. Several causes, both inherited and acquired, of hyperammonemia are known. Inherited deficiencies of urea cycle enzymes are the major cause of hyperammonemia in infants. The two major inherited disorders are those that involve the metabolism of the dibasic amino acids lysine and ornithine and those that involve the metabolism of organic acids, such as propionic acid, methylmalonic acid, isovaleric acid, and others (see Chapter 60 ). Insult to the liver, whether acute or chronic in nature, reduce its capacity to metabolize ammonia and this creates an ammonia burden on extrahepatic tissues which can result in hyperammonemia up to five times that of normal blood ammonia concentrations. The occurrence of hyperammonemia is not specific to liver dysfunction and can also be observed in various other disease states including, but not limited to, inborn errors of the urea cycle, Reye syndrome, and valproate poisoning.
The main acquired causes of hyperammonemia are advanced liver disease and renal failure. Severe or chronic liver failure (which occurs in fulminant hepatitis or cirrhosis, respectively) leads to significant impairment of normal ammonia metabolism. Reye syndrome, which is primarily a central nervous system disorder with minor hepatic dysfunction, is also associated with hyperammonemia. Hepatic encephalopathy in the cirrhotic patient is often precipitated by GI bleeding, which enhances ammonia production through bacterial metabolism of protein found in blood. Other precipitating causes of encephalopathy include excess dietary protein, constipation, infection, drugs (particularly central nervous system depressants and those that alter blood biochemistry such as diuretics), and electrolyte and acid-base imbalance (alkalosis). Because cirrhosis is accompanied by portosystemic shunting, ammonia clearance is impaired, leading to increased concentrations of blood ammonia. Impaired renal function also causes hyperammonemia. As blood urea concentration increases, more diffuses into the GI tract, where it is converted to ammonia.
The fasting venous plasma ammonia concentration is useful in the differential diagnosis of encephalopathy, when it is unclear whether encephalopathy is of hepatic origin. It is especially helpful in diagnosing Reye syndrome and the inherited disorders of urea metabolism, as well as increased ammonia concentrations due to drugs such as salicylates or valproate. In acute liver injury, ammonia concentrations more than 200 μmol/L (340 μg/dL) are associated with cerebral edema and a poor prognosis, and it has been suggested that ammonia concentrations should be used as part of the evaluation of prognosis in ALF. However, plasma ammonia is not useful in patients with known chronic liver disease. Although ammonia concentrations are higher as the degree of encephalopathy worsens, significant overlap between concentrations is seen in different stages of encephalopathy, and approximately 70% of those with cirrhosis without encephalopathy have increased ammonia concentrations. Ammonia concentrations may actually better reflect the presence of shunting blood around the portal veins than the degree of liver dysfunction. There is growing recognition of the complex and synergistic relationship between ammonia, inflammation (sterile and nonsterile), and oxidative stress in the pathogenesis of hepatic encephalopathy which develops in patients with liver dysfunction.
The concentrations of analytes may be affected by numerous factors that should be explored in order to establish ideal sampling conditions, processes, handling, and storage. These include diurnal variation, effects of feeding and fasting, donor position, sample containers, preservatives, sample handling, and sample processing and storage, including the influence of storage time, temperature, and freeze-thawing (see Chapter 5 ).
Both enzymatic and chemical methods are used to measure ammonia in body fluids. An enzymatic assay with glutamate dehydrogenase is the most frequently used method. Plasma ammonia measurement is particularly susceptible to contamination, leading to falsely increased concentrations. Common preanalytical problems are discussed in Chapter 4 on Specimen Collection and Processing.
For the enzymatic method, the reference interval is 15 to 45 μg/dL (11 to 32 μmol/L). For more details on age-dependent values, see the Appendix on Reference Intervals. Laboratories should verify that these ranges are appropriate for use in their own settings.
Because the liver is a major processor of dietary and endogenous carbohydrates, liver disease affects carbohydrate metabolism in a variety of ways (see Chapter 35 ). However, none of the conventional modes of evaluating carbohydrate metabolism has value in the diagnosis of liver disease. Because the liver is the major site of both glycogen storage and gluconeogenesis, hypoglycemia is a common complication in certain liver diseases, particularly Reye syndrome, fulminant hepatic failure, advanced cirrhosis, and HCC.
Xenobiotics are chemical substances that are foreign to the biological system. Biochemically, they are cleared and/or metabolized by the liver; some have been used as the basis of tests of liver function. Rates of metabolism of these compounds are sometimes referred to as quantitative liver function tests, to distinguish them from the more commonly used term, liver function tests, which is often used to refer to measurements of liver-associated enzymes. As liver disease progresses, quantitative liver function test results gradually worsen, but their measurement adds little to that obtained by widely used tests such as bilirubin, albumin, and INR measurement. Even when these tests are used, significant overlap of values is noted in persons with cirrhosis and less severe degrees of liver scarring, which limits their usefulness.
Dye excretion tests, such as BSP and indocyanine green clearance, were formerly used as indicators of liver disease. With the development of more sensitive and specific indicators of liver disease, dye excretion tests have become obsolete, although until the 1970s, BSP was the most frequently used dye excretion test. Because of reports of fatalities resulting from hypersensitivity and other adverse effects, BSP use has been discontinued. Indocyanine green clearance is still occasionally used for investigating hepatic blood flow and for predicting clearance rates of drugs that undergo first-pass clearance by the liver, such as lignocaine. Typical indocyanine green clearance values in healthy subjects range from 6.5 to 14 mL/min/kg body weight.
A variety of drugs that are metabolized by the liver have been used to study the action of various P 450 (mixed-function oxidase) enzymes. Aminopyrine is demethylated to form carbon dioxide and aminoantipyrine. With the use of 13 C- or 14 C-labeled aminopyrine, the resulting isotopically labeled carbon dioxide is measured in breath as a reflection of functioning liver mass. Decreases in metabolism are common in persons with cirrhosis, but metabolism is also affected by other factors such as cigarette smoking and use of drugs such as oral contraceptives; significant intraindividual variation in results has been noted. Overall diagnostic sensitivity is similar to that of other more routine laboratory tests.
Caffeine clearance is altered during hepatic injury; it is prolonged in both chronic hepatitis and cirrhosis. Caffeine is rapidly and nearly completely absorbed from the GI tract and then undergoes N-demethylation by the hepatic mixed-function oxidase system. A single dose of caffeine (3.5 mg/kg to a maximum dose of 200 mg, dissolved in water, fruit juice, or milk for oral administration) is administered. This caffeine dose is equivalent to that found in one cup of brewed coffee or in one can of a commercial soft drink.
Blood (or salivary samples) obtained before and at timed intervals after caffeine ingestion can be analyzed by reversed-phase HPLC or immunoassay. A close correlation is found between plasma and salivary caffeine concentrations. Caffeine half-life is approximately 5.5 hours in healthy adults and 3 hours in healthy children, with clearance of approximately 2 mL/min/kg in healthy adults and 10 mL/min/kg in healthy children. Caffeine clearance correlates with the aminopyrine breath test and has similar limitations, although it is less subject to effects of variables, such as smoking and oral contraceptive use. Lidocaine undergoes N-deethylation in the liver by cytochrome P 450 to form monoethylglycinexylidide (MEGX); the rate of appearance of MEGX in plasma reflects hepatic lidocaine clearance. Because lidocaine is highly extracted, its clearance is flow dependent. Thus alterations in hepatic blood flow also influence lidocaine elimination. Lidocaine (1 mg/kg) is given by intravenous bolus; plasma is obtained at baseline and at 15 minutes for MEGX concentration (time of plateau concentration in healthy individuals). MEGX is most commonly measured using an immunoassay. Lidocaine clearance has been used to assess liver transplantation function, but its use is limited by the effect of hypoperfusion (which occurs in sepsis or volume depletion).
Because individual cells are unable to store a sufficient supply of energy-rich carbohydrate substrates, the liver serves as the major site for their storage. For example, hepatic storage of glycogen allows the release of glucose to other tissue when the need exists (e.g., when plasma concentrations of glucose decrease). Other tissues, such as muscle and adipose tissue, store proteins and triglycerides, respectively, and are capable of adaptation. Depending on the availability of oxidizable fuels, these tissues also switch from the storage mode to the synthesis or release mode during periods of decreased carbohydrate intake.
Various characteristics indicate the presence of liver disease, including peripheral signs such as jaundice and spider naevi; evidence of fibrosis and portal hypertension and evidence of abnormalities of renal function, drug metabolism, hemostasis, metabolic abnormalities, and release of enzymes into various body fluids.
As the first solid organ beyond the gut to process ingested antigens, the liver is constantly exposed to antigen-rich blood; therefore it is a major line of defense against such antigens, especially microorganisms. Both the adaptive and innate immune systems of the liver are highly evolved to serve this function. Fibrosis should be considered as a normal component of the innate immune response to tissue injury, and as such, is controlled by the cells and products of the immune system. Both the innate and adaptive immune systems play an important role in hepatic fibrosis modulation. For example, in the liver, type I collagen (which predominates in fibrotic scar) protects hepatocytes against toxic stimuli. Inflammation and tumorigenesis are tightly linked pathways impacting cancer development. Inflammasomes are key signaling platforms that detect pathogenic microorganisms, including hepatitis C virus (HCV) infection, and sterile stressors (oxidative stress, insulin resistance, lipotoxicity) able to activate pro-inflammatory cytokines IL-1β and IL-18. In the liver, this inflammation can be due to acute or chronic viral hepatitis, AIH, alcohol or bile salt exposure, or fatty liver disease. Hepatic dysfunction is caused by degeneration and necrosis of epithelial cells (hepatocytes and/or cholangiocytes), replacement of liver parenchyma by fibrotic tissues and regenerative nodules, and loss of liver function. In the liver, when the inflammatory insult becomes chronic, fibrosis can then lead to apoptosis and loss of the architectural integrity of the liver and cirrhosis. Regeneration of these epithelial cells is essential for architectural and functional recovery of the organ.
Hepatic fibrosis is a dynamic process characterized by the net accumulation of extracellular matrix (ECM), or scar, resulting from chronic liver injury of any etiology, including chronic viral infection, alcoholic liver disease, bile salt exposure, and NASH. Fibrosis is a physiologic response to a wide range of stimuli including the degeneration and necrosis of epithelial cells (hepatocytes and/or cholangiocytes) that leads to replacement of liver parenchyma by fibrotic tissues and regenerative nodules, and a resulting loss of liver function. The remaining liver parenchymal cells may proliferate within regenerative nodules. Although fibrosis can reverse after elimination of the cause of injury, chronic injury left unchecked can lead to cirrhosis, which is characterized by distortion of the hepatic architecture associated with abnormal blood flow and eventually portal hypertension. Decompensated liver disease is characterized by the onset of reduced hepatic function and disordered blood flow leading to portal hypertension and complications including ascites, hepatic encephalopathy, and variceal hemorrhage. Cirrhosis is a major cause of mortality world-wide and is associated with increased individual risk of HCC.
The process of hepatic fibrosis involves the activation of hepatic stellate cells (HSCs) (or portal fibroblasts in biliary disease), Kupffer cells, and an array of other cells, proteins, and signaling pathways. The complexities of these interactions are becoming better understood, and the currently known roles of the many players are summarized in the following.
HSCs reside in the space of Disse, interposed between the endothelium and hepatocytes, where they encircle the liver sinusoids. After liver injury, HSCs become activated by the products of apoptotic mesenchymal cells, which leads to the conversion of a resting vitamin A–rich cell (a quiescent HSC) to one that has lost vitamin A droplets by autophagy, which leads to increased proliferation and contraction, and the release of proinflammatory, profibrogenic, and promitogenic cytokines. The activated HSCs become contractile myofibroblasts that generate a scar that forms around the injury site.
HSC activation can be divided into two phases: initiation and perpetuation. Initiation, which is also known as the preinflammatory stage, refers to early changes in gene expression and phenotype. It is the result of primarily paracrine stimulation from damaged parenchymal cells. Maintenance of these stimuli leads to a perpetuation phase that is regulated by autocrine and paracrine stimuli. Perpetuation involves at least six distinct changes in HSC behavior, including proliferation, chemotaxis, fibrogenesis, contractility, matrix degradation, and retinoid loss.
The profibrotic myofibroblasts are the master regulators of the fibrotic response because of their scar-producing, proliferative, migratory, contractile, immunomodulatory, and phagocytic properties. Myofibroblasts are the prototypical mesenchymal cell type that regulates repair after an injury in a range of tissues, including liver, kidneys, skin, lungs, and bone marrow, as well as the central nervous system. Myofibroblasts, once activated, are capable of enhanced migration and deposition of ECM components. Although HSCs are the primary source of this fibrogenic population in the liver, other cells such as bone marrow–derived cells, portal fibroblasts, and epithelial-to-mesenchymal transition from hepatocytes and cholangiocytes also contribute to fibrogenesis, although their exact role in disease is not completely understood.
In the normal liver, the ECM provides structural and biochemical support to the surrounding cells and is composed mainly of a number of structural proteins (including collagens IV and VI), as well as a range of growth factors and matrix metalloproteinases (MMPs) that are specifically bound and preserved in latent forms. The ECM can modulate the activation and proliferation of HSCs, angiogenesis, and the availability and activity of growth factors and MMPs. The ECM also provides cells with signals for polarization, adhesion, migration, proliferation, survival, and differentiation. ECM–cell interactions are determined largely by specific membrane adhesion receptors. The ECM may prevent apoptosis in the damaged liver and also prevent growth factor proteolysis. Interactions between ECM and its surrounding cells are bidirectional. After injury, the fibrillary collagens I and III predominate together with fibronectin. Liver fibrosis as a consequence of liver injury entails both qualitative and quantitative changes in ECM composition as a result of an imbalance between the rates of matrix synthesis and degradation. The ECM becomes progressively insoluble and resistant to protease digestion because of the thickening of fibrotic septae and increased cross-linking. ,
MMPs, also known as matrixins, are the major family of calcium-dependent enzymes that degrade collagenous and noncollagenous ECM substrates. There are 25 members of this tightly regulated family, which are classified on the basis of their substrate specificity: interstitial collagenases, gelatinases, stromelysins, membrane types, and metalloelastases. MMPs are secreted as inactive proenzymes, have complex transcriptional control, and their action is inhibited by a family of endogenous proteinase inhibitors known as tissue inhibitors of metalloproteinases (TIMPs). Four TIMP members bind reversibly to the active site of all MMPs and have different affinities for specific MMPs. Thus TIMPs play an important role in preventing degradation of the accumulating matrix during liver injury by antagonizing the activity of MMPs. TIMP-1 also has an antiapoptotic effect on HSCs; it prevents clearance of activated HSCs during injury and promotes their survival through induction of B-cell lymphoma. HSCs are a key source of MMPs, especially MMP-2, -3, -9, and -13. In chronic human liver disease and animal models of fibrosis, concentrations of MMP-1 and/or -13 do not change, but there is a progressive increase in TIMP-1 and -2 as fibrosis advances. TIMP expression can be detected soon (6 hours) after liver injury and may precede the induction of procollagen I.
Fibrosis usually follows an inflammatory insult; therefore certain cytokines secreted by a range of cells, including Kupffer cells, HSCs, hepatocytes, natural killer cells, lymphocytes, and dendritic cells play a key role in the response. These include the chemokines (monocyte chemotactic protein-1, RANTES [Regulated on Activation, Normal T Expressed and Secreted; also known as CCL5 or C-C motif chemokine ligand 5], IL-8), interferons (IFN-α, IFN-γ), ILs (IL-1, IL-6, IL-10), growth factors, adipokines, and soluble neurohumoral ligands (endocannabinoids). Adipokines (adipose tissue cytokines) are polypeptides secreted mainly by adipocytes, and to a lesser extent, by stromal cells, including macrophages, fibroblasts, and infiltrating monocytes. Leptin and adiponectin are the main adipokines implicated in liver injury. ,
The hepatocytes are the major cell type in the liver and are also involved in the process of fibrosis and cirrhosis. Normally, hepatocytes can regenerate removed liver tissue rapidly, but in chronic disease states they appear to become senescent with respect to this function; however, the HSCs become activated and are sufficient to regenerate the biliary and hepatocellular epithelium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here