Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although ultracentrifugation and electrophoretic techniques are of historical significance, most useful lipid and lipoprotein testing methods are now enzymatic.
Low-density lipoprotein (LDL) cholesterol is considered the primary target in assessing cardiac risk and directing therapy.
LDL cholesterol can be measured directly but is usually calculated using the Friedewald equation and more recently using the Martin-Hopkins formula. The Martin-Hopkins formula has demonstrated improved accuracy at lower LDL levels and in the setting of hypertriglyceridemia.
Given the cumulative impact of hyperlipidemia over a lifespan, identifying and treating hyperlipidemia early reduces an individual’s lifetime risk for cardiovascular disease.
New guidelines emphasize a personalized approach to the management of hyperlipidemia, incorporating more detailed risk assessments and new cholesterol-lowering drug options.
Disorders of lipid metabolism play a major role in atherosclerosis and coronary heart disease (CHD). A clear-cut relationship is evident between elevated serum cholesterol and myocardial infarction. At the tissue level, cholesterol deposits occur in areas of endothelial cell damage and are a prominent part of atherosclerotic lesions. Although cholesterol may be considered “bad” because of its association with myocardial infarction, it is actually a vital structural component of cell membranes and a precursor of steroid hormones and bile acids. Another lipid, triglyceride, is a major source of energy for cells. Cholesterol and triglycerides are the most important lipids in the study and management of CHD risk.
Lipids are soluble in nonpolar organic solvents such as chloroform and ether but relatively insoluble in polar solvents such as water. Thus, cholesterol and triglycerides travel in plasma not as free-floating molecules but rather as part of water-soluble complexes called lipoproteins . These particles contain cholesterol in two forms: free cholesterol, a polar nonesterified alcohol (about 30%), and cholesteryl ester, a hydrophobic form wherein cholesterol is linked to a fatty acid (about 70%). The lipoprotein is arranged like a micelle ( Fig. 18.1 ). The most hydrophobic lipids, such as cholesteryl esters and triglycerides, are located in the core of the particle. Lipids with some hydrophilicity, such as free cholesterol and phospholipids, are arranged on the surface with polar groups pointing outward. Apolipoproteins (apo), the protein moiety of lipoproteins, are arrayed on the surface. They show amphiphilic characteristics; hydrophobic residues interact with the hydrophobic core, whereas hydrophilic residues interact outside the aqueous environment.
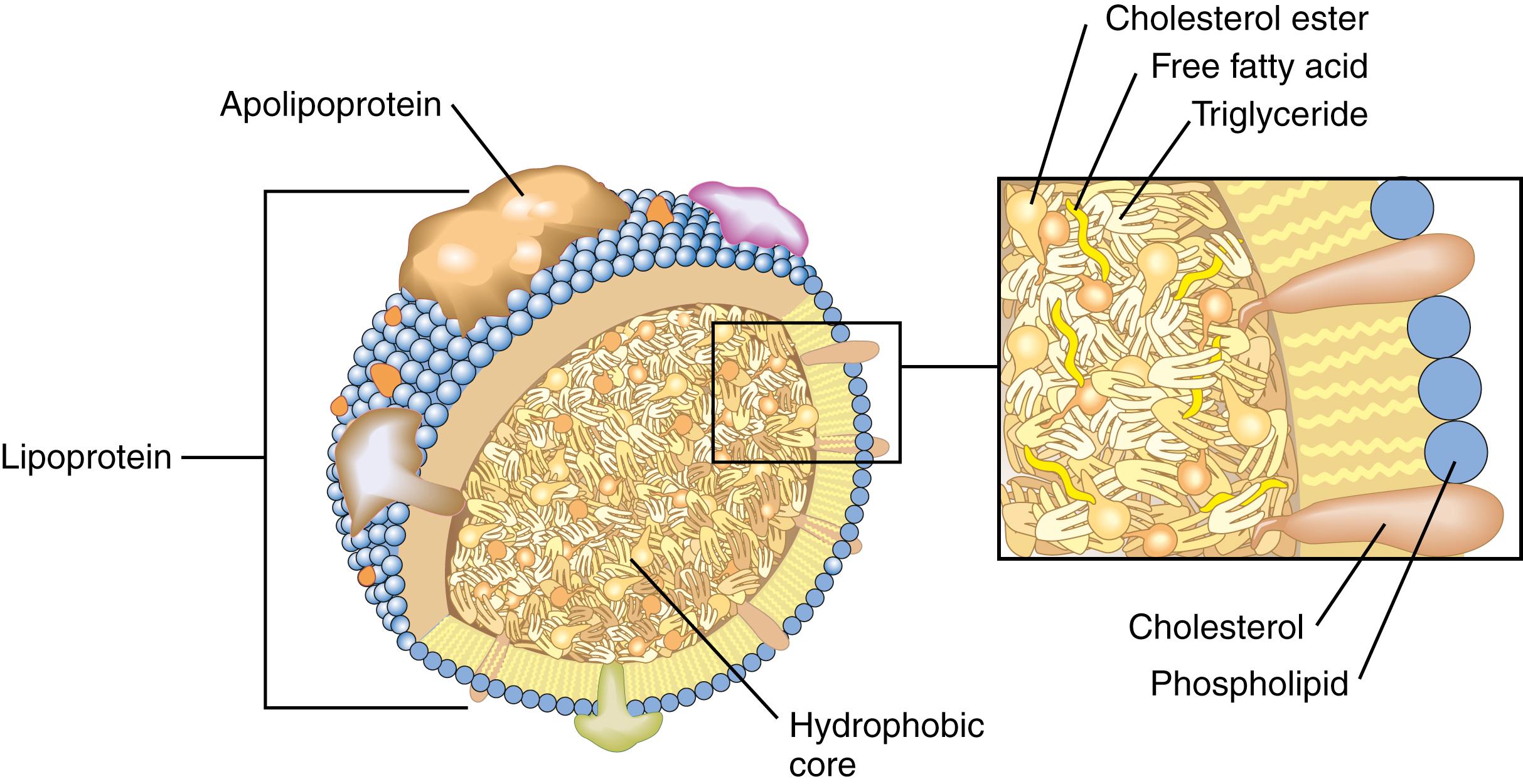
Four major lipoprotein classes are chylomicrons (CMs), very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). The classification is based on the buoyant density of the particles (see later discussion). Several minor lipoproteins have also been identified, including intermediate-density lipoprotein (IDL) and lipoprotein(a) (Lp[a]) ( Tables 18.1 and 18.2 ). Lipoproteins can be differentiated by density, particle size, chemical composition, and electrophoretic mobility. These physical properties are due to differences in protein, triglyceride, and cholesterol content and reflect the role of each lipoprotein in lipid metabolism (see later discussion). Each lipoprotein is associated with specific apos that play important roles in lipid transport, such as activating or inhibiting the enzymes involved in lipid metabolism and binding lipoproteins to cell surface receptors. The apo composition of the lipoprotein classes is summarized in Table 18.1 .
| Particle | Electrophoretic Mobility ∗ | Major Apolipoproteins | Diameter (Å) | Density (kg/L) | Sf † |
|---|---|---|---|---|---|
| Chylomicrons | Origin | ApoA-I, A-IV, B-48, C-I, C-II, C-III, E | 750–12,000 | <0.95 | >400 |
| VLDL | Pre-β | ApoB-100, C-I, C-II, C-III, E | 300–700 | 0.95–1.006 | 20–400 |
| IDL | β or pre-β | ApoB-100, E1.006–1.019 | 12–20 | ||
| LDL | β | ApoB-100 | 180–300 | 1.019–1.063 | 0–12 |
| HDL 2 | α | ApoA-I, A-II, E | 50–120 | 1.063–1.125 | |
| HDL 3 | α | ApoA-II, A-I, E | 50–120 | 1.125–1.210 | |
| Lp(a) | Pre-β | ApoB-100, Apo(a) | 1.045–1.080 |
| Protein (%) ∗ | Free Cholesterol (%) | Cholesteryl Esters (%) | Triglyceride (%) | Phospholipid (%) | |
|---|---|---|---|---|---|
| Chylomicrons | 1–2 | 1–3 | 2–4 | 80–95 | 3–6 |
| VLDL | 6–10 | 4–8 | 16–22 | 45–65 | 15–20 |
| IDL | Intermediate between VLDL and LDL | ||||
| LDL | 18–22 | 6–8 | 45–50 | 4–8 | 18–24 |
| HDL | 45–55 | 3–5 | 15–20 | 2–7 | 26–32 |
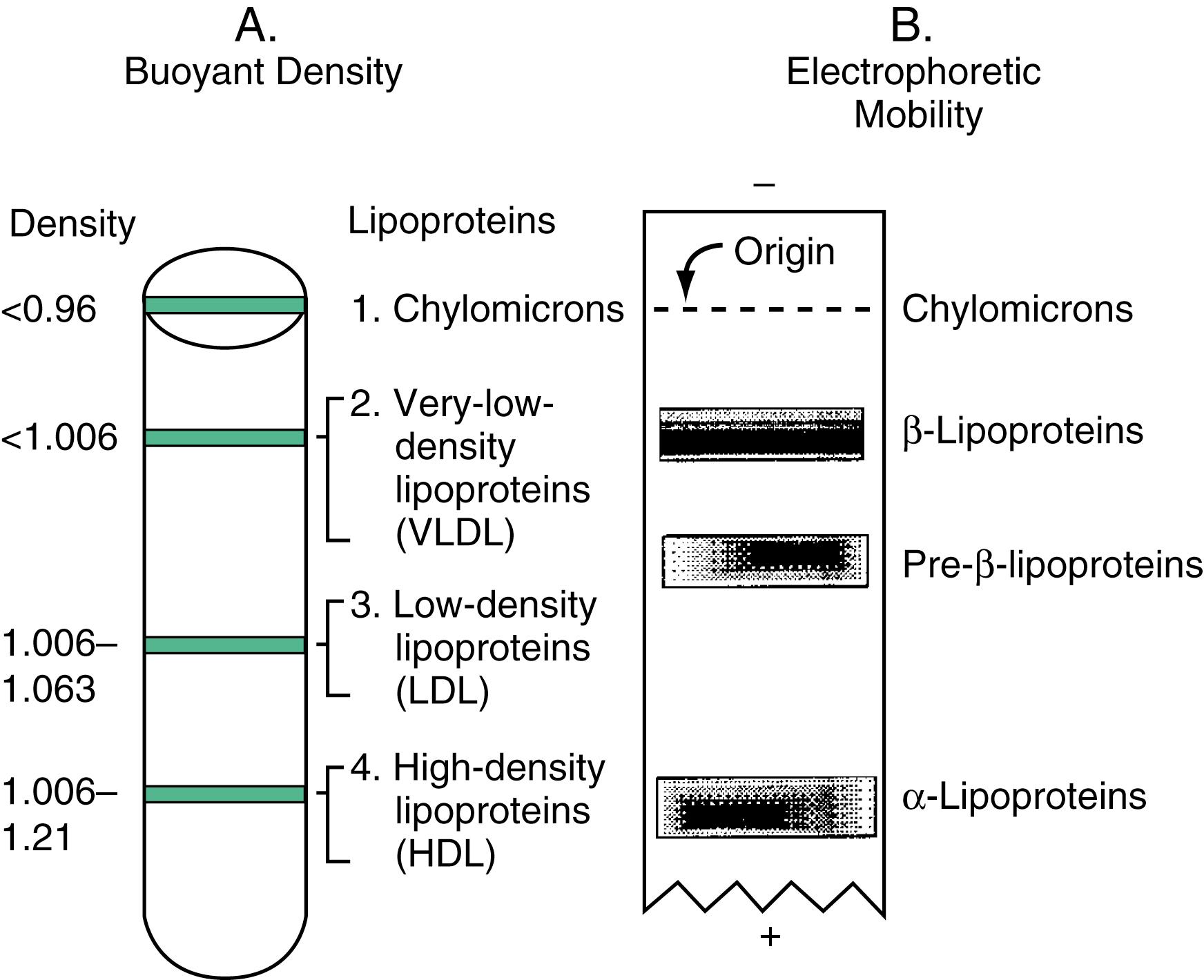
Lipoproteins are commonly differentiated from one another based on their buoyant density and electrophoretic mobility ( Fig. 18.2 ). Ultracentrifugation separates lipoproteins by buoyant density. Lipids and proteins have average densities of 1.0 and 1.4 g/mL, respectively. If centrifuged in solutions of different densities, these particles band at characteristic densities. The density of a lipoprotein particle is determined mostly by its protein and triglyceride content. As illustrated in Figure 18.2 , the higher the lipid content the less dense the lipoprotein particle, therefore, the closer they migrate to the top of the tube. Lipoproteins with high triglyceride and low protein content (CM and VLDL) are less dense than those with high protein and low triglyceride content (HDL). LDL and IDL are more dense than VLDL but less dense than HDL. When plasma lipoproteins are separated by agarose gel electrophoresis, CM remains at the origin and HDL migrates fastest in the α-region, followed by VLDL in the “pre-β” region and IDL and LDL in the β region.
Based on the presence and absence of apoB, lipoproteins can be classified as apoB-containing (CM, VLDL, IDL, LDL) and non–apoB-containing (HDL) lipoproteins. This classification is important because apoB is a nonexchangeable apolipoprotein, and only one molecule of apoB is present per lipoprotein. ApoB-containing lipoproteins (B-Lps) may or may not contain additional apolipoproteins such as apoA1, apoC, and apoE. ApoB serves as a structural protein for these lipoproteins and is always associated with these particles. B-Lps are synthesized mainly by hepatocytes and enterocytes.
B-Lps are mainly involved in delivering lipids to tissues for storage or use in energy production. CM (formed in the intestine from dietary fat) and VLDL (formed in the liver) are triglyceride-rich particles that are metabolized after entering the circulation. Through the action of lipoprotein lipase (LPL), found predominantly in microvasculature, they deliver fatty acids to tissues. The remnant CM and VLDL particle that have shed triglycerides and cholesterol esters are now transformed into denser lipoproteins with a higher percentage of cholesterol. The well-known LDL is the densest of these B-Lps, and elevated serum LDL-cholesterol (LDL-C) is a primary cardiac risk factor. Treatment of CHD-causing dyslipidemia is generally aimed at lowering LDL.
One way to understand heterogeneity in B-Lps is to view them as metabolic products in which CM and VLDL are acted upon by LPL, resulting in the release of free fatty acids to tissues with progressive decreases in their sizes. Because of this interaction with LPL, CM and VLDL become triglyceride depleted, denser, and relatively protein and cholesterol rich, giving rise to CM remnants and LDL. These particles are internalized and metabolized by cells—CM remnants by the liver and bone marrow cells and LDL by liver cells and by other cells throughout the body. LDL serves as the major source of cholesterol for tissues. This metabolic progression is presented in Figure 18.3 .
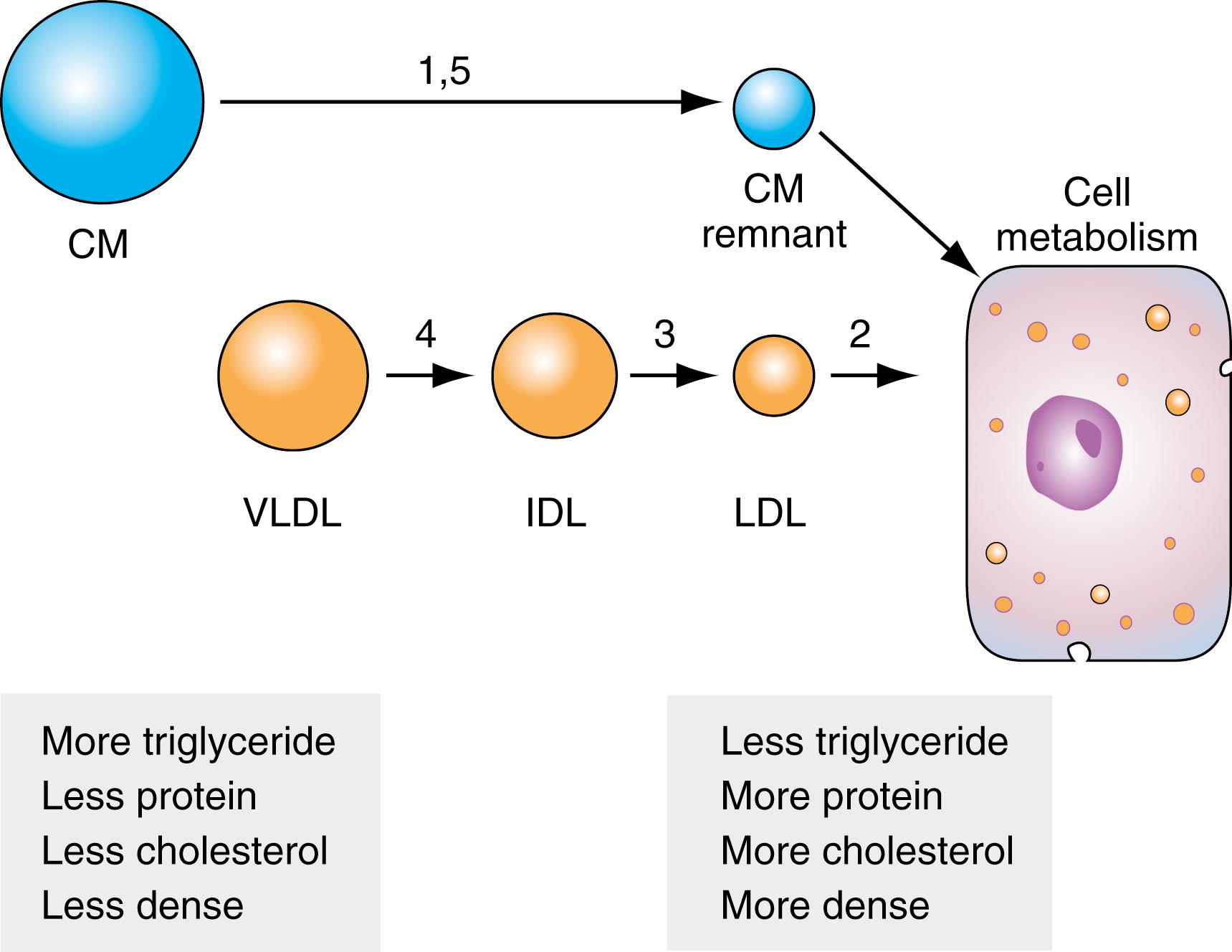
A block in any step of the pathway leads to the accumulation of one or more lipoproteins. In Figure 18.3 , the number over each step in the pathway represents the functional hyperlipoproteinemia (originally described by ) caused by a block between two intermediates. For example, a block in the progression from CM to CM remnants results in the accumulation of CM—Type 1 or 5 disease—and presents with high triglycerides and normal cholesterol. A block in the conversion of VLDL to IDL and LDL results in Type 4 disease (i.e., VLDL accumulation with elevated triglycerides and frequently elevated cholesterol). Often, the cause of Types 1, 5, and 4 disease is LPL deficiency and the resulting inability to break down triglycerides. Type 2 disease results from a block in LDL metabolism and may have a genetic basis. Four possible known defects include a defective apoB protein that does not bind to the LDL receptor (LDLR), a mutant LDLR that does not recognize apoB, a gain in function mutation in proprotein convertase subtilisin/kexin type 9 (PCSK9), or a defective LDL adaptor protein. Type 2 is further subcategorized based on triglyceride levels. Note that other studies (see later discussion) may be necessary to distinguish Type 2B and Type 3 because both present with elevated cholesterol and triglycerides. This functional classification is presented in Table 18.3 . No ideal system is known for classifying lipid disorders. They can be categorized in many different ways: primary versus secondary, hereditary versus acquired, and by lipoprotein fraction phenotypes. In general, each category is heterogeneous with respect to genetic, clinical, and pathologic factors. These are discussed in greater detail at the end of the chapter.
| WHO ICD and OMIM Numbers | Type | Particle | Triglycerides | Cholesterol | Comments |
|---|---|---|---|---|---|
| E78.3 238600 |
1 (familial chylomicronemia or LPL deficiency) | CM | High | Normal | Low cardiac risk; hereditary, found mostly in pediatric patients and young adults; autosomal-recessive mutation in LPL or APOC2 ; APOA5 , LMF-1 , and GPIHBP1 mutations are linked to this phenotype. |
| E78.0 143890 |
2A (heterozygous and homozygous familial hypercholesterolemia) | LDL | Normal | High | High cardiac risk; mostly polygenic disease; about 10% are monogenic; heterozygous form is due to mutations in LDLR , APOB , or PCSK9 ; homozygous form is due to mutations in LDLR or LDLRAP1 (ARH). |
| E78.4 144250 |
2B (combined hyperlipoproteinemia) | VLDL, LDL | High | High | High cardiac risk; polygenic disease; links to mutations in USF1 , APOB , and LPL . |
| E78.2 107741 |
3 (dysbetalipoproteinemia) | IDL | High | High | High cardiac risk; mutations in APOE gene or homozygous for E2 allele of APOE . |
| E78.1 144600, 145750 |
4 (primary hypertriglyceridemia) | VLDL | High | Normal | Lower cardiac risk than type 2 or 3; polygenic disease |
| E78.3 144650 |
5 (mixed hyperlipidemia) | VLDL, CM | High | High | Low cardiac risk; polygenic disease; 10% of patients have mutations in LPL , APOC2 , and APOA5 ; mutations in APOE , TRIB1 , CHREBP , GALNT2 , GCKR , and ANGPTL3 are thought to contribute to this disease. |
The major protein present in non–apoB-containing HDL is apoA-I, which makes up about 70% of the HDL protein. HDL is formed in the liver and small intestine. HDL plays a key role in reverse cholesterol transport, the process by which excess cholesterol is returned from tissues to the liver, where it is reused or excreted in bile. Besides apoA-I, HDL contains several apos. In addition to reverse cholesterol transport, other functions of HDL based on in vitro assays include anti-inflammatory, antioxidant, antithrombotic, and nitric oxide–inducing activities. Although the role of reverse cholesterol transport is suggested as a protective mechanism against atherosclerosis, these other properties of HDL might also contribute to the “protective” effect. Consequently, such protection may stem from HDL function rather than from increased plasma levels of HDL. In fact, interventions increasing HDL-C levels in plasma have not consistently shown clinical benefit ( ).
Lipoprotein particles are dynamic entities that acquire and shed protein and lipid components as they circulate in the body. As mentioned earlier, CM and VLDL particles lose triglycerides and become smaller as they are catabolized. Thus, young VLDL and CM particles are larger and less dense than their more mature counterparts. For this reason, it is best to view each of the lipoprotein classes not as a collection of identical particles but as a heterogeneous group. In fact, distinct lipoprotein subfractions or subclasses have been well described.
CMs are large particles produced by the intestine that transport lipids of dietary origin to the tissues of the body. They are very rich in triglycerides but relatively poor in free cholesterol, phospholipids, and protein. These particles are secreted into mesenteric lymphatics and reach circulation at the thoracic duct. These lipoproteins acquire apoC-II, a cofactor for LPL, from plasma. The interaction of CMs with LPL at the luminal surface of capillary endothelium results in the depletion of triglycerides and surface elements. The resulting smaller particles, called CM remnants, are removed from circulation by the liver ( ), primarily through the interaction of apoE with receptors such as proteoglycans, the LDLR, and the LDLR-related protein (LRP). The half-life of the CM in human circulation is short—only minutes. Because of the very high lipid/protein ratio, CMs are considerably less dense than water and float without centrifugation. When present at high levels, CMs result in “milky” plasma and accumulate as a floating creamy layer when left undisturbed for several hours. The apolipoproteins in CMs include apoB-48, apoA-I, apoA-IV, apoC-I, apoC-II, apoC-III, and apoE. In metabolism of CMs, apoC-II serves as an activator of LPL.
VLDL particles are produced by the liver and supply the tissues of the body with triglycerides of endogenous, primarily hepatic origin, and cholesterol. As compared with CMs, VLDL particles are smaller and produce turbid plasma when present in excessive amounts. They are rich in triglycerides, although to a lesser extent than CMs, and have a higher buoyant density because of their lower lipid/protein ratio. By mass, VLDL particles contain approximately 50% triglyceride, 40% cholesterol and phospholipid, and 10% protein, mostly apoB-100 and apoC-I, apoC-II, and apoC-III, but also apoE. VLDL particles vary widely in size and chemical composition. Larger particles are rich in triglycerides and apoC. Smaller particles have less of these two components. LPL hydrolyzes VLDL, and this produces highly atherogenic, smaller triglyceride and surface material–depleted particles called VLDL remnants and IDL. LDLRs on hepatocytes recognize apoE in CM remnants and VLDL remnants and remove them from plasma.
LDL is produced through the metabolism of VLDL/IDL in circulation and constitutes about 50% of the total lipoprotein mass in human plasma. The particles are much smaller than the triglyceride-rich lipoproteins (VLDL and CM) and do not scatter light or alter the clarity of plasma even at greatly increased concentrations. LDL consists of approximately 50% cholesterol, mostly esterified; 25% protein, mostly apoB-100 with traces of apoC; 20% phospholipid; and some triglycerides. Although each VLDL and LDL particle is thought to contain only one apoB-100 molecule, the extraordinary size of this protein allows it to be the largest protein component of these particles. The liver takes up most of the LDL in circulation (≈75%), with apoB-100 serving as a ligand for the hepatic LDLRs. The remaining LDL is delivered to other tissues. Some LDL is modified and is removed from the circulation by scavenger cells such as those found in atheromatous plaque. Small, dense LDL contains less cholesteryl ester, has a lower cholesterol/apoB ratio, and is believed to be atherogenic. Increased amounts of the small particles have been found in patients with several common forms of dyslipoproteinemia that are associated with CHD. People with high levels of small, dense LDL are at high risk of a CHD event, which is independent of total LDL concentration.
HDL is a small particle, consisting mostly of protein, cholesterol, and phospholipids, with only traces of triglycerides. ApoA-I is the major apolipoprotein for HDL, which is synthesized in the liver and small intestine. Different from B-Lps, most of HDL particles have more than one apoA-I molecule. Produced by the liver and intestine, HDL is involved in reverse cholesterol transport. In vitro studies suggest that HDL is involved in anti-inflammatory, antioxidant, antithrombotic, and nitric oxide–inducing mechanisms. The major site for the clearance of HDL cholesterol is the liver, and the best-understood mechanism is the selective uptake of cholesteryl esters from HDL by the SR-B1 receptor expressed in the liver. In the selective uptake process, SR-B1 promotes cholesteryl ester uptake without apoA-I degradation. Cholesteryl esters are removed from the internalized HDL particles, and cholesterol-depleted HDLs are re-secreted. Mouse models that lack SR-B1 exhibit increased plasma HDL, slower HDL uptake by the liver, and increased atherosclerosis. Reverse cholesterol transport is reduced. In contrast, mouse models overexpressing SR-B1 in the liver show increased reverse cholesterol transport with reduced HDL plasma levels; however, these same mice also exhibit reduced atherosclerosis. In studies done on humans who maintain normal lipid profiles, the contribution of SR-B1 to HDL uptake is minimal. The cholesteryl ester transfer protein (CETP) pathway is considered an alternative pathway for HDL metabolism. CETP transfers triglycerides from B-Lps in exchange for cholesteryl esters in HDL. The result is an HDL particle depleted of cholesteryl esters but enriched with triglycerides. Cholesterol transferred to B-Lps is then cleared by LDLRs.
HDLs are also heterogeneous particles. Discrete HDL particle subpopulations have been identified on the basis of differences in size or charge, including two major ultracentrifugation subclasses, HDL 2 and HDL 3 ( ; ; ). HDL 3 is smaller than HDL 2 .The distinction is significant because HDL 2 is thought to be more cardioprotective than HDL 3 , and people with low levels of HDL 2 are thought to be at increased risk for premature CHD. By surface charge, HDL is divided into three different subpopulations, from small to large: pre-β, pre-α, and α. Pre-βHDL is also called lipid-poor apoA-I . Each pre-βHDL contains one apoA-I molecule, three to four phospholipid molecules, and one to two cholesterol molecules. Additionally, HDL has been subfractionated into particles that contain apoA-I but not apoA-II, and those that contain both apoA-I and apoA-II ( ). ApoA-I is present on virtually all HDL particles and makes up 70% of the protein content. ApoA-II makes up about 20% of the HDL lipoprotein and is present on about two-thirds of all HDL particles in humans. The physiologic function of apoA-II is not fully understood. In a study with 306 individuals from 25 families with CHD family history, the apoA-II plasma level is significantly correlated with the serum free fatty acid level. In a mouse model, human apoA-II inhibits hepatic lipase activity on hydrolysis of HDL triglyceride ( ). ApoA-II plays an important role in maintaining HDL particle size and levels of HDL in plasma. It is known that apoA-II is associated with insulin resistance and increased body fat ( ). ApoE also associates with HDL particles. Laboratory measurement of particles with different apolipoprotein composition may eventually prove to be clinically useful ( ).
Formed through the metabolism of VLDL in circulation, IDL can be removed from circulation quickly through interaction with the LDLR or it can be further metabolized to LDL. As expected, the lipid content, size, and density of IDL are intermediate between VLDL and LDL. Concentrations of IDL also contribute to the development of CHD, together with VLDL and LDL in humans.
Lp(a) is similar to LDL in terms of density and overall composition. It can be thought of as an LDL particle to which a large glycoprotein, apo(a), has been linked to apoB-100 via a disulfide bond ( ; , ). The electrophoretic mobility of Lp(a) is usually pre-β but can vary between that of LDL (β) and albumin (pre-α). Lp(a) is generally present in much lower concentrations than LDL. However, in normal subjects, values can range from less than 20 to 1500 mg/L or more. Increased levels are primarily genetically determined and its distribution varies between racial groups. Lp(a) levels show an autosomal-dominant pattern of inheritance and have been associated with an increased risk of CHD, cerebrovascular disease, and stroke ( ). When concentrations in the plasma are increased to above 200 to 300 mg/L, Lp(a) appears electrophoretically as a lipid-staining pre-β band in the plasma fraction containing lipoproteins of density greater than 1.006 g/mL.
Lp(a) is synthesized in the liver. It binds to the LDLR by virtue of its apoB-100 component, albeit with lower affinity than LDL ( ). Several mechanisms exist for the association of Lp(a) with cardiovascular disease (CVD). The removal of apo(a) from Lp(a) increases the affinity of the residual apoB-containing particle for the LDLR ( ), and it has been suggested that apo(a) may interfere with the uptake of apoB-100–containing particles ( ). Additionally, the VLDL receptor on macrophages in atherosclerotic lesions can bind and mediate the catabolism of Lp(a), leading to accumulation of lipids within macrophages. Supporting this hypothesis is the observation that Lp(a) is ubiquitous in human coronary atheroma ( ). It has been speculated that Lp(a) and apo(a) might interfere with normal thrombolysis by virtue of their similarity to plasminogen. In vitro, it competes with plasminogen and tissue-type plasminogen activator for fibrin binding ( ). Human apo(a) transgenic mice are resistant to tissue plasminogen activator–mediated thrombolysis.
Screening for Lp(a) is considered when either familial or personal history of premature atherosclerotic cardiovascular disease (ASCVD) is not explained by major risk factors ( ). Niacin, estrogen, and Lp(a) apheresis have been shown to lower Lp(a). However, apart from the newer PCSK9 inhibitors, traditional LDL-lowering therapies are not effective in this regard. Antisense gene therapy to lower Lp(a) is an area of active research. At present, no clinical trials have demonstrated that lowering Lp(a) influences clinical outcomes.
LpX is an abnormal lipoprotein found in patients with obstructive biliary disease and in patients with familial lecithin/cholesterol acyltransferase (LCAT) deficiency. Lipids account for more than 90% of its weight (mostly phospholipids: phosphatidylcholine, unesterified cholesterol, and very little esterified cholesterol). Proteins, primarily apoC and smaller amounts of albumin, constitute less than 10% of LpX by weight. A study has shown that LpX formation associates with a high level of hepatic cholesterol synthesis, but it is independent of LCAT activity, as well as ABCA1 and SR-BI expression ( ).
β-VLDL (floating β lipoprotein) is an abnormal lipoprotein that accumulates in type 3 hyperlipoproteinemia. It is richer in cholesterol than VLDL and apparently results from the defective catabolism of VLDL. The particle is found in the VLDL density range but migrates electrophoretically with or near LDL. β-VLDL is enriched in apoE. Cholesterol-fed animals accumulate β-VLDL in plasma. Uptake of cholesteryl ester-rich β-VLDL by macrophages induces foam cell formation.
As mentioned previously, apos constitute the major protein component of lipoproteins. They are commonly referred to using the nomenclature introduced by . Some significant properties of the apos are in Tables 18.4 and 18.5 .
| Apolipoprotein | Major Lipoproteins | Mr ∗ (kDa) | Amino Acids | Chromosome | Plasma Concentration | |
|---|---|---|---|---|---|---|
| mmol/L | mg/dL | |||||
| A-I | HDL | 29 | 243–245 | 11 | 32–46 | 90–130 |
| A-II | HDL | 17.4 | 154 | 1 | 18–29 | 30–50 |
| A-IV | HDL, LDL | 44.5 | 396 | 11 | ||
| (a) | Lp(a) | 350–700 | Variable | 6 | ||
| B-100 | VLDL, IDL, LDL | 512.7 | 4536 | 2 | 1.5–1.8 | 80–100 |
| B-48 | CM | 240.8 | 2152 | 2 | <0.2 | <5 |
| C-I | CM, LDL | 6.6 | 57 | 19 | 6.1–10.8 | 4–7 |
| C-II | CM, LDL | 8.9 | 78 or 79 | 19 | 3.4–9.1 | 3–8 |
| C-III | CM | 8.8 | 79 | 11 | 9.1–17.1 | 8–15 |
| D | HDL | 19 | 169 | 3 | ||
| E | CM, LDL, IDL | 34.1 | 299 | 19 | 0.8–1.6 | 3–6 |
| F | HDL, LDL, VLDL | 29 | 162 | 12 | 8.35 | |
| H | VLDL | 50 | 326 | 17 | 20 | |
| J | HDL | 80 | 449 | 8 | ||
| L | HDL | 39–42 | 383 | 22 | Absent in plasma | |
| M | HDL, LDL, VLDL, CM | 26 | 188 | 6 | 2–15 | |
| O | HDL, LDL, VLDL | 22.3 | 198 | X | ||
| Apolipoprotein | Main Distribution | Function (If Known) | Comments |
|---|---|---|---|
| A-I | HDL | Activates LCAT that esterifies cholesterol in plasma. Ligand for ABCA1 . |
Synthesized in liver and intestine; HDL biosynthesis. |
| A-II | HDL | May inhibit lipoprotein and hepatic lipases and increases plasma triglyceride. | |
| A-IV | HDL, CM, and free in plasma | May be a cofactor for LCAT; increased during fat absorption; HDL biosynthesis. | |
| B-100 | VLDL and LDL | Synthesis of VLDL; carboxy-terminal recognition signal targets LDL to the LDL (apoB, E) receptor. | Very large structural protein, synthesized in liver with lipids of endogenous origin (i.e., not chylomicrons). |
| B-48 | CM | Synthesis of CM; not recognized by LDLR. | Synthesized in intestine, encoded by same gene and same amino terminus as apoB-100. Differential production of the two proteins involves RNA editing. |
| C-I | CM and VLDL | May inhibit hepatic uptake of VLDL and cholesteryl ester transfer protein. | |
| C-II | CM and VLDL | Activates lipoprotein lipase. | Deficiency causes reduced clearance of triglyceride-rich lipoproteins. |
| C-III | VLDL, HDL | Inhibits lipolysis of triglyceride-rich lipoproteins; decreases clearance rate of remnant particles. | Deficiency causes reduced clearance of triglyceride-rich lipoproteins. |
| D | HDL | Activates LCAT. | |
| E | CM, VLDL, IDL, remnants and HDL | Recognition factor that targets CM and VLDL remnants to hepatic receptor; also binds to cell surface LDL receptors and proteoglycans. | E-2, E-3, and E-4 isoforms; E-4 is associated with high LDL-C, higher risk of CHD and Alzheimer’s disease; E-2 associated with type 3 hyperlipoproteinemia. |
| F | HDL, LDL, VLDL | Regulates CETP function. | |
| H | VLDL | Related to activation of LPL; triglyceride metabolism. | Antibodies against apoH or β 2 -glycoprotein-I are a subset of antiphospholipid antibodies, and may be associated with hyperthrombosis and stroke. |
| J | Cell-aggregating factor in Sertoli cells; inhibitor of the C5b∗7 complement complex; beta-amyloid clearance in glial cells; cholesterol trafficking in brain. | Involved in apoptosis; linked to neurologic diseases like Pick and Alzheimer; also known as clusterin. | |
| L | HDL | May be linked to reverse cholesterol transport | |
| M | HDL, CM, LDL, VLDL | May be linked to HDL remodeling. | |
| Apo(a) | Lp(a) | Homologous to plasminogen, may be prothrombotic; bound to apoB-100 by disulfide linkage. |
The major enzymatic systems that are known to participate in plasma lipoprotein metabolism are the LCAT and lipolytic enzymes, LPL, hepatic triglyceride lipase (HL), and endothelial lipase (EL). Many other proteins are also involved in lipoprotein metabolism. Some significant attributes of these proteins are in Table 18.6 .
| Enzyme | Gene Location | Function | Deficiency | Tissue Expression |
|---|---|---|---|---|
| ABCG5 | 2p21 | Forms heterodimers with ABCG8 to pump out plant sterols back into the intestinal lumen. | Increased plant sterol levels in plasma that can disrupt cell membranes and cause sitosterolemia; influences cholesterol levels in plasma. | Tissue expression in liver, colon, and intestines |
| ABCG8 | 2p21 | Forms heterodimers with ABCG5 to pump out plant sterols back into intestinal lumen; also associated with cholesterol and sterol excretion in bile. | Increased plant sterol levels in plasma that can disrupt cell membranes and cause sitosterolemia; influences cholesterol levels in plasma. | Tissue expression in the liver, intestines, and gallbladder |
| ABCA1 | 9q22-31 | Efflux of cholesterol from peripheral cells into HDL. | Tangier disease, with very low HDL and accumulation of lipids in peripheral cells. | Many cell types, prominently in the liver, testis, and adrenal |
| CETP | 16q21 | Transfers CE, PL, and TG among lipoproteins, especially the transfer of CE from HDL to apoB-100–containing lipoproteins in exchange for TG. | Deficiency results in large cholesterol-laden HDL. | Produced in liver and circulates with HDL. |
| EL | 18q21.1 | Hydrolysis of PL and TG in lipoproteins, especially PL in HDL. Homologous to LPL and EL and pancreatic lipase. | Increased levels of HDL 2 , and large buoyant LDL. Overexpression in mice, decreased TC, PL, and HDL-C. | Expressed in many tissues, including liver. Synthesized by endothelium. |
| HL | 15q22-23 | Hydrolysis of TG and PL, especially from HDL 2 , and may be necessary for HDL metabolism. Also active on lipids in VLDL remnants and IDL. Not very active on newly released VLDL or CM. | Increased TC, TG, and HDL-C in deficiency. | Associates with nonparenchymal liver cells. |
| LCAT | 16q22.1 | Catalyzes the esterification of cholesterol, especially in HDL, by promoting transfer of fatty acids from lecithin to cholesterol. Enables HDL to accumulate cholesterol as CE. Activated by apoA-I. |
Deficiency results in decreased HDL. | Produced in liver and circulates with HDL. |
| LPL | 8q22 | Hydrolysis of TG in lipoproteins (especially VLDL and CM), releasing free fatty acids and glycerol to tissues. ApoC-II are essential cofactors. | Large CM and VLDL with very high TG levels. | Present on surface of capillary endothelial cells in adipose tissue and skeletal and heart muscle, but not produced by endothelial cells. |
| LDLR | 19p13.2 | Binds apoE and apoB-100 and mediates endocytosis of lipoproteins, mostly LDL, but also VLDL, IDL, and CM remnants. | Familial hypercholesterolemia results primarily in elevated LDL. | Expressed on most cell types, but hepatic receptors clear 70% of LDL. |
| MTP | 4q24 | Lipidates and regulates secretion of ApoB particles from the liver and intestines. | Deficiency of MTP function leads to abetalipoproteinemia, in which ApoB lipoproteins are virtually undetectable in plasma. | Expression is seen in liver, intestines, heart, kidney, and eye. |
| PLTP | 20q12 | Transfer of PL to and from HDL. Important for HDL growth and remodeling. | Deficiency in mice results in low HDL. | Expressed on many cell types. |
| PCSK9 | 1p32.3 | Influences the number of LDLRs expressed on cell surface. | Depending on mutation—either gain of function or loss of function—the presence of PCSK9 affects availability of LDLR on cell surface and, consequently, the levels of LDL in plasma; gain of function leads to more LDL in plasma; loss of function associates with increased LDLR expression and thus less LDL in plasma. | Secreted protein by the liver cells; expressed in pancreatic islet beta cells and neuronal cells. |
| SR-B1 | 12q24.31 | Binds HDL on cell surface. Plays a role in selective uptake of CE from HDL in liver and steroidogenic tissues. May also enable macrophages to bind oxidized LDL. |
Accumulation of large CE-rich HDL, and accelerated atherosclerosis in mice. | Macrophage, adrenal, liver and testis |
Triglycerides and cholesterol enter circulation as part of triglyceride-rich lipoprotein particles, CMs produced in the intestine, and VLDLs produced primarily by the liver. Their synthesis requires an intracellular chaperone microsomal triglyceride transfer protein (MTP) that transfers different lipids in in vitro systems ( , ). In addition, MTP physically interacts with apoB. These two properties of MTP play a critical role in the biosynthesis of B-Lps. It is believed that MTP physically associates with nascent apoB in endoplasmic reticulum and lipidates it, forming a lipoprotein particle that is further matured and secreted ( ; ). Enterocytes synthesize CMs under postprandial conditions. In fasting conditions, they synthesize VLDL and smaller lipoproteins ( ). These lipoproteins are concentrated into mesenteric lymphatics and are delivered to blood at the thoracic duct. Hepatocytes synthesize VLDL and directly secrete into the circulation. Their synthesis is also dependent on MTP activity. The main function of B-Lps is to deliver cholesterol and triglyceride to different tissues. CMs are primarily involved in the absorption and delivery of dietary fat and fat-soluble vitamins, whereas VLDLs deliver endogenous lipids to other tissues.
Only one APOB gene is present in the human genome. Nevertheless, enterocytes and hepatocytes make two different forms of apoB protein to synthesize CMs and VLDL, respectively. In both tissues, the human APOB gene is transcribed into messenger ribonucleic acid (mRNA) that is 15 kilobases long. This transcript is translated into a single polypeptide in the liver, called apoB-100. In the intestine, however, APOB mRNA undergoes a posttranscriptional change. One cytosine residue (at position 6666) is deaminated to a uracil. This changes a glutamine codon into a stop codon. Translation of the edited mRNA gives rise to a polypeptide of 2152 amino acids that is 48% of the apoB-100 (synthesized by the liver). This editing enzyme is not expressed in the human liver; therefore, the liver synthesizes only full-length apoB-100.
These lipoprotein particles begin to undergo intravascular change almost immediately after entry into the circulation through the action of LPL. This enzyme hydrolyzes triglycerides and diglycerides, releasing fatty acids and monoglycerides, which are taken up by cells and used as a source of energy. ApoC-II stimulates the hydrolysis of triglycerides. In addition to losing triglycerides by LPL-mediated hydrolysis, the CM loses surface lipids and apos by transfer of these components to HDL. Overall, CMs lose significant mass in the form of triglyceride and the A and C apolipoproteins. The depleted CM remnant particle contains apoB-48 and apoE as its major apolipoproteins. These particles are rapidly removed from plasma and are sequestered in the space of Disse by binding to proteoglycans or taken by hepatocyte after binding to the LDLRs. The sequestered particles can undergo further hydrolysis by hepatic lipase and are enriched with apoE. Eventually, they are internalized and degraded by means of a rapid and specific receptor-mediated endocytic process involving LDLRs, LRP, and proteoglycans. C apos inhibit the uptake of CM remnants, allowing them to remain in the circulation long enough to complete the hydrolysis of triglycerides. In the fasting state, the intestine continues to make apoB and secretes “intestinal VLDL” (small CMs). These particles may constitute up to 10% or 20% of the circulating “VLDL,” but they are probably metabolized as CMs ( ; ; ; ).
VLDL is synthesized in the liver. Similar to CM, VLDL is catabolized upon entry into the circulation, in part by LPL, and is converted to cholesterol-enriched VLDL remnants, IDL. Some of these remnants are removed from circulation by the liver via receptor-mediated endocytosis; others are further catabolized to LDL ( ). LDL carries most of the circulating cholesterol and transports cholesterol to hepatic and extrahepatic tissues, where it is taken up by LDLR-mediated endocytosis ( ). LDL binds to the LDLR via apoB-100 and is subsequently internalized and directed to the lysosome, where apoB-100 is degraded and cholesteryl ester and other lipids are hydrolyzed. LDLRs are recycled back to the cell membrane ( Fig. 18.4 ).
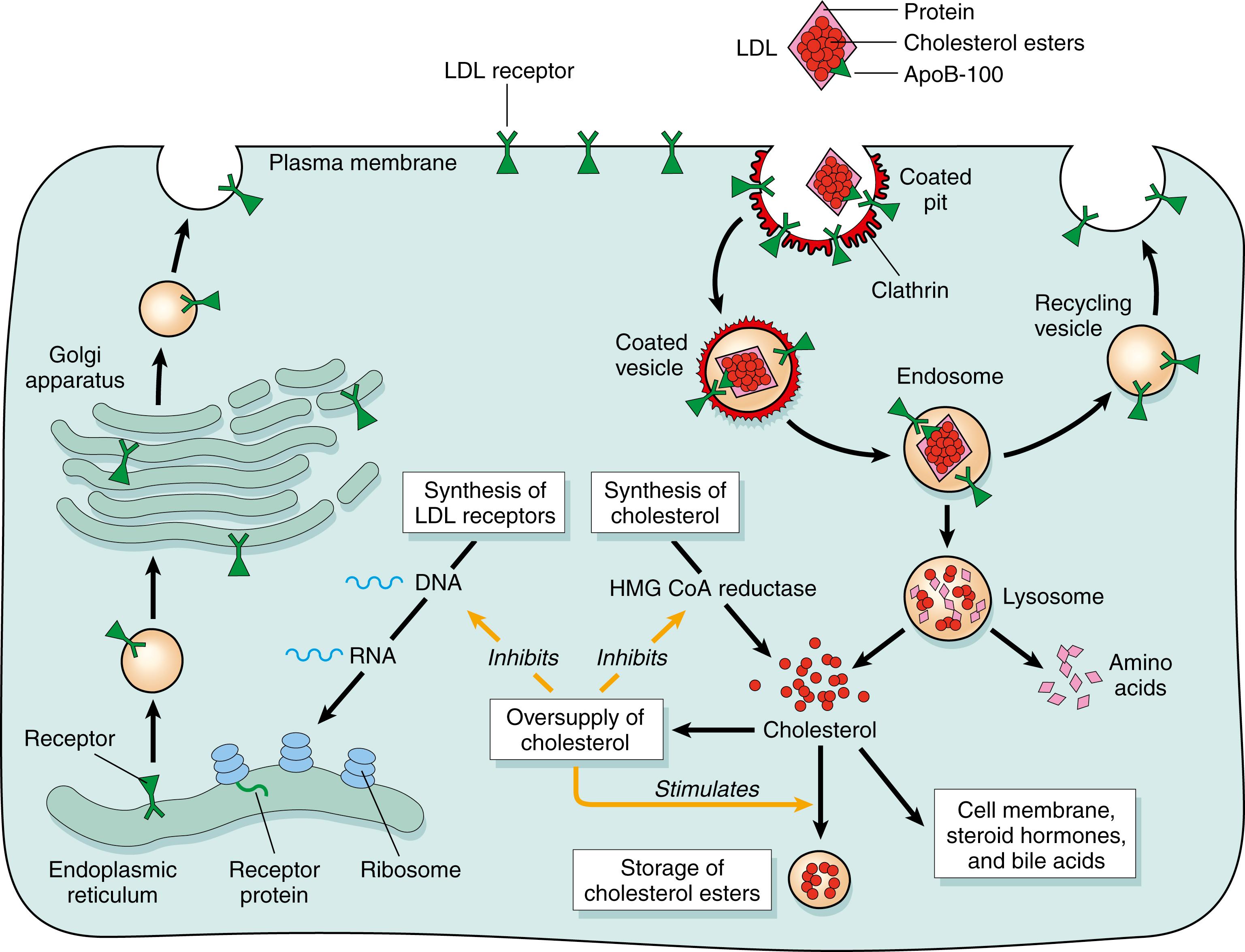
The unesterified cholesterol produced via lysosomal hydrolysis becomes available for membrane, hormone, and bile acid synthesis. Excess cholesterol is reesterified by the microsomal enzyme acyl:cholesterol acyl transferase (ACAT) and is stored until it is needed. High cellular free cholesterol downregulates the expression of LDLR on the cell surface, leading to reductions in LDL uptake. About two-thirds of LDL is removed from plasma via hepatic LDLRs. Although most tissues use cholesterol only for membrane synthesis or store it as cholesteryl ester, the liver can utilize cholesterol to make bile acids and excrete it into the bile both as unesterified cholesterol and after conversion to bile acids. The liver also reuses cholesterol for lipoprotein synthesis and secretes it with VLDL into the circulation. Steroid-secreting tissues use cholesterol as a precursor for the synthesis of steroid hormones.
LDL and CM remnants deliver lipids to the tissues. In contrast, HDL is thought to be the vehicle for reverse cholesterol transport, the process by which excess cholesterol is removed from peripheral tissues and transported back to the liver. HDL is secreted from both the liver and the intestine as nascent, disk-shaped particles that contain apos, cholesterol, and phospholipid ( ; ; ; ). The formation of nascent HDL particles is almost exclusively dependent on the synthesis and release of apoA-I. HDL also arises de novo in circulation from excess surface material (e.g., free cholesterol, apoA-I, apoA-II, apoC, phospholipid) removed from the triglyceride-rich lipoproteins as they are catabolized. In peripheral tissues, excess cholesterol is exported from cells (including macrophages), in part through the action of the protein ATP-binding cassette transporter 1 (ABCA1) to apoA-I or HDL. The free cholesterol in nascent HDL particles is esterified by LCAT into cholesteryl esters. As cholesteryl esters move into the hydrophobic core, the particle becomes spherical and larger, developing eventually into HDL 3 and then HDL 2 . Two plasma proteins are involved in the remodeling of HDL: phospholipid transfer protein (PLTP) and CETP. CETP catalyzes the transfer of cholesteryl esters to apoB-100–containing particles in exchange for triglycerides ( ). PLTP facilitates the transfer of phospholipids from other lipoproteins to HDL, allowing the particle to grow by acquiring surface phospholipid as it accumulates esterified cholesterol and triglyceride in its core. Once formed, HDL delivers excess lipids, especially cholesterol, to the liver and other tissues ( ; ; ). This may occur directly when HDL gives cholesterol to the hepatocytes, mainly via Scavenger receptor class B member 1 (SR-B1). Lipids may also return to the liver indirectly or be directed to peripheral tissues via B-Lps to which they are transferred from HDL by CETP.
Although HDL particles may return to the liver soon after formation, the bulk of HDL seems to remain in circulation for several days, continuously exchanging lipids and apos with other lipoprotein particles, retrieving additional cholesterol from peripheral tissues, and delivering those lipids to the liver and steroid-producing tissues. This is supported by the fact that apoA-I has a half-life of several days in circulation. Eventually, mature HDL is internalized by the liver SR-B1 or small lipid-depleted HDL may be catabolized in the kidney following filtration and cubulin-mediated reuptake in the proximal tubule. Lipid-poor apoA-I is eliminated from the body mainly by the kidney through the renal glomerulus. Patients with kidney disease exhibit impaired cholesterol efflux capacity and are at high risk of cardiovascular disease ( ).
Lipoprotein concentrations have been measured and described in several ways. Some of these measurements, including particle mass and mass concentration (the mass of each lipoprotein particle as solute per liter of solution), are not easily applied for screening or routine clinical purposes. Fortunately, other methods may be used to describe the lipoprotein content of blood. Because the cholesterol composition of each lipoprotein class is similar from individual to individual, lipoprotein cholesterol is commonly used to evaluate lipoprotein concentration. For example, it is easier to determine the amount of LDL-C in a specimen than it is to determine the mass of LDL (cholesterol + triglyceride + protein) in solution, yet both measurements provide similar information about the LDL content of plasma. Lipoprotein-cholesterol concentrations correlate well with analytic ultracentrifugation values. Also, because these values have been used in most population studies of cardiovascular risk, they have documented predictive value.
When various methods of lipid analysis are considered, several issues should be kept in mind. First, the more complicated the analytic procedures, the greater the variability of the analyses ( ; ). For example, the measurement of plasma lipoproteins usually requires two steps: separating lipoprotein classes and measuring the class of interest. Both steps contribute to the error in the measurements. Consistent with this, lipoprotein cholesterol analyses are generally more variable than total cholesterol (TC) analyses because of the additional manipulations required in preparing the lipoprotein-containing fractions. Second, in addition to analytic sources of error, significant preanalytic variables may affect measured lipid and lipoprotein levels. In fact, plasma lipoprotein concentrations can change dramatically as a result of normal physiologic variation. In this section, issues of sampling and storage are considered, along with methods for measuring lipids and lipoproteins.
Variation and error may occur before or during venipuncture or when the samples are handled and stored before analysis. Therefore, it is important to standardize conditions under which blood specimens are drawn and prepared for analysis.
Physiologic variations in cholesterol, triglyceride, and lipoproteins have been examined in a number of studies ( ; ; ; ; ). For cholesterol, the coefficient of physiologic variation within an individual averages about 6.5%, but it can be higher in certain individuals ( Tables 18.7 and 18.8 ). When measured in serial samples from the same person, cholesterol levels in 95% of the samples will vary by about 13% above or below that person’s mean level. As a result, physiologic variation can be several times greater than analytic error; thus, measurements must be made in several blood samples taken at least a week apart to establish the individual’s usual lipoprotein concentration. Determinations of these levels is important for consideration of appropriate therapy for documented hypercholesterolemia, such as use of statins shown in Table 18.9 . Table 18.10 summarizes levels of serum total cholesterol and their significance. Table 18.11 summarizes some of the clinical presentations of hypercholesterolemias and/or hypertriglyceridemias.
| Component | CVP (%) ∗ | CVP (%) † |
|---|---|---|
| Total cholesterol | 5.0 | 6.4 |
| Triglycerides | 17.8 | 23.7 |
| LDL-cholesterol | 7.8 | 8.2 |
| HDL-cholesterol | 7.1 | 7.5 |
| ApoA-I | 7.1 | — |
| ApoB | 6.4 | — |
∗ Data from patients of a lipid clinic ( ).
† Data from the National Cholesterol Education Program 1995 Working Group on Lipoprotein Measurement.
A variety of biological factors can affect lipid and lipoprotein levels. Cholesterol levels rise with age, starting in early adulthood, in both sexes. Women have lower cholesterol levels than men, except in childhood and after the early fifties. Age-related variation forms the basis for the National Cholesterol Education Program (NCEP) recommendation that cholesterol screening should be repeated every 5 years. Seasonal variation also occurs, such that cholesterol levels are slightly higher in the winter ( ). In addition, dietary intake of saturated fat and cholesterol significantly influences plasma lipid levels. The effects of dietary modification take several weeks to become apparent; thus before individuals’ cholesterol levels are ascertained, it is important that they be on their usual diet for 2 weeks and are neither gaining nor losing weight. Several common medications, including oral contraceptives, postmenopausal estrogens, and some antihypertensive drugs, significantly alter lipid levels. Medical disorders that lead to secondary dyslipoproteinemia include thyroid, hepatic, and kidney disease (see Table 18.12 ). In such cases, management of hyperlipidemia is predominantly a function of treating the underlying disorder. Lifestyle and biological factors that produce short-term deviations from baseline lipid values include alcohol consumption, fasting, posture, venous occlusion, anticoagulants, recent myocardial infarction, stroke, cardiac catheterization, trauma, acute infection, and pregnancy. It is recommended that lipoprotein measurements be made no sooner than 8 weeks after any form of trauma or acute bacterial or viral infection and 3 to 4 months after childbirth.
| Lipoprotein Profile | Secondary Causes | Lifestyle Factors |
|---|---|---|
| High cholesterol and high LDL-C with or without low HDL-C | Hypothyroidism and nephrotic syndrome Medications such as thiazide diuretics and steroids Chronic obstructive liver disease Cholestatic liver disease (primary biliary cirrhosis and related diseases) ApoE-4 may increase susceptibility |
Obesity Excess dietary cholesterol and/or saturated fat |
| High triglycerides with normal total and LDL-C with or without low HDL-C | Medications such as thiazide diuretics, estrogens, corticosteroids, retinoids, cyclosporine, and beta blockers (without intrinsic sympathomimetic) Insulin resistance/diabetes Chronic renal failure and nephrotic syndrome Antipsychotic drugs (clozapine/olanzapine) |
Obesity Physical inactivity Cigarette smoking Excess alcohol intake High-carbohydrate diets |
| High cholesterol and high triglycerides with or without low HDL-C | Medications—notably, high-dose steroids or cyclosporine Severe hypothyroidism, diabetes/insulin resistance, and nephrotic syndrome |
Obesity HIV antiretroviral drugs |
| Isolated low HDL-C | Medications such as isotretinoin, probucol, anabolic steroids, beta blockers, and certain progestogens | Physical inactivity Increased body weight High-carbohydrate, low-fat diets |
| Isolated high HDL-C | Medications such as phenytoin, phenobarbital, rifampicin, griseofulvin, and estrogens | Alcohol intake |
Patients should fast for 12 hours before venipuncture. CMs are usually present in postprandial plasma and, depending on the type and amount of food ingested, can markedly increase the plasma triglyceride (TG) concentration. Concentrations of LDL- and HDL-cholesterol (LDL-C and HDL-C) also decline transiently after eating, in part as a consequence of CETP-mediated compositional changes that occur during the catabolism of CMs ( ). CMs are almost completely cleared within 6 to 9 hours; their presence after a 12-hour fast is considered abnormal. Generally, TC and HDL-C levels can be measured in nonfasting individuals, greatly facilitating screening and monitoring. Fasting has little effect on plasma TC levels; although nonfasting HDL-C levels can be a few mg/dL lower than fasting levels, this should not lead to misclassification of patients with low HDL levels. When TG and LDL-C are being measured, fasting becomes a requirement.
When a standing patient reclines, extravascular water transfers to the vascular system and dilutes nondiffusible plasma constituents. Decreases of as much as 10% in the concentrations of TC, LDL-C, HDL-C, apoA-I, and apoB have been observed after a 20-minute period of recumbence ( ). The decrease in TG is about 50% greater, suggesting that factors other than simple hemodilution may also be involved. These effects are about half as great in a standing subject who sits ( ). Postural changes are reversible when the patient resumes the standing position. Current NCEP guidelines recommend that patients be seated for 5 minutes before sampling to prevent hemoconcentration. If it is necessary to use the recumbent position, it should be used each time the patient is sampled to minimize postural change. Prolonged venous occlusion can lead to hemoconcentration and cholesterol increases of 10% to 15%. Tourniquets should not be applied for longer than a minute or two, if possible.
Although it is generally assumed that venous and capillary samples are equivalent, the available information at present is limited and somewhat contradictory. Some investigators have found that cholesterol measurements in the two kinds of samples agreed in about 4% ( ) or less ( ; ), but others have reported differences of 8% to 12% ( ). In general, measurements in capillary blood samples seem to be a little lower than in venous samples. Additionally, measurements in fingerstick samples tend to be more variable than in venous samples obtained at the same time, probably as the result of preanalytic sources of error. Estimates for the biological component of within-subject variations have been made for lipid and lipoprotein in venous and fingerstick samples and are similar in both kinds of samples for cholesterol, TG, HDL, and LDL ( ). Although the use of capillary samples may be unavoidable under some conditions, it is good to keep in mind, first, that the epidemiologic data from which risk levels for lipids and lipoproteins are derived are based on measurements in venous samples and, second, that for various physiologic and methodologic reasons, the measurements in the two kinds of samples may differ.
Plasma or serum can be used when only cholesterol, TG, and HDL-C are measured, and LDL-C is calculated from these three measurements (see later discussion). However, plasma is preferred when the lipoproteins are measured by ultracentrifugal or electrophoretic methods because the samples can be cooled to 4° C immediately to retard changes that can occur in the lipoproteins at room temperature. When plasma is to be used, blood is cooled in an ice bath as soon as it is drawn, and the cells are removed as soon as possible, generally within 3 hours. The plasma is then stored at 4° C until it is analyzed. Plasma should not remain in contact with the cells overnight. Even in the presence of the anticoagulant, protein aggregation can occur in plasma that is stored in the refrigerator for a few days or frozen for longer periods. This can make it difficult to obtain a homogeneous aliquot for analysis and can interfere with the flow of sample in automated analyzers, resulting in inaccurate or variable results. Protein aggregation occurs less frequently in serum.
The choice of anticoagulant is also important. Some anticoagulants, such as citrate, exert rather large osmotic effects that result in falsely low plasma lipid and lipoprotein concentrations. Heparin, because of its relatively high molecular weight, has little effect on plasma volume but can alter the electrophoretic mobilities of the lipoproteins. Ethylenediaminetetraacetic acid (EDTA) is the preferred anticoagulant even though cholesterol and triglyceride concentrations in EDTA plasma are about 3% lower than in serum (Laboratory Methods Committee of the Lipid Research Clinics Program, 1977). This anticoagulant retards certain kinds of oxidative and enzymatic alterations that occur in the lipoproteins during storage.
Generally, TC, triglycerides, and HDL-C can be satisfactorily analyzed in frozen samples, and LDL-C concentrations can be estimated. Apolipoproteins can also be measured in frozen samples (see later discussion). Frozen samples are not appropriate for ultracentrifugal analysis because the triglyceride-rich lipoproteins do not withstand freezing. HDL free cholesterol and VLDL free fatty acid decrease by about 35%, and LDL cholesteryl ester increases by 35% upon single freeze and thaw ( ). When serum or plasma must be stored for long periods, it should be maintained at temperatures of –70° C or lower. For short-term storage (up to 1 or 2 months), the samples can be kept at –20° C, but they should not be stored in a self-defrosting freezer. The temperature in a self-defrosting freezer actually cycles between about –20° C and 2° C during the defrost cycle and effectively subjects the samples to daily freeze–thaw cycles, which can hasten their deterioration and cause the lipid and lipoprotein measurements to become variable (i.e., less reproducible). Quantification of lipid and apos are not affected within three freeze-and-thaw cycles. In most lipid classes, the effect of storage temperature over 1 week is minimal between 4° C, –20° C, and –80° C ( ).
Cholesterol and triglycerides are the plasma lipids of greatest interest in the diagnosis and management of lipoprotein disorders. Phospholipid analyses generally provide little additional information and are seldom required. Occasionally, they may be requested in cases of obstructive liver disease or disorders associated with abnormally low lipoprotein levels.
Cholesterol accounts for almost all of the sterol in plasma. It exists as a mixture of unesterified (30%–40%) and esterified (60%–70%) forms; the proportion of the two forms is fairly constant among normal individuals. TC and lipoprotein-cholesterol concentrations are usually expressed in terms of the sterol nucleus without distinguishing the esterified and unesterified fractions. In general, it is not necessary to distinguish the two forms, except in cases in which the contribution of the fatty acid moiety to cholesteryl ester mass must be accounted for or when the cholesterol/cholesteryl ester mass ratio is of interest.
This discussion considers primarily the enzymatic methods of cholesterol quantification, which have virtually replaced the chemical methods that were used for most clinical and research purposes ( ; ; ). However, one chemical method, a modification of the Abell-Kendall method ( ), continues as the reference method for cholesterol used by the Centers for Disease Control and Prevention (CDC) and a network of secondary reference laboratories ( ). In the Abell-Kendall method, cholesteryl esters are hydrolyzed with alcoholic potassium hydroxide (KOH), and the unesterified cholesterol is extracted with petroleum ether and measured with the Liebermann-Burchard reagent using purified cholesterol standards. This method can be accurate within about 0.5% of true value.
These methods measure TC directly in plasma or serum through a series of reactions in which cholesteryl esters are hydrolyzed. The 3-OH group of cholesterol is oxidized, and hydrogen peroxide, one of the reaction products, is quantified enzymatically:
To complete the enzymatic measurement of TC, there must be a way to quantify the by-products of the reaction. Currently, the most common method of quantifying the cholesterol oxidase reaction is to measure the amount of hydrogen peroxide produced ( Eq. 18.3 ). The hydrogen peroxide produced in the cholesterol oxidase reaction can be oxidatively coupled to two chromogenic substrates by catalysis with a peroxidase, most commonly horseradish peroxidase (HRP). The chromogens used are usually phenol and 4-aminoantipyrine. When coupled with hydrogen peroxide, they produce a quinoneimine dye that can be read photometrically at 500 nm. The catalytic properties of HRP are very nonspecific; therefore, this step is most subject to interference from other components of serum/plasma.
Enzymatic methods are less subject to interference by nonsterol substances that react in the chemical methods; however, they are not absolutely specific for cholesterol. Cholesterol oxidase ( Eq. 18.2 ) can react with sterols other than cholesterol that are present in plasma such as plant sterols, which are present in appreciable concentrations in the circulation of patients with β-sitosterolemia. These sterols also contribute to the cholesterol values measured via most chemical methods. Another type of interfering agent is ascorbic acid, a reducing agent, which competes with the chromogenic substrates of Equation 18.3 for hydrogen peroxide. Therefore, plasma/serum samples with elevated levels of ascorbic acid can result in lower measured levels of TC. It is currently accepted that ascorbic acid levels over 30 mg/dL should be accounted for in TC measurements, although levels that high are infrequent ( ). Another interfering agent that acts in a similar manner is bilirubin. Bilirubin can interfere with TC measurements because of its own spectral properties; bilirubin absorbs light at 500 nm ( ). This tends to increase measured cholesterol values. However, bilirubin is also oxidized by H 2 O 2 ; as a result, it loses its absorbance at 500 nm. This complicates the application of a serum blank to correct for bilirubin absorbance. Overall, interference by bilirubin seems to be significant only at concentrations exceeding 5 mg/dL, at which level it has been reported to decrease apparent cholesterol values by 5% to 15% ( ; ; ). Sample turbidity because of hypertriglyceridemia can also interfere with enzymatic methods ( ). Hemoglobin is another possible interfering agent in the measurement of TC. It has a pseudo-peroxidase activity that can consume the hydrogen peroxide produced in Equation 18.2 . More important, however, is that hemoglobin has an inherent color that can falsely elevate TC levels. Just as hemoglobin is a product of red blood cell hemolysis, other products of hemolysis, such as catalase, can compete with the peroxidase for hydrogen peroxide. However, hemolyzed products do not significantly affect cholesterol measurements even at abnormally high plasma concentrations ( ; ).
In addition to their relative resistance to interference, enzymatic methods have other significant benefits. They consume only microliter quantities of sample and do not require a preliminary extraction step. They are rapid, and if the cholesteryl ester hydrolase step is omitted, they can be used to measure unesterified cholesterol. Finally, enzymatic methods are precise, with coefficients of variation generally in the range of 1% to 2%. For the most part, they use stabilized pure cholesterol standards or serum calibration standards for which the stated values are traceable to the CDC reference method for cholesterol ( ). Enzymatic values generally agree with reference values within 1% to 2% when measured in a laboratory setting with modern equipment. Serum-based calibrators are inherently preferable to pure cholesterol because they are subject to all analytic reactions undergone by patient samples.
A wide variety of methods have been used to measure plasma triglycerides, but the methods most commonly used for clinical or epidemiologic purposes are based on the hydrolysis of triglycerides and the measurement of glycerol that is released in the reaction:
Because each glycerol molecule is calculated to represent a triglyceride molecule, TG concentrations are often overestimated if endogenous unesterified glycerol is not subtracted. Even after correction for glycerol, TG levels are overestimated because of the presence of monoglyceride and diglyceride molecules. Despite this knowledge and the availability of reagents and methods to correct for endogenous glycerol, only about 5% of American clinical laboratories actually correct for endogenous glycerol. The reason for this is that in most normal individuals, endogenous glycerol levels are negligible.
Enzymatic methods ( ) are now universally used for TG analysis in the clinical laboratory. They are relatively specific, rapid, and easy to use. The analyses are performed directly in plasma or serum and are not subject to interference by phospholipids or glucose. Common to most enzymatic methods is the hydrolysis of triglycerides to free fatty acids and glycerol, followed by the phosphorylation of glycerol to glycerophosphate.
However, several methods may be used to quantify the amount of glycerolphosphate formed and thus the amount of triglyceride in plasma. In one approach, glycerophosphate reacts as follows:
NADH formation can be measured spectrophotometrically at 340 nm. In other methods, Equation 18.8 has been added so that absorbance readings can be made in the 500- to 600-nm region of the spectrum, using instruments that are more commonly available in the clinical laboratory.
In a common variation, the glycerophosphate formed in Equation 18.6 is oxidized by the action of glycerophosphate oxidase:
The resulting H 2 O 2 is measured as described previously for cholesterol methods ( Eq. 18.3 ).
In a third approach, adenosine diphosphate (ADP) rather than glycerophosphate is formed in Equation 18.6 , and is quantified:
In this method, the disappearance of NADH is measured at 340 nm.
Enzymatic TG methods generally perform well. The reagents are available commercially as lyophilized preparations that need only to be reconstituted before use. Based on recent College of American Pathologists (CAP) surveys, the interlaboratory coefficients of variation (CVs) for TG measurement using a variety of enzymatic methods were about 5% to 6%. It is prudent before selecting an enzymatic method to evaluate its accuracy and precision over the range of triglyceride concentrations likely to be encountered most frequently (1.299–12.987 mmol/L; 50–500 mg/dL).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here