Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
‘Limbus’ is the Latin word for ‘border’. When the eye is viewed macroscopically from a social viewing distance of, say, 50 cm, the limbus appears as a reasonably clear circle that forms the outer limit (or ‘border’) of the visible iris. However, defining the exact position of the limbus is more problematic from both a clinical and a histological perspective. When the limbus is viewed under magnification with a slit lamp biomicroscope, the transition zone between the cornea and conjunctiva is gradual and occurs over a span of about 0.2 to 0.4 mm, generally being slightly greater in the vertical meridian.
From a histological perspective, defining the location of the limbus is even more problematic. The limbus is formally defined as being about 1.5 mm wide. This is because various histological features that define the limbal region start and finish at different locations, and some changes are abrupt and some are gradual; a 1.5-mm zone encompasses all these features. Specifically, changes that take place in the transition from the cornea to the conjunctiva and sclera include
abrupt termination of Bowman’s layer,
gradual thickening of the epithelium,
introduction of loose connective tissue underlying the conjunctival epithelium,
increasing irregularity of anterior stromal lamellae, and
appearance of blood vessels in the stroma. ( Fig. 15.1 )
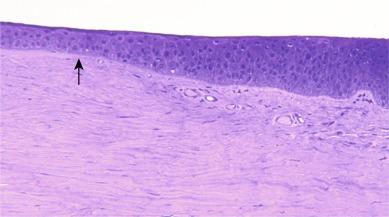
It is the last of these features that is the topic of this chapter, and for the purposes of discussion of the limbal redness response to contact lens wear, it is convenient to regard the limbus as the region within which the vascular network of the conjunctiva gives way to the avascularity of the cornea.
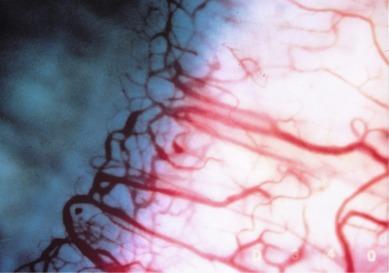
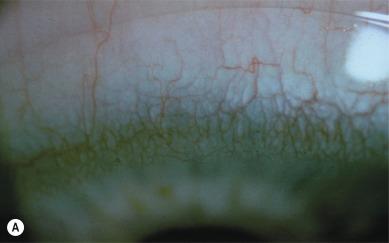
The fact that the limbus displays a distinct response to contact lens wear was first recognised by Müller over a century ago. Careful inspection of the superficial blood vessels at the limbus reveals the presence of ‘anterior limbal loops’ ( Fig. 15.2 ). In some patients, a series of two or three layers of anterior limbal loops can be observed to build on top of each other as the limbal vascular plexus extends towards the cornea, with the vessels constituting each successive inwardly progressing loop becoming finer and finer. The innermost series of loops are termed ‘terminal arcades’.
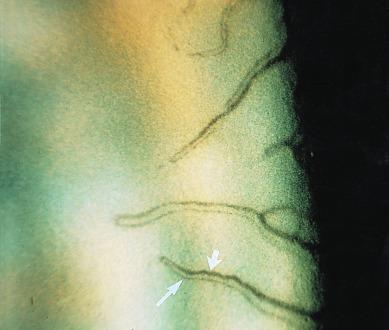
When the limbus has been physically and/or physiologically stressed – for example, by some types of contact lens – the limbal vessels dilate and two types of vascular features connected to the terminal arcades can be readily visualised – ‘recurrent limbal vessels’ and ‘spike vessels’. On casual inspection with the slit lamp biomicroscope, a recurrent limbal vessel may appear as a single vessel, with an accompanying shadow, that connects tangentially from a terminal arcade. On closer inspection, however, it can be seen that two vessels of different calibre are present – a thicker arteriole and a much thinner venule (the ‘shadow’) ( Fig. 15.3 ).
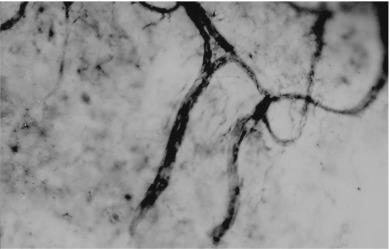
Vessel spikes look similar to recurrent limbal vessels, the distinction being that the vessel spike is a single arteriole that does not have a returning venule. The lumen of vessel spikes and recurrent limbal vessels can become so narrow that only clear serum (not red blood corpuscles) can pass through. This raises the question as to whether a vessel spike really only represents the visible arteriolar component of a recurrent limbal vessel, with the venular component being too narrow to allow red blood cells to pass through (and thus remaining invisible). However, electron microscopic evidence ( Fig. 15.4 ) has confirmed the structure of vessel spikes as single arterioles.
Holden et al. measured the extent of bulbar conjunctival and limbal redness in a group of patients who had worn a conventional (low-Dk) high-water-content soft lens on an extended-wear basis in one eye only (the other eye being emmetropic or amblyopic) for an average of 5 years. Bulbar conjunctival redness was graded as 0.9 (vs. 0.7 in non-lens-wearing control eyes) and limbal redness was graded as 1.1 (vs. 0.3 in the non-lens-wearing control eyes). From these data, it can be inferred that extended wear of conventional soft lenses has a much greater effect on limbal redness than on bulbar conjunctival redness.
The time course of development of limbal redness in response to conventional hydrogel lenses and silicone hydrogel (Si-H) (high-Dk) lenses during open and closed eye wear has been investigated by Papas et al. Subjects were first monitored without lenses. They then wore a conventional hydrogel lens in one eye and a Si-H lens in the other eye and were observed after 4 and 16 hours. When not wearing lenses, limbal redness changes averaged 0.2 ± 0.2 and 0.4 ± 0.2 grades at 4 and 16 hours, respectively (inferior quadrant). The corresponding values for conventional hydrogel lens wear were 1.0 ± 0.6 and 1.1 ± 0.6, whereas for Si-H lens wear, they were 0.2 ± 0.4 and 0.5 ± 0.5, respectively ( Fig. 15.5 ). Both for the non-lens-wearing eyes and eyes wearing Si-H lenses, increases in limbal redness were significant only during eye closure. During conventional hydrogel lens wear, significant and larger limbal redness increases were seen after 4 hours open-eye wear, with only relatively small further changes being observed over the next 12 hours. Papas et al. concluded that (a) conventional hydrogel lens wear induces a marked increase in limbal redness during open-eye wear, which is not seen either in the no-lens situation or when Si-H lenses are worn, and (b) the pattern of limbal redness for both the open and closed eyes during Si-H lens wear is very similar to that for the no-lens situation.
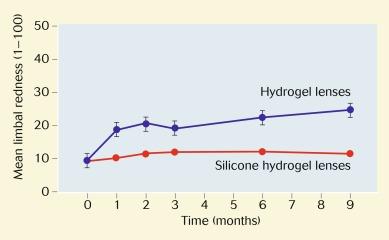
A similar study to that of Papas et al. was conducted by Dumbleton et al., and the subjects were monitored over a 9-month period rather than a few hours. On a 0-to-100 scale, extended wear of conventional hydrogel lenses resulted in a 16-point increase in limbal redness; no significant change occurred with the Si-H lenses ( Fig. 15.6 ). Interestingly, the difference was greatest for subjects wearing conventional hydrogel lenses initially presenting with lower levels of redness. There was a slight resolution of redness in participants who initially presented with higher levels of redness after wearing the Si-H lenses.
Covey et al. were unable to detect any difference in the grade of limbal redness between patients wearing Si-H lenses (2.6 ± 0.3) on an extended-wear basis versus patients who wore no lenses (2.4 ± 0.4), over a 9-month period.
Numerous other studies have confirmed that silicone hydrogel lenses result in significantly reduced levels of limbal redness compared with hydrogel lenses, for both daily and extended lens wear. This is not just a short-term effect; reductions in limbal redness are still evident after 3 years of silicone hydrogel lens wear. Improved limbal redness with silicone hydrogel lenses is equally evident in Caucasian and Asian eyes.
Szczotka-Flynn et al. also confirmed that two silicone hydrogel lens types – lotrafilcon B (Air Optix Aqua) and comfilcon A (Biofinity) – induce less limbal redness (0.10 and 0.18 grade difference, respectively) than a mid-water hydrogel lens (etafilcon A; Acuvue 2). However, they deemed these differences to be clinically inconsequential and suggested that etafilcon A was ‘non-inferior’ to lotrafilcon B and comfilcon A in this regard.
The development of limbal redness is not thought to be associated with any subjective symptoms, although patients with severe contact lens–induced complications may coincidentally have limbal redness and be suffering from discomfort or pain. None of the short-term or long-term studies referred to earlier reported any symptoms relating to limbal redness during uncomplicated lens wear.
Throughout the vascular system, blood flow is constantly adjusted to meet the local tissue needs. This is achieved via four basic regulatory mechanisms:
Neural control – the calibre of vessels is controlled by balancing the input of parasympathetic and sympathetic innervation to vascular smooth muscle in the vessel wall.
Myogenic control – vessel diameter is altered in response to changes in blood pressure within the vessel. Thus, distension of the vessel wall with increased pressure will cause smooth muscle contraction. In the limbus, this mechanism dampens the tendency for increased fluid filtration to cause interstitial oedema during periods of raised blood pressure.
Metabolic control – the accumulation of waste products, such as carbon dioxide and lactic acid surrounding the vessels, causes the vessels to dilate, increasing blood flow and removing the metabolic waste. Local hypoxia has a similar effect.
Humoral control – agents circulating in blood, such as epinephrine, norepinephrine, histamine, serotonin, angiotensin, bradykinin and vasopressin.
However, because microcirculatory fields, such as the limbus, are devoid of direct neural control and lack smooth muscle in the vessel walls, alternative mechanisms must come into play to control blood flow. Two mechanisms are thought to be involved. First, it is thought that a system of precapillary sphincters – defined as the last (most peripheral) smooth muscle cell along any branch of a terminal arteriole – control blood flow into the limbal plexus in a co-ordinated manner. This implies that capillary flow is not continuous but will start and stop according to local needs and conditions. Second, small cells which surround capillary vessels, known as pericytes , and vascular endothelial cells appear to have contractile properties that allow them to alter the calibre of limbal vessels.
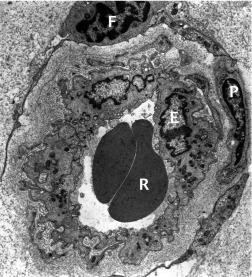
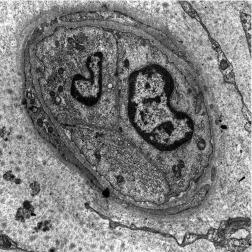
When fine terminal vessels – comprising either vessel spikes or recurrent limbal vessels – are viewed with the electron microscope, they are found to display the usual features of normal limbal capillaries. They are non-fenestrated, with thick endothelial walls surrounded by pericytes and their processes, and connective tissue cells. Although many vessels possess an open lumen capable of allowing the passage of formed elements of blood ( Fig. 15.7 ), many others are observed in which the calibre is reduced so as to prevent the passage of these elements, and in extreme cases, the lumen is completely closed ( Fig. 15.8 ). These observations highlight the dynamic growth patterns of capillaries.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here