Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Limbs are remarkable structures that are designed almost solely for mechanical functions: motion and force. These functions are achieved through the coordinated development of various tissue components. No single tissue in the limb takes shape without reference to the other tissues with which it is associated. The limb as a whole develops according to a master blueprint that reveals itself sequentially with each successive stage in limb formation. Many of the factors that control limb development cannot be seen by examining morphology alone, but rather must be shown by experimental means or through the localization of molecules. Despite remarkable progress in understanding the molecular basis of the tissue interactions that control limb development, many fundamental questions remain poorly understood. Limb anomalies are common and highly visible. Many of these anomalies are now known to be reflections of disturbances in specific cellular or molecular interactions that are fundamental to limb development. These are discussed in Clinical Correlation 10.1 at the end of the chapter.
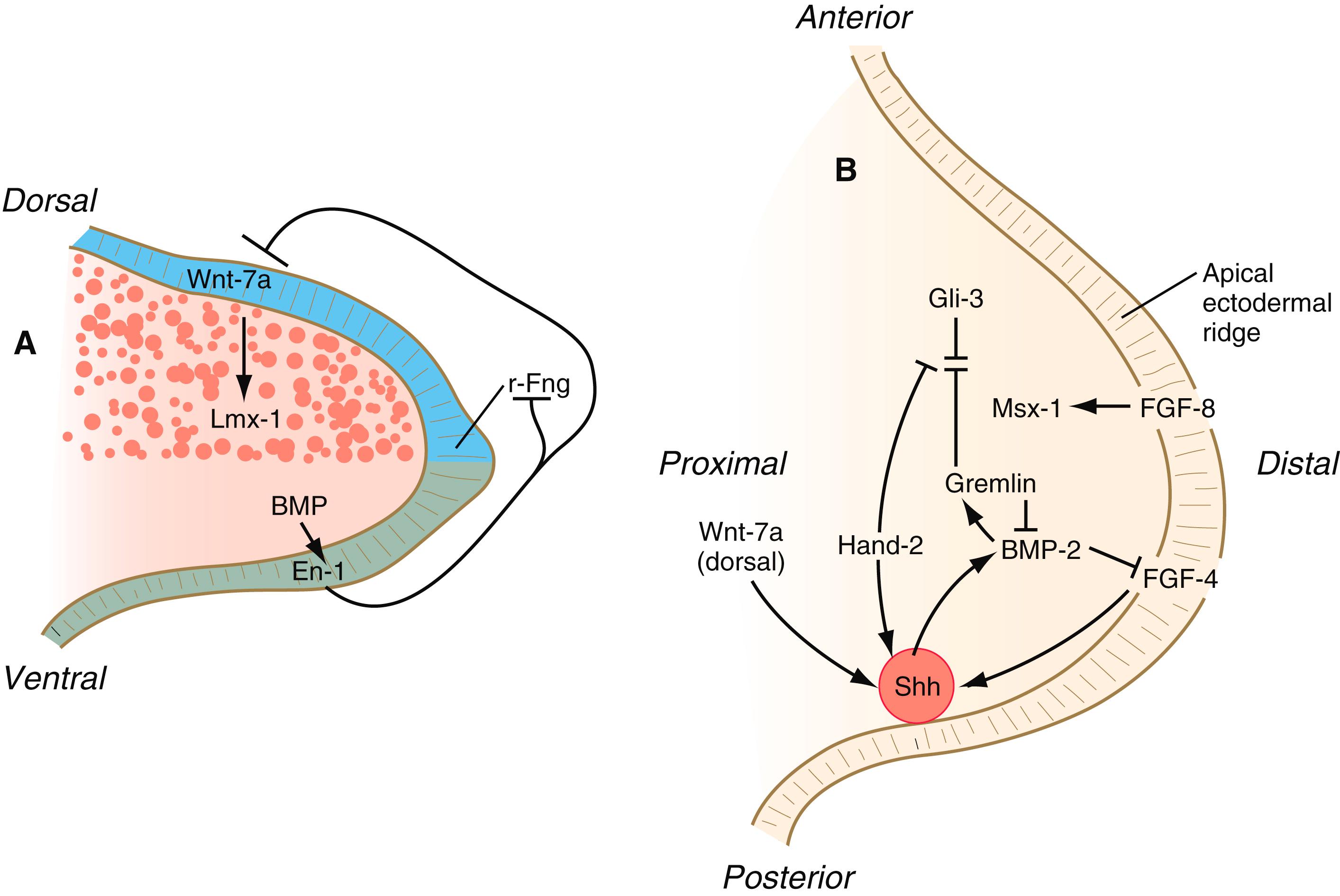
Limb formation begins relatively late in embryonic development (at the end of the fourth week in humans) ( Figure 10.1 ). One of the earliest steps in the initiation of limb formation is the expression of a linear array of Hox genes in the lateral plate mesoderm. The levels of the limbs along the body axis are determined by the activation of Hox genes— Hox-4,5 to Hox-9 , which delineate the boundaries for the forelimb, and Hox-11, 12, 13 for the hindlimb. At the level where the forelimbs will form, retinoic acid , produced by the paraxial mesoderm, stimulates the expression of the T-box transcription factor, Tbx-5 ( Figure 10.2A ). Tbx5 in the area of the future forelimb and Tbx4 (along with Pitx-1) in the area of the future hindlimb stimulate the expression and secretion of fibroblast growth factor-10 (FGF-10) by the local mesodermal cells ( Figure 10.2A ). FGF-10 stimulates the overlying ectoderm to produce FGF-8. Soon a positive feedback loop involving FGF-10 and FGF-8 is established, and limb development begins. Paradoxically, FGF-8, produced in the anterior and posterior trunk, is inhibitory to the initiation of forelimb development through its inhibition of expression of Tbx5. Retinoic acid inactivates these sources of FGF-8 and induces the expression of Tbx5 through both direct induction and the inhibition of the inhibitory influence of trunk FGF-8.
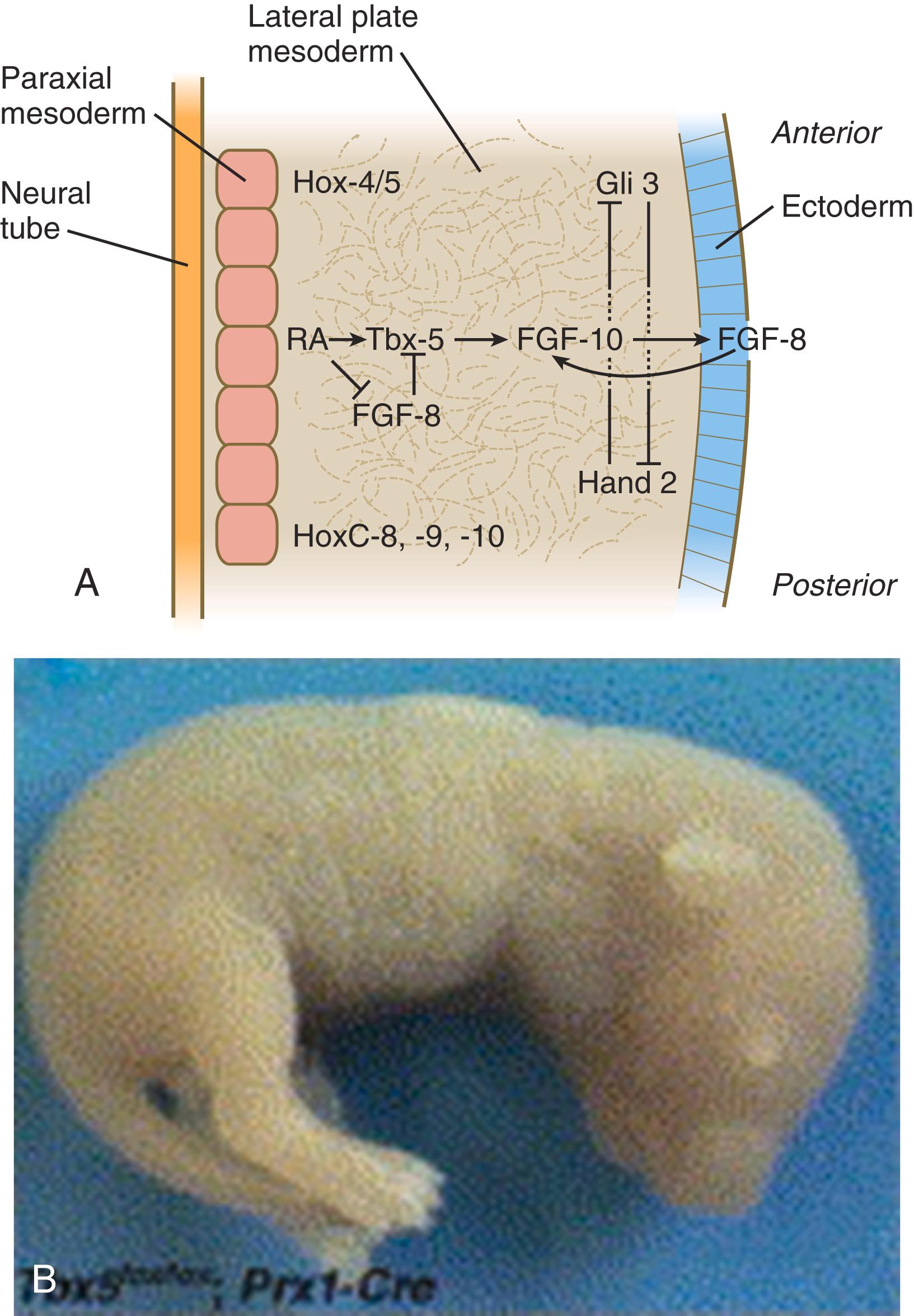
The Tbx transcription factors appear to be the local driving forces of limb development. If Tbx5 expression in a mouse is prevented, forelimbs fail to develop ( Figure 10.2B ). Similarly, in FGF-10 knockout mice, limbs (and lungs) do not form. Conversely, if a bead soaked with FGF-10 is implanted within the future flank region of a chick embryo, a supernumerary limb develops at the spot. Once the interaction between ectoderm and mesoderm begins, the limb primordium contains sufficient developmental information to form a limb even if isolated from the rest of the body (a so-called self-differentiating system ).
When the molecular stimulus for the initiation of limb development first occurs, the mesodermal somatopleure is in an epithelial configuration. At the cellular level, one of the first changes in limb development is an epitheliomesenchymal transformation (EMT) resulting in the conversion of somatopleural epithelial cells to early limb bud mesenchyme. Only after this step does proliferation play a major role in the formation of the early limb bud. Corresponding to the overall sequence of limb development, the EMT of the forelimb occurs before that of the hindlimb, and while these are occurring, the somatopleure of the flank region remains epithelial.
The primacy of the early limb mesoderm was shown long ago by transplantation experiments on amphibian embryos. If early limb mesoderm is removed, a limb fails to form. If the same mesoderm is transplanted to the flank of an embryo, however, a supernumerary limb grows at that site. In contrast, if the ectoderm overlying the normal limb mesoderm is removed, new ectoderm heals the defect, and a limb forms. If the original ectoderm that was removed is grafted to the flank, no limb forms. These experiments show that in early limb development, mesoderm is the primary bearer of the limb blueprint, and ectoderm is only secondarily coopted into the system.
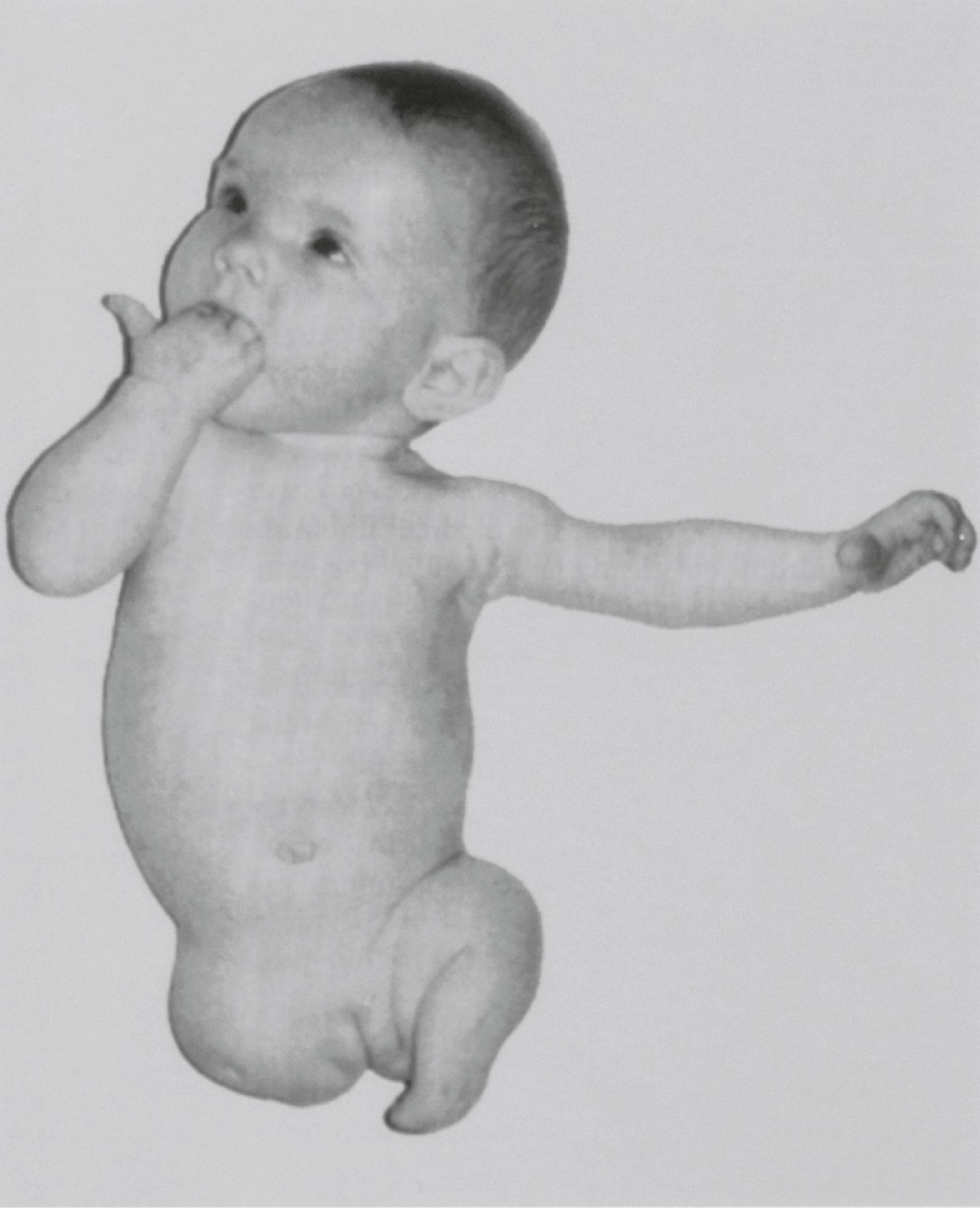
In rare instances, individuals are born without one or sometimes all limbs ( amelia ) ( Figure 10.3 ). In some cases, this situation may reflect a disturbance in the production of the transcription factors or signaling molecules that initiate limb development or the cellular receptors for these molecules.
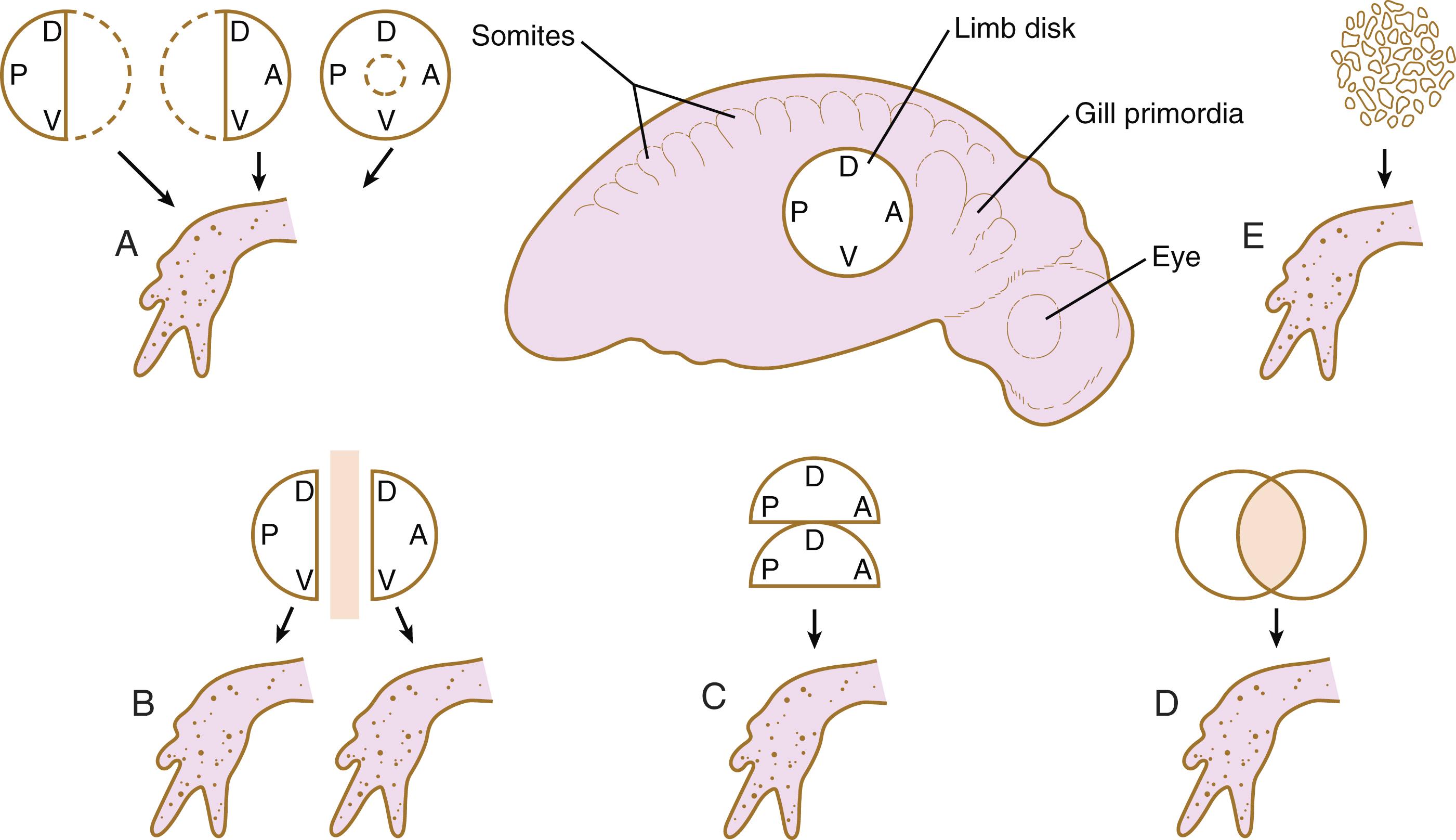
The early limb primordium is a highly regulative system, with properties similar to those described for the cleaving embryo (see p. 61). These properties can be summarized with the following experiments ( Figure 10.4 ):
If part of a limb primordium is removed, the remainder reorganizes to form a complete limb.
If a limb primordium is split into two halves, and these are prevented from fusing, each half gives rise to a complete limb (the twinning phenomenon).
If two equivalent halves of a limb primordium are juxtaposed, one complete limb forms.
If two equivalent limb disks are superimposed, they reorganize to form a single limb (see the section on tetraparental embryos [p. 62]).
In some species, disaggregated limb mesoderm can reorganize and form a complete limb.
The organization of the limb is commonly related to the Cartesian coordinate system. The anteroposterior ∗
∗ Because of different conventions in the use of axial terms, some human embryologists would take exception to the axial terminology presented here. Specifically, according to strict human terminology, anterior means “ventral,” and posterior means “dorsal.” However, the axial terminology used in this chapter (anterior means “cranial,” and posterior means “caudal”) is so uniformly used in the experimental and comparative embryological literature that a student referring to the original literature in the field of limb development would find it confusing to use adult human axial terminology.
axis runs from the first (anterior) to the fifth (posterior) digit. The back of the hand or foot is dorsal, and the palm or sole is ventral. The proximodistal axis extends from the base of the limb to the tips of the digits.
Experiments involving the transplantation and rotation of limb primordia in lower vertebrates have shown that these axes are fixed in a sequential order: anteroposterior to dorsoventral to proximodistal. Early fixation of the anteroposterior axis results from the expression of the transcription factors Gli-3 in the anterior and Hand-2 in the posterior part of the early limb field (see Figure 10.2A ). These two molecules mutually oppose each other’s actions. Before all three axes are specified, a left limb primordium can be converted into a normal right limb simply by rotating it with respect to the normal body axes. These axes are important as reference points in several aspects of limb morphogenesis. Evidence indicates a similar sequence of axial specifications in certain other primordia, such as those of the retina and inner ear.
Shortly after its initial establishment, the limb primordium begins to bulge from the body wall (late in the first month for the human upper extremity [ Figure 10.5 ]). At this stage, the limb bud consists of a mass of similar-looking mesodermal cells covered by a layer of ectoderm.
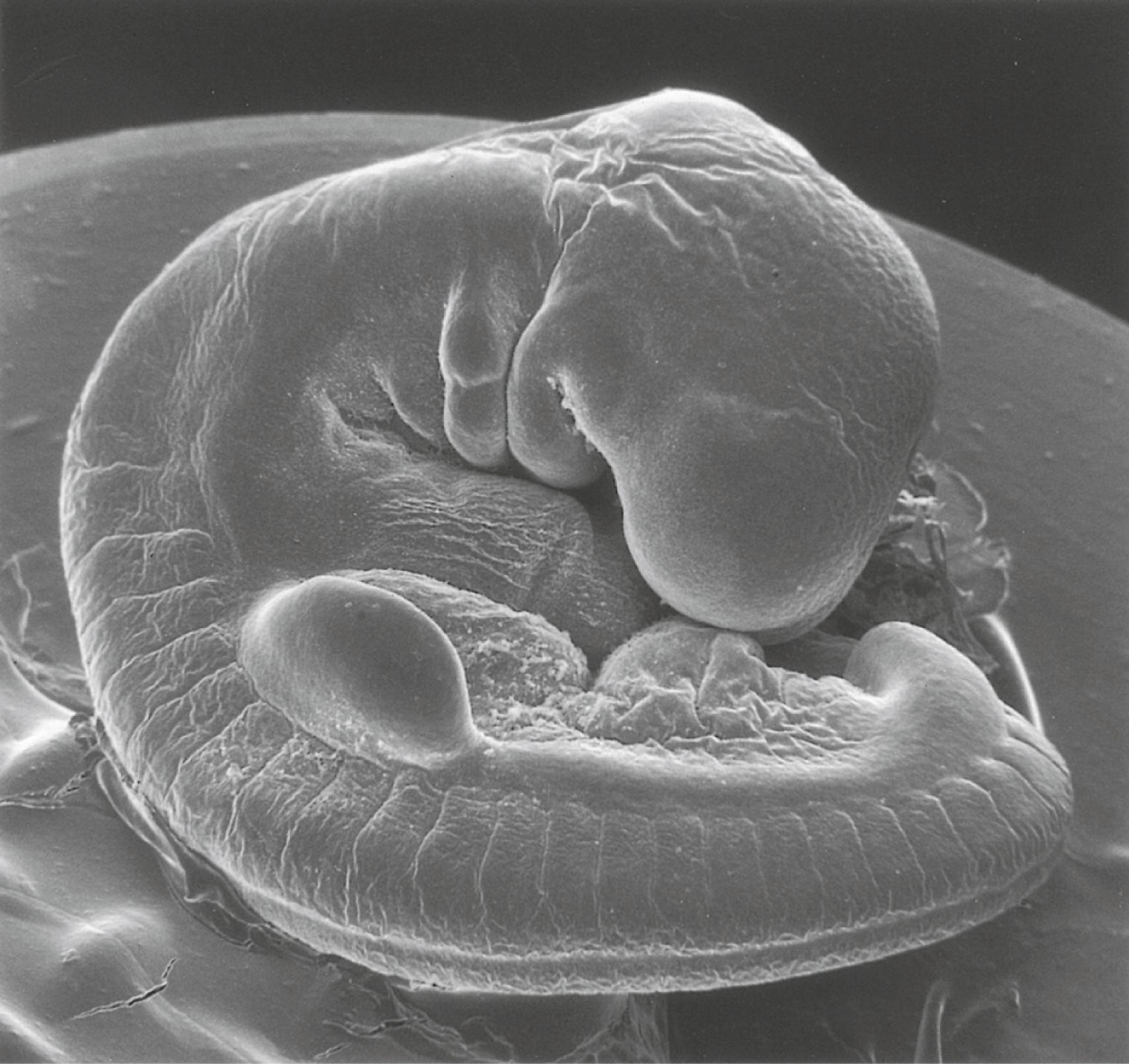
A distinctive feature is the presence of a ridge of thickened ectoderm ( apical ectodermal ridge [AER] ) located along the anteroposterior plane of the apex of the limb bud ( Figure 10.6 ) . During much of the time when the AER is present, the hand-forming and foot-forming regions of the developing limb bud are paddle shaped, with the apical ridge situated along the rim of the paddle ( Figure 10.7 ). Experiments have shown that the AER interacts with the underlying limb bud mesoderm to promote outgrowth of the developing limb. Other aspects of limb development, such as morphogenesis (the development of form), are guided by information contained in the mesoderm.
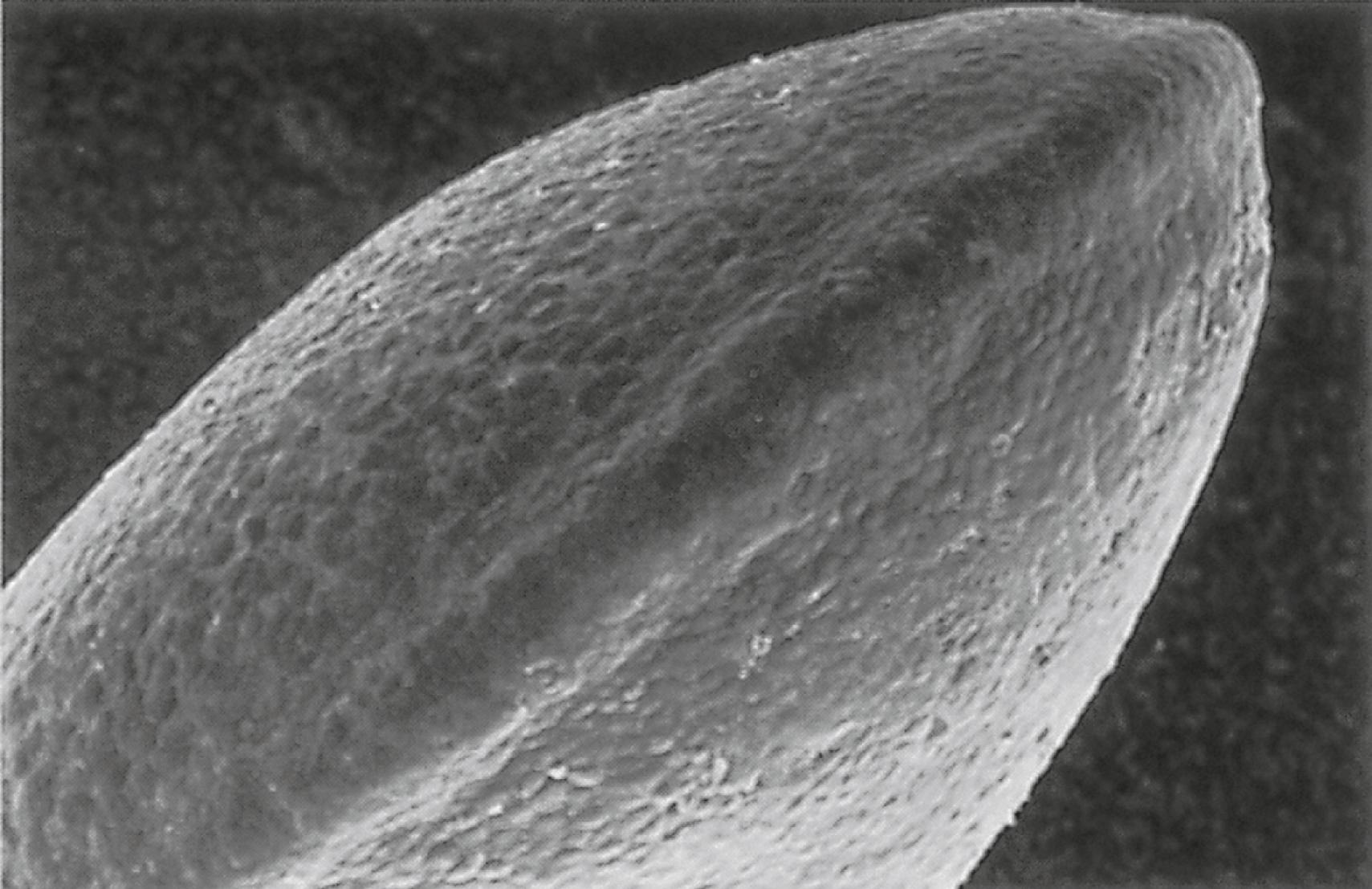
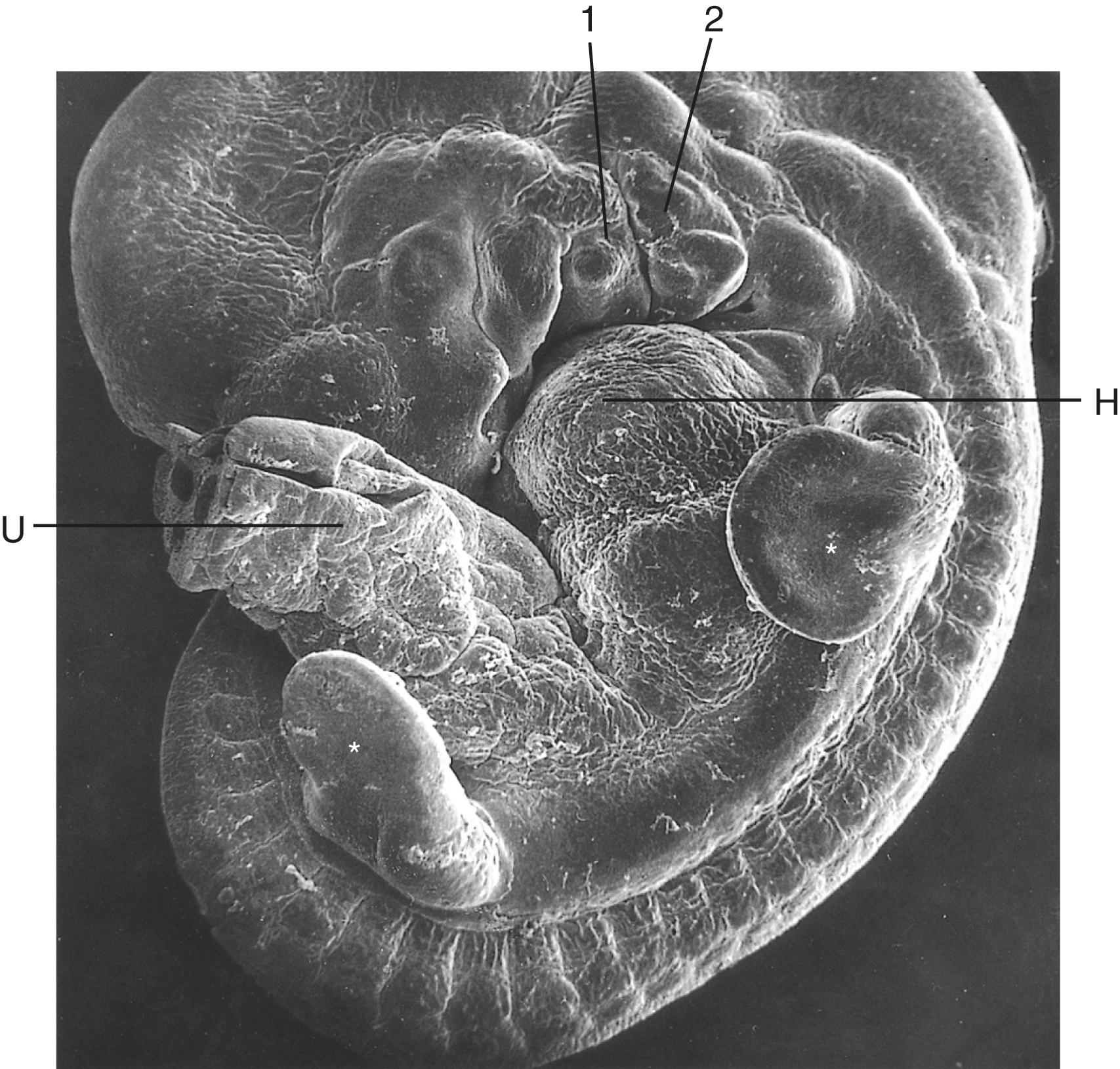
This section outlines many of the ways in which the limb bud mesoderm and ectoderm interact to control limb development. Recognition of these developmental mechanisms is important in understanding the genesis of many limb malformations.
The earliest limb bud begins to form before an AER is present, but soon a thickened AER appears along the border between dorsal and ventral limb ectoderm. Molecular studies have shown that the position of the AER corresponds exactly to the border between dorsal ectoderm , which expresses the signaling molecule radical fringe , and ventral ectoderm , which expresses the transcription factor Engrailed-1 (En-1) (see Figure 10.16A ).
Although the AER had been recognized for years, its role in limb development was not understood until it was subjected to experimental analysis. Removal of the AER results in an arrest of limb development, thus leading to distal truncation of the limb ( Figure 10.8 ). In the limbless mutant in chickens, early limb development is normal; later, the AER disappears, and further limb development ceases. If mutant ectoderm is placed over normal limb bud mesoderm, limb development is truncated, whereas combining mutant mesoderm with normal ectoderm results in more normal limb development. These findings suggest that the ectoderm is defective in this mutant.
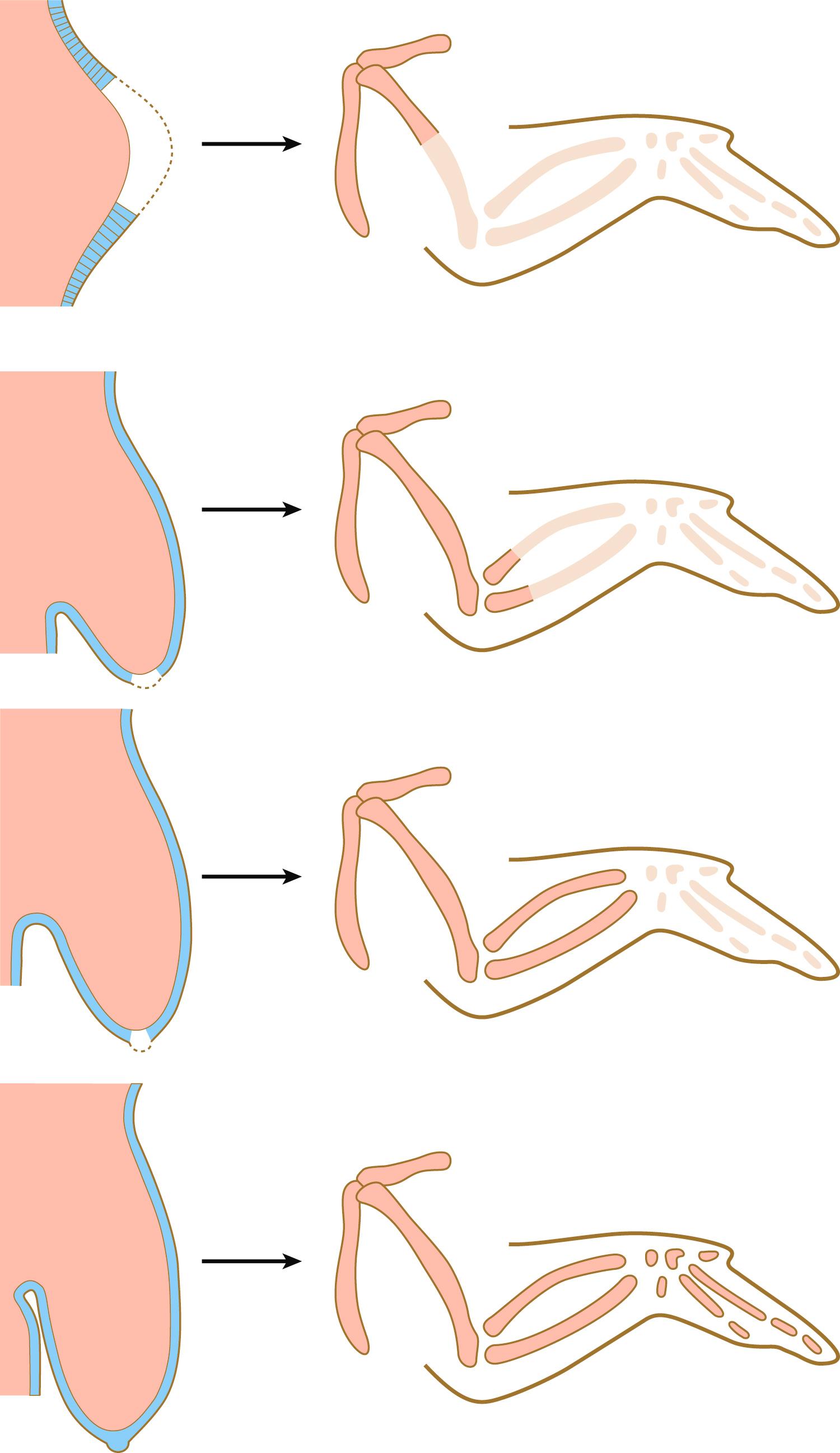
Further analysis has shown that in the limbless mutant, the entire ectoderm of the limb bud displays a dorsal character; that is, radical fringe and other “dorsal” molecules are expressed in dorsal and ventral ectoderm. Correspondingly, En-1 is not expressed in ventral ectoderm. In the absence of the juxtaposition of ectoderm with dorsal and ventral properties, an AER cannot be maintained.
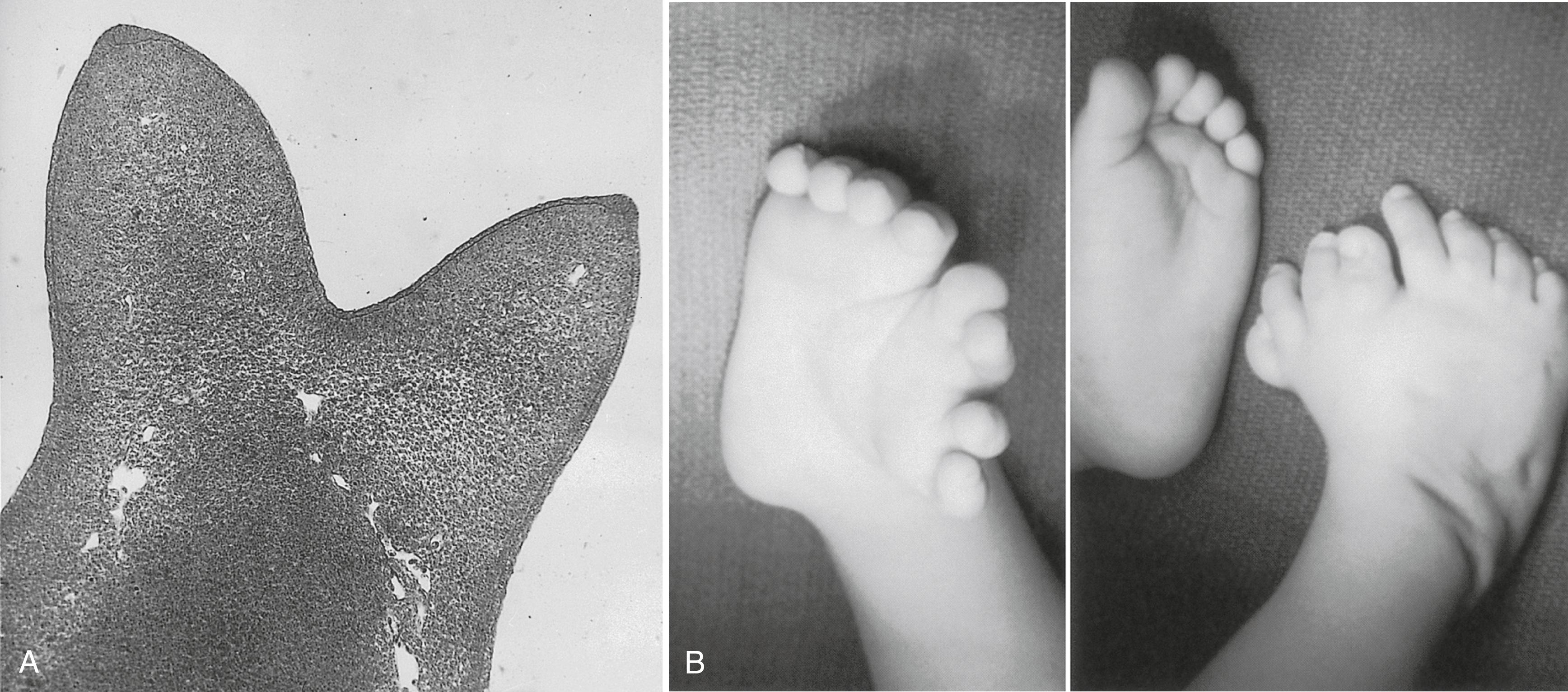
The power of the AER is shown by experiments or mutants that result in the formation of two AERs on the limb bud. This situation leads to a supernumerary limb, as is illustrated by the mutants eudiplopodia in chickens and diplopodia in humans ( Figure 10.9 ).
The outgrowth-promoting signal produced by the AER is FGF. In the earliest stages of limb formation, the lateral ectoderm begins to produce FGF-8 while it thickens to form an AER. While the limb bud begins to grow out, the apical ridge also produces FGF-4, FGF-9, and FGF-17 in its posterior half. If the AER is removed, outgrowth of limb bud mesoderm can be supported by the local application of FGFs. Other studies have shown that in many mutants characterized by deficient or absent outgrowth of the limb, the mutant ectoderm fails to produce FGF. The effects of the FGF produced by the apical ectoderm on the underlying mesoderm are discussed later in this chapter.
The mesoderm of the early limb bud consists of homogeneous mesenchymal cells supplied by a well-developed vascular network. The mesenchymal cells are embedded in a matrix consisting of a loose meshwork of collagen fibers and ground substance, with hyaluronic acid and glycoproteins prominent constituents of the latter. There are no nerves in the early limb bud.
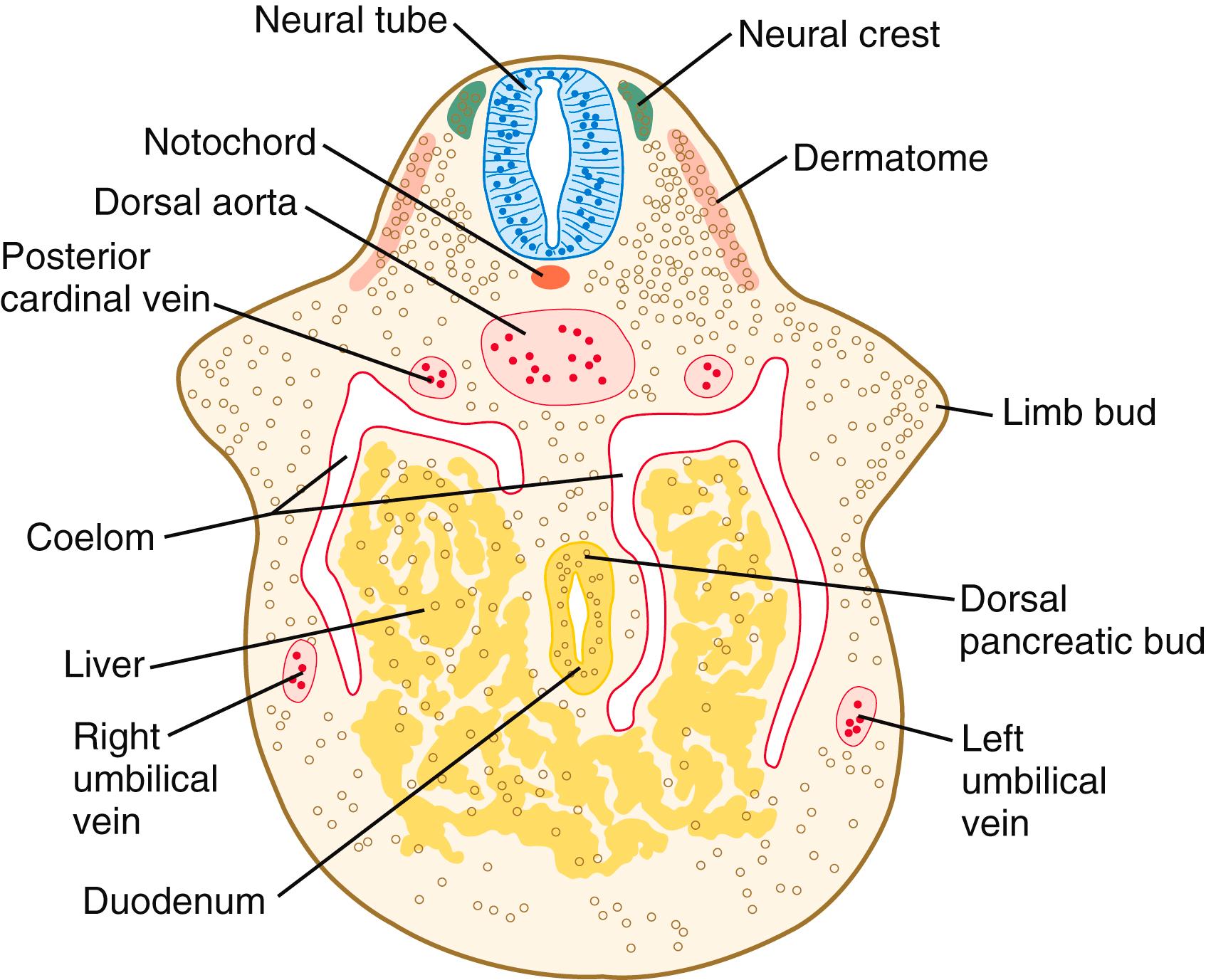
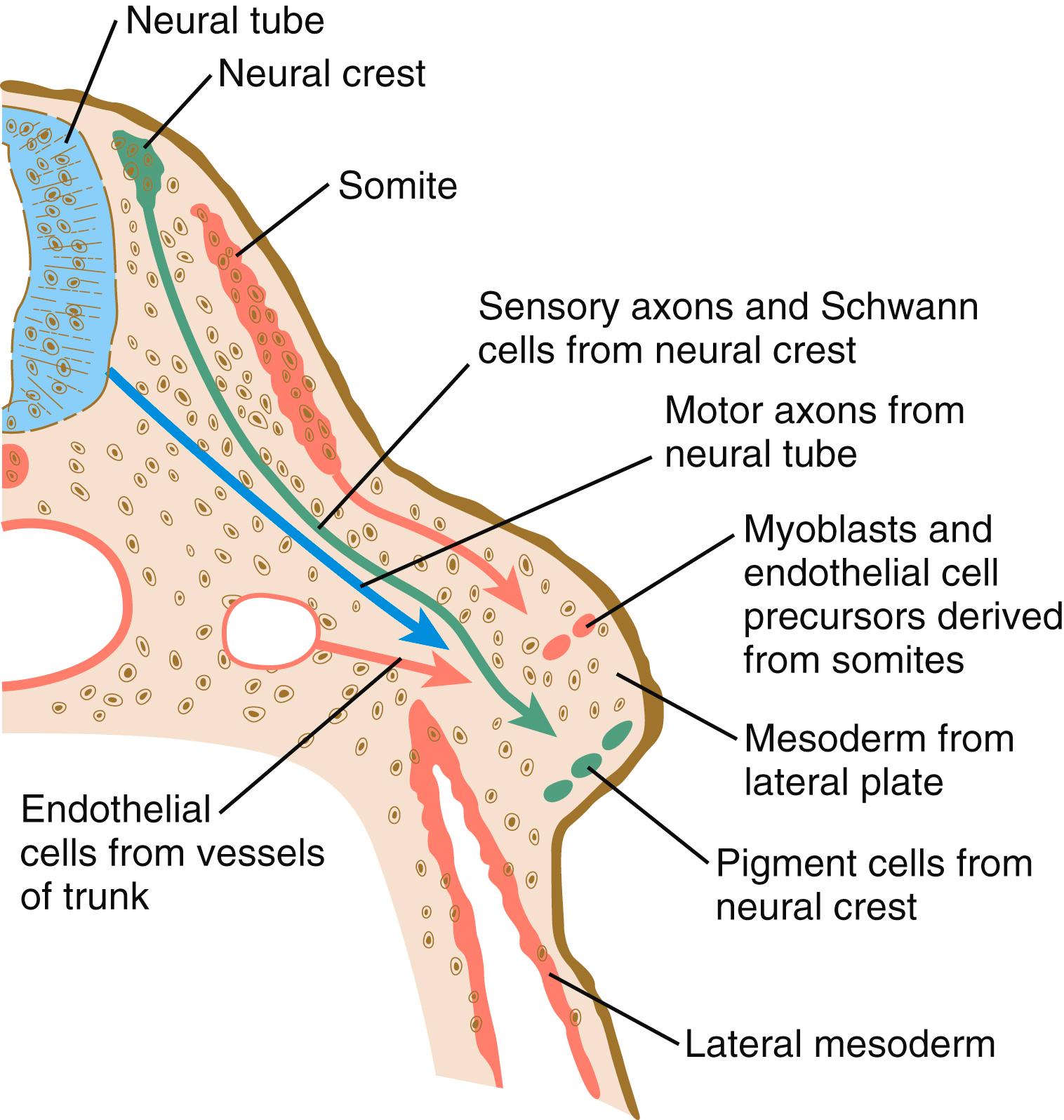
It is impossible to distinguish different cell types within the early limb bud mesenchyme by their morphology alone. Nevertheless, mesenchymal cells from several sources are present ( Figure 10.10 ). Initially, the limb bud mesenchyme consists exclusively of cells derived from the lateral plate mesoderm. These cells give rise to the skeleton, connective tissue, and some blood vessels. Mesenchymal cells derived from the somites migrate into the limb bud as precursors of muscle and endothelial cells. Another population of migrating cells is that from the neural crest; these cells ultimately form the Schwann cells of the nerves, sensory nerves, and pigment cells ( melanocytes ).
Limb development occurs as the result of continuous interactions between the mesodermal and ectodermal components of the limb bud. The apical ectoderm stimulates outgrowth of the limb bud by promoting mitosis and preventing differentiation of the distal mesodermal cells of the limb bud. Although the AER promotes outgrowth, its own existence is reciprocally controlled by the mesoderm. If an AER from an old limb bud is transplanted onto the mesoderm of a young wing bud, the limb grows normally until morphogenesis is complete. If old limb bud mesoderm is covered by young apical ectoderm, however, limb development ceases at a time appropriate for the age of the mesoderm and not that of the ectoderm.
Similar reciprocal transplantation experiments have been used to show that the overall shape of the limb is determined by the mesoderm and not the ectoderm. This is most dramatically represented by experiments done on birds because of the great differences in morphology between the extremities. If leg bud mesoderm in the chick embryo is covered with wing bud ectoderm, a normal leg covered with scales develops. In a more complex example, if chick leg bud ectoderm is placed over duck wing bud mesoderm, a duck wing covered with chicken feathers forms. Such experiments, which have sometimes involved mosaics of avian and mammalian limb bud components, show that the overall morphology of the limb is determined by the mesodermal component and not the ectoderm. In addition, the regional characteristics of ectodermal appendages (e.g., scalp hair versus body hair in the case of mammals) are also dictated by the mesoderm. Cross-species grafting experiments show, however, that the nature of the ectodermal appendages formed (e.g., hair versus feathers) is appropriate for the species from which the ectoderm was derived.
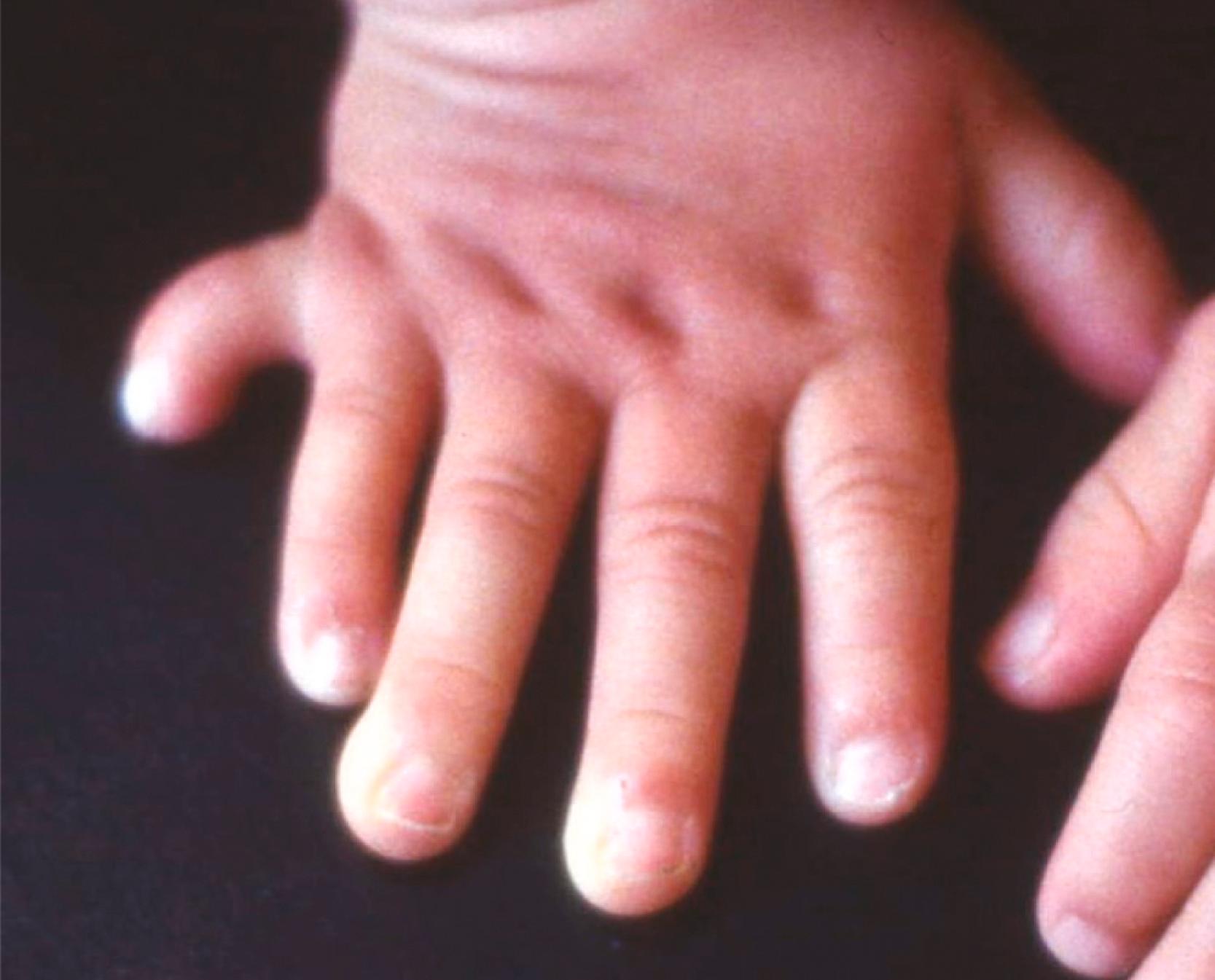
Polydactyly is a condition characterized by supernumerary digits and exists as a mutant in birds. Reciprocal transplantation experiments between mesoderm and ectoderm have shown that the defect is inherent in the mesoderm and not the ectoderm. Polydactyly in humans ( Figure 10.11 ) is typically inherited as a genetic recessive trait and is commonly found in populations such as certain Amish communities in the United States in which the total genetic pool is relatively restricted (see Clinical Correlation 10.1 for further details).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here