Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Normal development of the limbs begins at the end of the fourth week after fertilization, with limb buds forming in the mesoderm along the flank of the embryo ( Fig. 21.1 ). The limb bud is divided into three major regions. The apical ectodermal ridge (AER), in which several fibroblast growth factors (FGFs) are expressed, keeps the adjacent mesenchymal cells in an undifferentiated, rapidly proliferating state. This mesenchyma is known as the progress zone. The third zone is the zone of polarizing activity (ZPA) and this region is responsible for anteroposterior polarization as the limb develops. The cells that remain in this region the longest populate the distal portion of the extremity. The limbs develop in a proximodistal direction from the limb girdle to the digits. In embryologic terminology, a limb consists of four segments: a root (the zonoskeleton); a proximal segment (stylopodium) with one bone; a middle segment (zeugopodium) with two bones; and a distal, more complex part (autopodium) with many bones.
Early in the development of the limb bud, mesoderm thickens into a lateral plate, which induces thickening of the overlying ectoderm. This thickened ectoderm becomes the AER. The proximal bones of the limb girdle and the humerus or femur form before the differentiation of ridge ectoderm, whereas development of the remaining bones and digits depends on the AER. ,
Molecular organization of limb bud development occurs along three axes and is controlled by three separate organizing centers. Prior to limb bud development, the highly conserved Hox genes specify the segmentation of the embryo (neck, thorax, abdomen, etc.) and define the position of the limb buds. Discussion of the three axes and their controls follow.
The proximal distal axis is induced by a number of factors still being clarified. Hox transcription factors, Wnt, and retinoic acid, induce the Tbx5 transcription factor. This factor upregulates Fgf10, which induces Wnt3- in the distal ectoderm, which, in turn, induces secretion of fibroblast growth factor 8 (Fgf8). Fgf8 helps establish and maintain the AER. Fgf8 also induces Fgf10, which works in concert with Fgf8 to promote proliferation and limb outgrowth patterns in the limb by exerting a distalizing influence during progressive outgrowth. This development occurs in a proximal to distal fashion with progressive increase in complexity distally. Fgf8 is the key signaling element of the AER.
As the limb grows, the FGFs from the AER maintain a portion of the limb in an undifferentiated state to promote limb outgrowth as well as patterning the limb along its proximal distal axis. This portion of the limb is known as the progress zone. Proliferation of this pleuripotentian mesoderm is dependent on vascular ingrowth. Recently, a two-signal mechanism has been proposed by which the distalizing influence of FGFs is countered by a proximalizing influence of retinoic acid. With further growth these two factors separate to create a tripartite pattern resulting in delineation of the arm, forearm and hand (see Fig. 21.3C ).
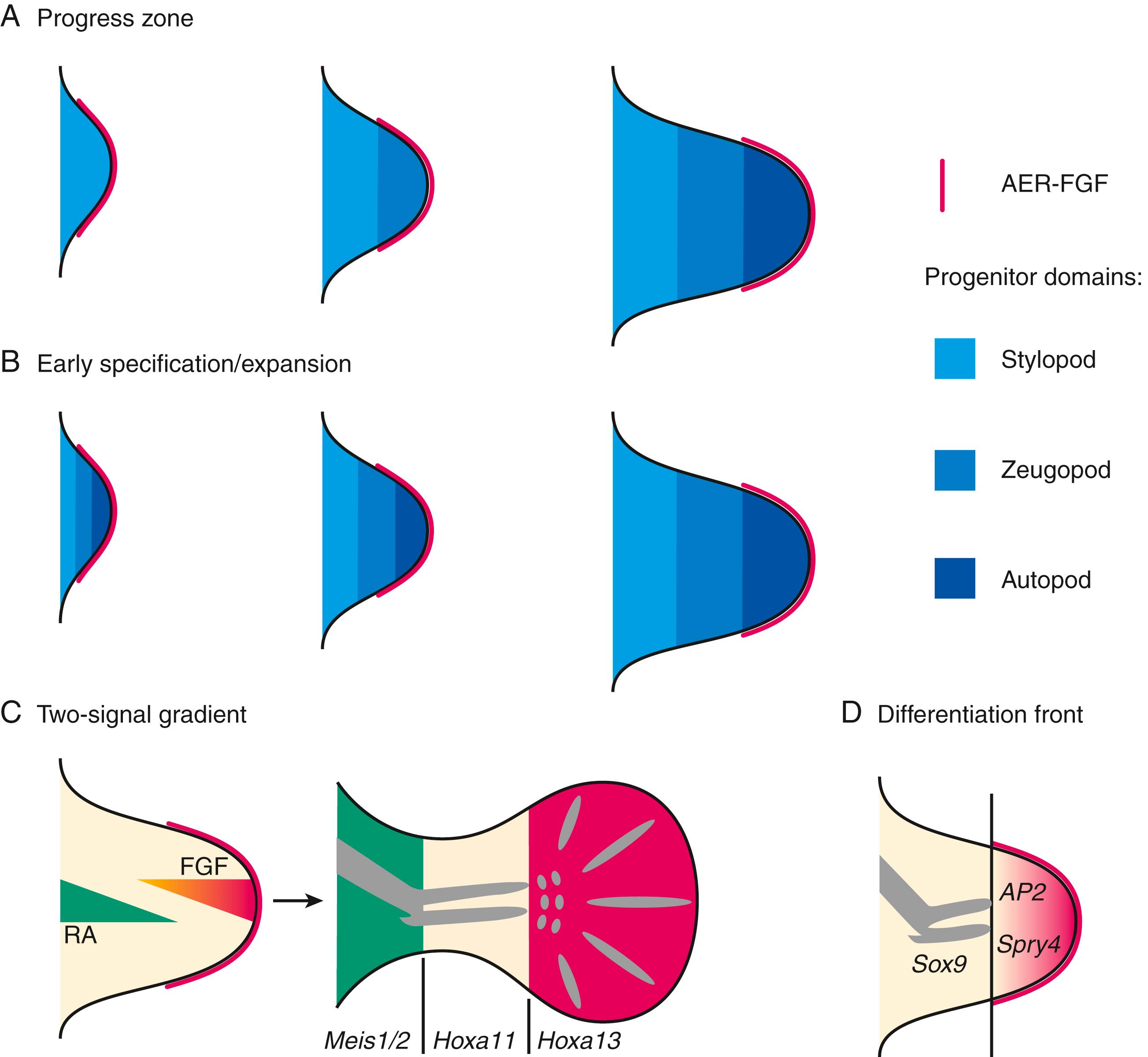
HOX factors also initiate the signaling center of the anterior posterior axis. HOX induces Hand2 transcription factor, which induces secretion of sonic hedgehog (SHH), which establishes the ZPA. SHH is the defining molecule of the ZPA. SHH acting with GLI3 and other factors also induces proliferation-expanding limb bud width in an anterior posterior axis going from the first to the fifth digit. The zone of polarizing activity regulatory sequence (ZRS), located 800 kb away from SHH, is an enhancer and regulates the expression of SHH.
This pathway of the dorsal ventral axis (back of hand to palm) is regulated by Wnt7a, which is secreted by the dorsal ectoderm. Wnt7a is the critical molecule in the development of this axis. The ZPA receives input from Wnt7a, establishing a reciprocal feedback loop with the AER to precisely coordinate limb development.
The bones and connective tissues of the limbs are formed by lateral plate mesoderm, and the muscles originate from myotome regions of the somitic mesoderm. Forelimb and hind limb development occurs via similar mechanisms, with upper limb growth preceding lower limb growth by 1 to 2 days. By 6 weeks, as the buds extend distally, the terminal parts of the limbs flatten to form handplates and footplates, complete with distal rays, and cartilage begins to appear in the proximal portions of the limbs. During the seventh week, the limbs begin to rotate, with the forelimb turning 90 degrees laterally (positioning the thumb laterally) and the hind limb turning 90 degrees medially (positioning the big toe medially). Digital rays appear in the handplates and footplates. By the eighth week, the limbs have rotated to their final position, and all segments are complete, including the digits. During this time, ossification starts. By 12 weeks, ossification centers are present in all the long bones.
When pathways are disrupted, certain patterns emerge. If the induction pathway is fully disrupted, the result will be tetra amelia. Complete loss of FGFs can also result in tetra amelia. If Fgs are partially disrupted, then a transverse deficiency will result. The location of the defect will be determined by the timing of the insult relative to the limb bud development.
Disruption in the proximal distal axis results in a rizomelic dysplasia if Shox2 is affected. Mesomelic dysplasia occurs if Shox is not functional, and phocomelia occurs if both are involved.
Loss of FGF function in SHH-independent areas results in loss of radial structures as in radial hemimelia. Loss of SHH function leads to ulnar deficiencies. Disruption of regulatory sequences can lead to excess SHH function, which results in polydactyly. Dorsal ventral disruption with loss of Lmxb results in the Nail-Patella disorder. A new classification is based on the axis of disruption ( Box 21.1 ).
Malformations
Entire upper limb
Proximo-distal axis defect causes transverse defects
Radioulnar axis defect causes dysplasias, polydactylies
Dorso-ventral axis defect causes nail-patella syndrome, Fuhrmann disorder
Unspecified defect causes Sprengel disorder
Handplate
Proximo-distal axis causes transverse defects
Radioulnar axis causes dysplasias, polydactylies
Dorso-ventral axis causes nail-patella syndrome, Fuhrmann disorder
Unspecified defect causes Sprengel disorder
The proximal bones of the limb girdle and the humerus or femur form before the differentiation of ridge ectoderm, whereas development of the remaining bones and digits depends on the AER. ,
The AER is formed by the thickening of lateral plate mesoderm, which signals the overlying ectoderm to thicken and establish a ridge over the tip of the limb bud. The AER regulates the proximodistal growth of the limbs ( Fig. 21.2 ). Although the AER causes outgrowth of the limbs, the mesenchyma determines the type of limb that will develop. , ,
The anteroposterior axis, going from digit 1 to 5, is specified by the ZPA signaling center, which is governed by SHH. The third axis, the dorsal-ventral axis (back of hand to palm) is regulated by WNT protein 7A. The bones and connective tissues of the limbs are formed by lateral plate mesoderm, and the muscles originate from myotome regions of the somitic mesoderm. Forelimb and hind limb development occurs via similar mechanisms, with upper limb growth preceding lower limb growth by 1 to 2 days. By 6 weeks, as the buds extend distally, the terminal parts of the limbs flatten to form handplates and footplates, complete with distal rays, and cartilage begins to appear in the proximal portions of the limbs. During the seventh week, the limbs begin to rotate, with the forelimb turning 90 degrees laterally (positioning the thumb laterally) and the hind limb turning 90 degrees medially (positioning the big toe medially). Digital rays appear in the handplates and footplates. By the eighth week, the limbs have rotated to their final position, and all segments are complete, including the digits. During this time, ossification starts. By 12 weeks, ossification centers are present in all the long bones.
Extensive research has provided increasing insight into the genes and factors involved in the development of the limbs in humans and other species. , A series of hypotheses has been developed to explain increasingly complex understanding of the interplay among factors that control development. An initial model, known as the progress zone model, has been useful in explaining many experimental observations. Subsequent discoveries have led to newer theories and models.
In this model the limb bud, in its early state, consists of a central group of cells, the progress zone, in which the most distal rim of cells makes up the AER. A group of cells on the posterior margin comprises the ZPA, which regulates anteroposterior axis development. Retinoic acid stimulates the ZPA via sonic hedgehog genes (Shh), which also regulate downstream genes including homeobox genes, bone morphogenetic proteins (BMPs), Wnt genes, and Gli transcription factors.
The more proximal cells proliferate and push the progress zone cells more distally so that they are controlled by the AER to form middle and distal segments, whereas the proximal cells develop into more proximal structures. The proximal structures (humerus, femur, or stylopod) develop earlier than the middle and distal structures.
In this model the three segments are considered to be predetermined at the outset and become specific structures as growth and differentiation occur.
In this model a group of cells known as the differentiation front divides the more differentiated cells from the less differentiated. The AER via FGFs keeps the distal mesenchyme in an undifferentiated state. Differentiating chondrocytes proximal in the limb bud express Sox9, whereas more distal undifferentiated cells express Sprouty4 and adipocyte protein 2 (AP2). The differentiation front separates these two zones of cells and moves more distally as the limb bud grows.
In this model the development of cells is specified by two signals, a proximal-to-distal retinoic acid gradient and a second distal-to-proximal gradient of FGFs from the AER. The interaction of these two signals over time directs development. Meis1/2 , Hoxa11 , and Hoxa13 genes control this development.
Current work favors the following concepts: (1) progenitor cells are specified early with regard to their proximodistal and anteroposterior fates; (2) regulatory signaling is required for subsequent proliferation and expansion of the progenitor pools; (3) regulation of the BMP antagonist Gremlin1 via feedback loops integrates inputs from the BMP, Shh, and FGF pathways; and (4) vertebrate limb-bud development is controlled by a four-dimensional patterning system with integrated positive and negative regulatory feedback loops, rather than thresholds set by morphogen gradients ( Fig. 21.3 ).
One type of malformation, split hand and foot malformation associated with tibial hemimelia, has been associated with specific chromosomal region mutations.
The classification system proposed by Frantz and O’Rahilly in 1961 continues to be widely used as a method of grouping congenital skeletal limb deficiencies ( Fig. 21.4 ). , Extremity anomalies are categorized as either terminal deficiencies or intercalary deficiencies. Terminal deficiencies are those in which the missing segment extends to the end of the extremity. Intercalary deficiencies occur within the extremity and do not extend to its end. These deficiencies may be transverse, as if the limb were cut across its long axis, or paraxial (formerly longitudinal) if the deficit is parallel to the long axis ( Figs. 21.5 and 21.6 ).
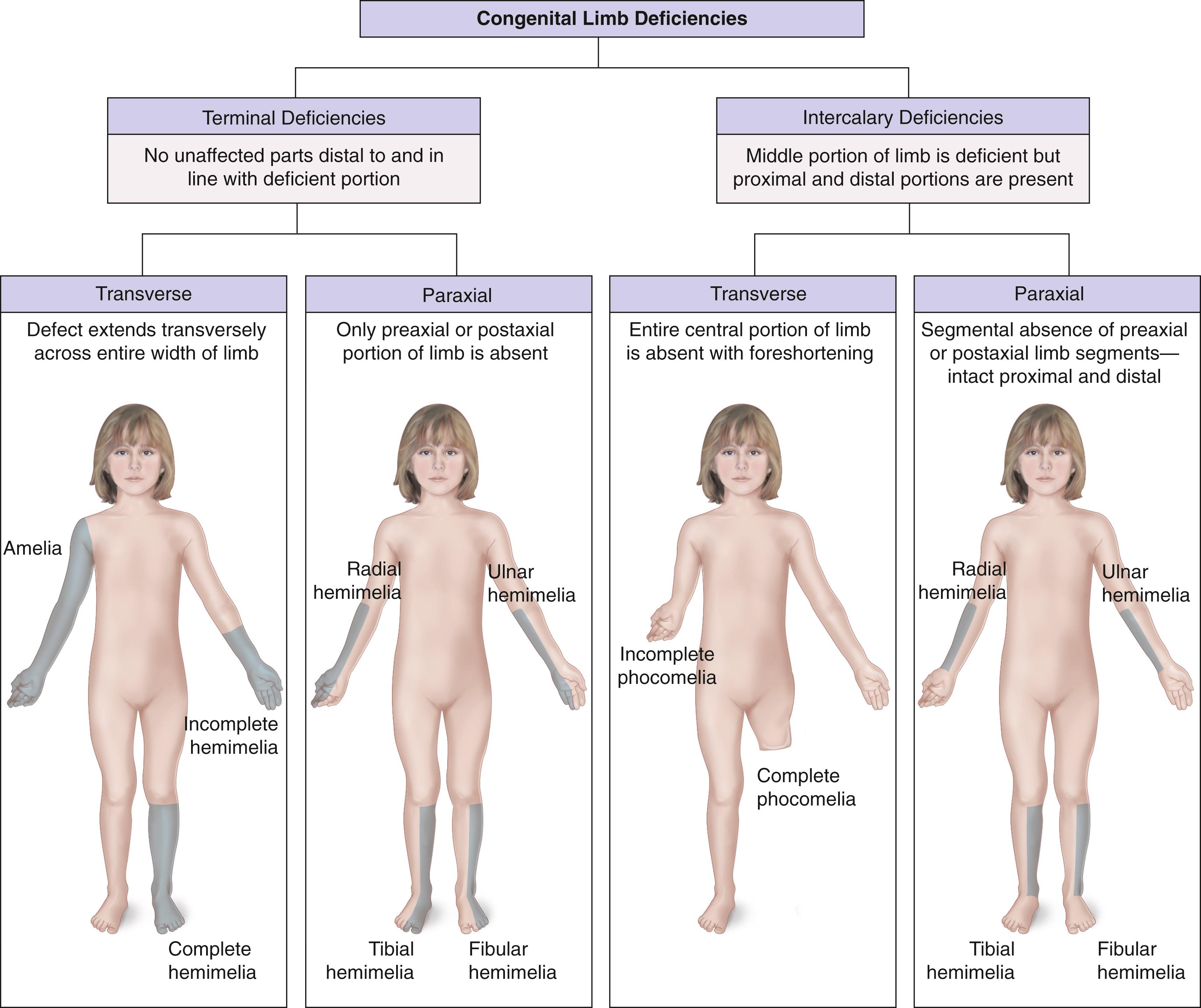
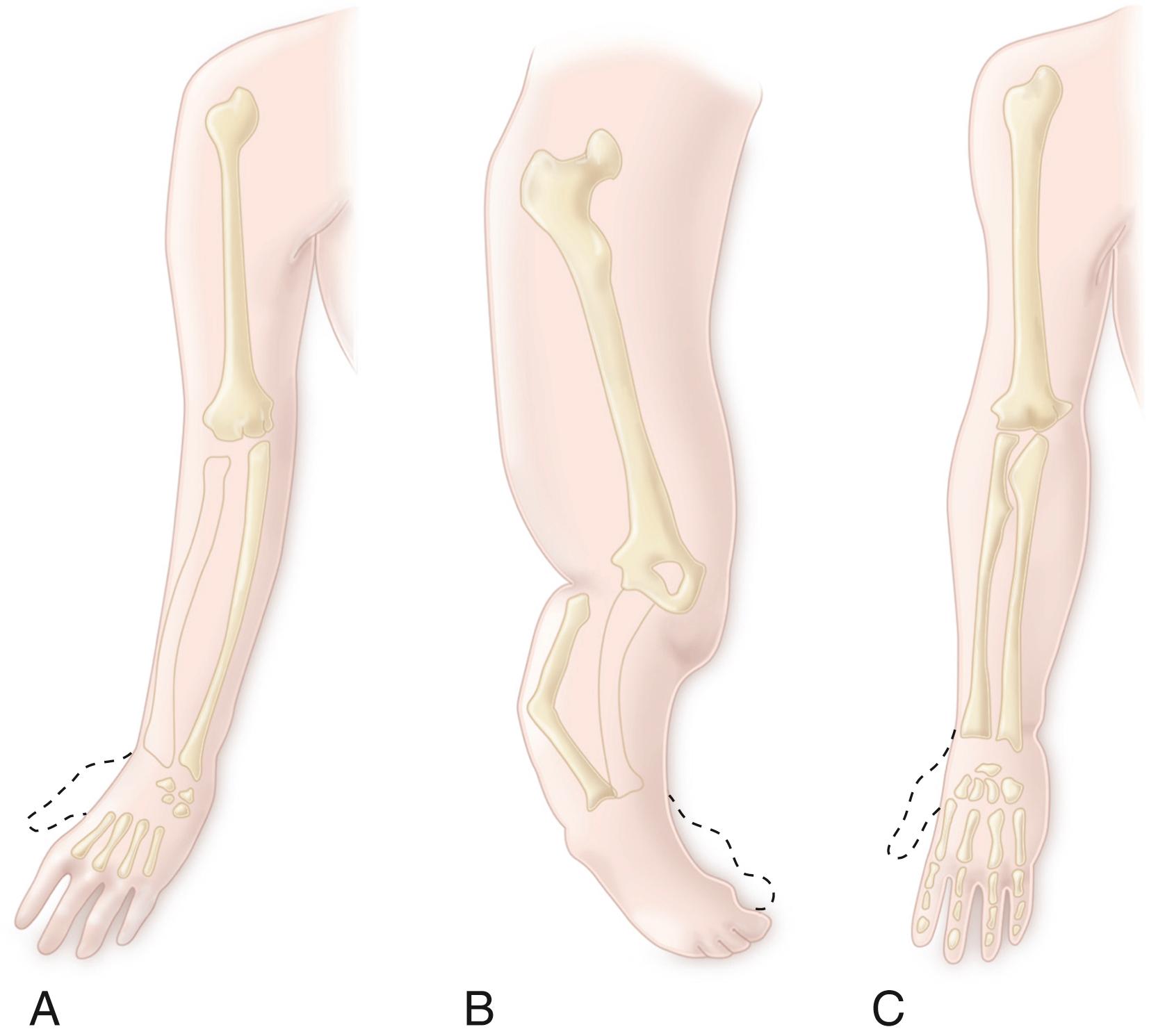
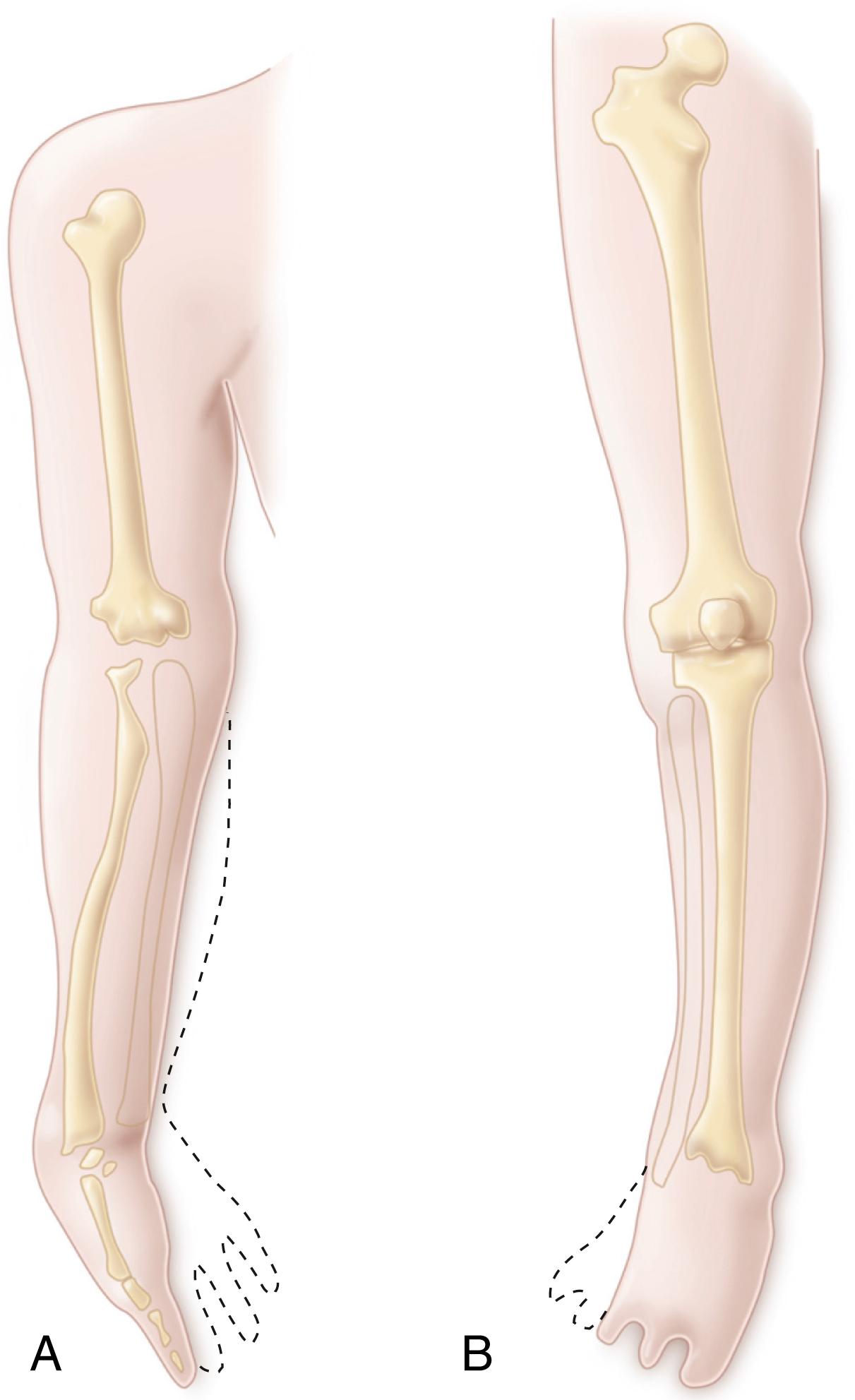
Amelia is the complete absence of a limb. Hemimelia, which means half a limb, is used when one of the paired bones of the upper or lower extremity is absent. The terms complete and incomplete are applied to hemimelias to indicate the absence of all or only part of the affected bone. Deficiencies are designated according to the anatomic part that is absent. For example, when the tibia is absent, the deficiency is termed tibial hemimelia . A glossary of terms associated with limb deficiencies is provided in Box 21.2 .
Acheiria (or achiria): absence of the hand
Acheiropodia: absence of the hands and feet
Adactyly (or adactylia): absence of the fingers or toes
Agenesis: absence or no development
Amelia: complete absence of a limb
Amelia totalis: complete absence of all four limbs
Amputation: absence of a distal part of a limb
Aphalangia: absence of the phalanges
Aplasia: absence of a specific bone or bones
Apodia: absence of the foot
Ectrocheiria: total or partial absence of the hand
Ectrodactyly: total or partial absence of the fingers
Ectromelia: total or partial absence of one or more long bones or limbs
Ectrophalangia: absence of one or more phalanges
Ectropodia: total or partial absence of the foot
Hemimelia: absence of one of the paired bones of the limbs
Hypophalangia: fewer than normal phalanges
Intercalary deficiency: absence of the middle portion of a limb, when proximal and distal portions are present
Longitudinal deficiency: absence of a limb extending parallel to the long axis (may be preaxial, postaxial, or central)
Meromelia: partial absence of a limb
Oligodactyly: absence of some of the fingers
Paraxial deficiency: absence of the preaxial or postaxial portion of a limb
Peromelia: hemimelia, especially hands ending in a stump
Phocomelia: absence of the arm and forearm in the upper limb or the thigh and leg in the lower limb (i.e., the hands or feet sprout directly from the trunk); the deficiency may be proximal (arms or thighs missing) or distal (forearms or legs missing)
Postaxial: pertaining to the ulnar side of the upper limb and the fibular side of the lower limb
Preaxial: pertaining to the radial side of the upper limb and the tibial side of the lower limb
Terminal deficiency: absence of a limb with all portions in line with and distal to the defect involved
Transverse deficiency: absence of the entire width of a limb
An international classification system was proposed in 1991 by the International Society for Orthotics (ISO) and the International Society for Prosthetics and Orthotics (ISPO) in an attempt to improve communication among researchers in different countries by standardizing the terminology for congenital deficiencies ( Box 21.3 and Table 21.1 ). In the international system, all limb deficiencies are classified as either transverse or longitudinal. Missing bones are named and described as either complete or partial in their absence. The authors of this system theorized that no true intercalary deficits exist and, instead, treated such deficits as variable degrees of longitudinal deficiencies.
The limb has developed normally to a particular level beyond which no skeletal elements are present, although there may be digital buds.
The deficiency is described by naming the segment at which the limb terminates and the level within the segment beyond which no skeletal elements exist.
Reduction or absence of an element or elements within the long axis of the limb in which normal skeletal elements may be present distal to the affected bone or bones.
The deficiency is described by naming the bones affected in a proximodistal sequence; any bone not named is present and normal.
The affected bone is described as totally or partially absent.
For partial deficiencies, the approximate fraction and position of the absent part may be stated.
The number of the digit is stated in relation to the metacarpal or metatarsal and phalanges, with numbering beginning from the preaxial, radial, or tibial side.
The term ray may be used to refer to a metacarpal or metatarsal and its corresponding phalanges.
| Standard | ISO/ISPO |
|---|---|
| Congenital “Chopart” | Transverse, tarsal, partial |
| Congenital absence of forefoot | Transverse, metatarsal, complete |
| Congenital “Syme” | Transverse, tarsal, complete |
| Congenital below-knee, long | Transverse, leg, lower third |
| Congenital below-knee, short | Transverse, leg, upper third |
| Congenital knee disarticulation | Transverse, leg, complete |
| Congenital above-knee, short | Transverse, thigh, upper third |
| Congenital hip disarticulation | Transverse, thigh, complete |
| Upper limb amelia, no scapula | Transverse, shoulder, complete |
| Upper limb amelia, scapula present | Transverse, forearm, upper third |
| Congenital below-elbow, long | Transverse, forearm, lower third |
| Congenital wrist disarticulation | Transverse, carpal, complete |
| Congenital partial hand (with carpals) | Transverse, carpal, partial |
| Congenital partial foot (with metatarsals) | Transverse, phalangeal, complete |
| Fibular hemimelia (foot unaffected) | Longitudinal, fibular, complete |
| Partial fibular hemimelia | Longitudinal, fibular, partial |
| Tibial hemimelia | Longitudinal, tibial, complete |
| Absent tibia and first two toes | Longitudinal, tibial, complete; rays 1, 2 |
| Absent radius, ulna (index finger present) | Longitudinal, radius and ulna, complete; carpals, complete; rays 1, 3, 4, and 5, complete |
| Upper limb phocomelia (hand intact, scapula present) | Longitudinal, humerus, complete; radius and ulna, complete |
| Clavicle absent, humerus short, radius short, ulna absent, carpals absent, fifth finger absent | Longitudinal, clavicle, complete; humerus, partial; radius, partial; ulna, complete; ray 5 |
In this international classification system, transverse deficiencies are named according to the level of absence ( Table 21.2 ). Therefore, a short congenital below-elbow absence is termed “transverse right Fo (forearm), upper,” whereas an elbow disarticulation level is a “transverse right Fo, complete.” Longitudinal deficiencies are named for the missing parts, and parts not named are assumed to be present ( Table 21.3 ). For example, fibular hemimelia is described as a “complete deficiency, Fi (fibula),” implying a normal foot. If the lateral two rays are also missing, one adds “MT (metatarsal) 4, 5, Ph (phalanges) 4, 5” to designate that condition. Although the terminology is laudable for its anatomic accuracy, the lengthy designations have limited its general acceptance.
| Upper Limb (UL) | Lower Limb (LL) | Level of Absence |
|---|---|---|
| Arm (Ar) or forearm (Fo) | Thigh (Th) or leg (Le) | Complete, upper third, middle third, or lower third |
| Carpal (Ca), metacarpal (MC), phalangeal (Ph) | Tarsal (Ta), metatarsal (MT), phalangeal (Ph) | Complete or partial |
| Location | Deficiency |
|---|---|
| Upper Limb (UL) | |
| Proximal | Humeral (Hu) |
| Distal | Radial (Ra); central (Ce); ulnar (Ul); carpal (Ca); metacarpal (MC) 1, 2, 3, 4, 5; phalangeal (Ph) 1, 2, 3, 4, 5 |
| Combined | Radial (Ra); humeral (Hu); ulnar (Ul); carpal (Ca); metacarpal (MC) 1, 2, 3, 4, 5; phalangeal (Ph) 1, 2, 3, 4, 5 |
| Lower Limb (LL) | |
| Proximal | Femoral (Fe) |
| Distal | Tibial (Ti); central (Ce); fibular (Fi); tarsal (Ta); metatarsal (MT) 1, 2, 3, 4, 5; phalangeal (Ph) 1, 2, 3, 4, 5 |
| Combined | Tibial (Ti); femoral (Fe); fibular (Fi); tarsal (Ta); metatarsal (MT) 1, 2, 3, 4, 5; phalangeal (Ph) 1, 2, 3, 4, 5 |
The timing of nonsurgical and surgical corrections of limb deficiencies should accord with the child’s developmental milestones and social needs. For example, children who are treated with Syme amputation for fibular hemimelia do remarkably well with amputation at the age when they are pulling to stand and beginning to walk. When fitted with a prosthesis at this age, these children almost immediately begin to walk as if they recognize the prosthesis as a part of their body.
For many lower limb deficiencies, surgical interventions should be planned early and completed, if possible, before the child starts to walk, usually around 11 months of age. This allows time for the wound to heal properly and for a prosthesis to be ready when the child starts to walk.
The child’s developing anatomy is another important factor in the timing of surgical interventions. A Syme amputation in a child with a proximal focal femoral deficiency (PFFD) may be performed when the child is young, but knee fusion is best deferred until the femoral condyles and proximal tibia have sufficiently ossified (at approximately 4–5 years of age). Alternatively, the child may be treated initially with an equinus prosthesis to allow ambulation, followed by amputation and knee fusion at 3 to 4 years of age. In a patient with a partial tibial hemimelia, fusion of the proximal tibia to the fibula is more easily achieved after the tibial anlage has adequately ossified, rather than when it is still primarily cartilaginous.
For children and adolescents, many important social factors come into play when deciding on interventions to treat limb deficiencies. For example, a young child may discard an upper extremity prosthesis and then request one when starting school. When planning surgery, the surgeon should factor in sufficient recovery time so that the child does not have to enter a new school while still walking on crutches. For an adolescent, every attempt should be made to minimize any embarrassment, such as having to go to school without a prosthesis.
Difficult decisions often must be made regarding the best long-term plans for the child. This is especially true when the choice is between a prolonged course of multiple corrective procedures and a less involved, early, single intervention such as amputation. In our institution, new patients are evaluated by both the limb lengthening team and the prosthetic team, and at those encounters, the patients meet other children and families who have had similar interventions. Treatment decisions are usually not made until the family has had several such visits. Psychologic evaluation and intervention is a vital part of the decision-making process as well.
Such a dilemma typically arises in a child with fibular hemimelia. If a Syme amputation is performed just before the patient starts walking, the child will probably not require any additional operations or hospitalizations for the deformity and will be able to function at a nearly normal level in sports activities. However, the child will have to deal with wearing a prosthesis in place of a foot.
An alternative approach is to use limb-lengthening techniques to correct the deformity, which will maintain the limb and eliminate the need for a prosthesis. However, limb lengthening requires that the child endure two or three periods in an external apparatus (each of which may last 6 months or longer) for a successful outcome; the procedures are often difficult and painful, and the social and psychological costs of these interventions can be substantial. , , , A study of patients treated by limb-lengthening procedures at Texas Scottish Rite Hospital for Children in Dallas found that half of them experienced a deterioration in mental status, often with signs of depression. The medical outcome was compromised in approximately 20% of patients because of a psychological or behavioral factor. The investigators recommended that, in addition to arranging for psychological counseling, the orthopaedist should aggressively treat any pain, sleep, or appetite problems experienced by the patient during limb lengthening and reconstruction.
Although the anatomic results of lengthening procedures are well known, long-term functional assessments of patients who have undergone such procedures are not available. Thus Damsin and associates made a good point when they stated that “surgeons should remain humble and wise during decision making” and “not yield to the temptation offered by a brilliant equalization procedure.” Whenever a choice must be made between amputation and lengthening, the role of the orthopaedist is to make sure that the patient and the parents fully understand the risks and benefits of the options available. In that way, informed decisions can be made based on knowledge and realistic expectations, not ignorance or wishful thinking. This goal may be best accomplished by introducing the child and family to another child and family who have faced similar decisions.
By 7 weeks of embryonic life, the formation of all parts of the upper and lower limbs is essentially complete. Most limb deficiencies occur early in the period of limb morphogenesis, during rapid proliferation and differentiation of cells and tissues. This “sensitive period” of limb formation peaks during the fifth and sixth weeks after fertilization. Thus, major malformations (e.g., absence of a long bone) appear by 7 weeks of fetal development. Major upper limb deficiencies occur at 28 days, major lower limb deficiencies at 31 days, distal upper abnormalities on the 35th day, and distal lower limb deformities on the 37th day. Depending on the timing and severity of the insult, abnormalities develop in a predictable manner. Unlike malformations, deformations—changes in formed structures resulting from external forces—can occur at any time during fetal development. Distal deformity secondary to a constricting amniotic band is an example of a deformation ( Fig. 21.7 ).

Advances in molecular biology have provided new information about the genes and gene products responsible for coordinating normal limb development. This knowledge has enabled scientists to better identify genes that may be directly responsible for limb defects or indirectly responsible through the effects of teratogens.
At this time, however, the specific cause of congenital limb deficiencies is unknown in most cases. Although a few limb abnormalities have genetic bases, most limb deformities develop sporadically, with no identifiable environmental factors, trauma, or familial incidence. Most single-limb anomalies have a very small chance of recurring in subsequent children of the same parents or in the children of the affected person. The incidence of recurrence of the same anomaly is 1% to 3%, only slightly greater than that among the general population.
In a population-based review of birth defects in Norway, Lie and associates reported that in families of children with limb abnormalities, a second child has a significantly greater chance of being born with a similar anomaly compared with the expected rate in the general population. However, these investigators also noted that the risk of a birth defect in subsequent children decreases if the mother moves to another part of the country after the first child is born, a finding that suggests an environmental influence.
Many drugs are known teratogens; however, the only drug specifically identified with a large number of limb abnormalities is thalidomide. , , , , A possible mechanism of the thalidomide effect is the production of free radicals and hydrolysis products (2-phthalimidoglutaramic acid [PGMA] and 2-phthalimidoglutaric acid) that cause misregulation of limb growth pathways. A case of fibular hemimelia with focal femoral deficiency has been reported in a case of a woman who attempted abortion by taking the drug misoprostol.
Amniotic bands (Streeter band syndrome or congenital constriction band syndrome) are another potential cause of prenatal limb amputation (see Fig. 21.7 ). The prevailing theory is that early compression of the embryo is believed to cause early rupture of the amniotic membrane, with subsequent formation of aberrant amnion bands (strands) that can disrupt structures in the craniofacial area, abdominal wall, or limbs. However, this extrinsic theory does not explain some of the clinical findings occasionally seen in these patients and, therefore, intrinsic (germline developmental abnormality) and vascular theories have been proposed to better explain these. Amniotic bands also occur occasionally following intrauterine interventions such as chorionic villus sampling and laser coagulation for twin–twin transfusion syndrome. When these bands of tissue form constriction rings around different parts of the limbs, they can impede venous drainage (resulting in edema), remain as deep clefts in the soft tissue, or completely amputate parts of the limb distal to the band. It is uncommon for only one limb to be affected. In most cases, early intrauterine disturbance of the limb bud results in failure of the limbs to develop further. Any part of the body can be constricted by amniotic bands, including the head, neck, and trunk. Investigators have estimated that this syndrome usually occurs at approximately 6 weeks of fetal development.
Upper limb deficiencies are more likely to have associated abnormalities (particularly in patients with genetic disorders), and humeral defects are the most predictive of concomitant anomalies.
Poland syndrome consists of unilateral absence of the pectoralis minor and the sternal portion of the pectoralis major muscles, along with some type of coexisting ipsilateral hand abnormalities, including hypoplasia of the hand and digits with syndactyly, brachydactyly, and reduction deformities.
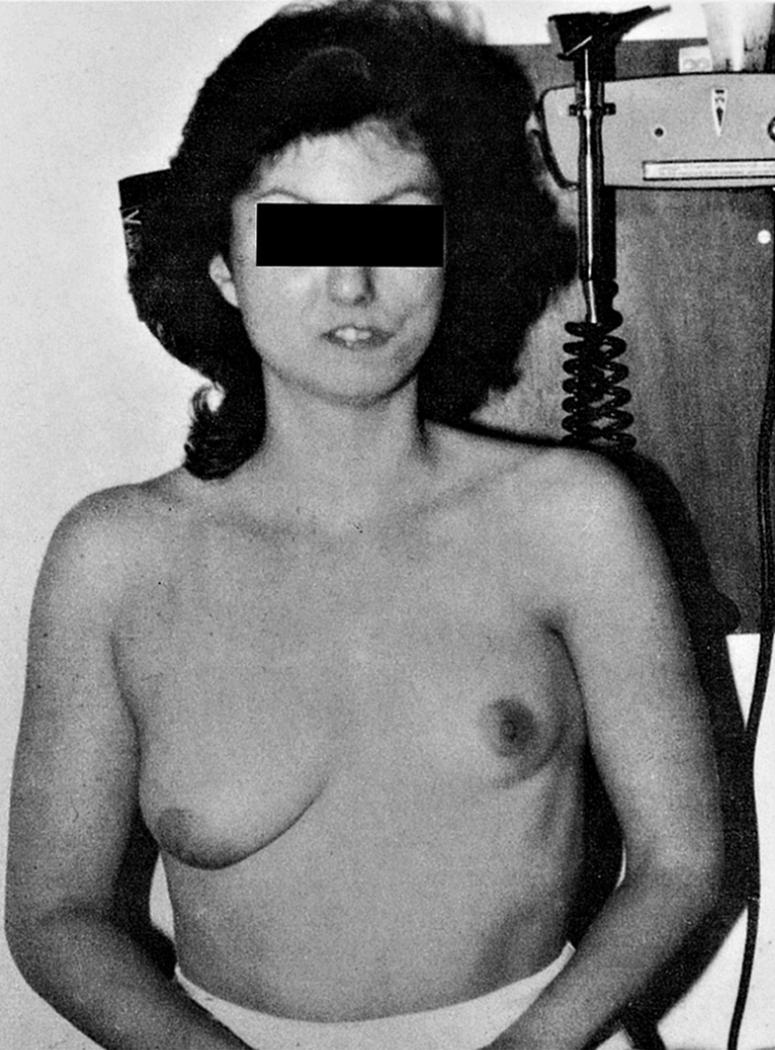
Patients with thrombocytopenia-absent radius syndrome usually have bilateral absence or severe hypoplasia of the radius, along with a radially deviated hand, deformed or absent thumb, and hypoplasia of the ulna ( Fig. 21.8 ). Associated anomalies include short stature, strabismus, micrognathia, dislocated hip, clubfoot, congenital heart disease, foreshortened humeri and hypoplastic shoulder girdles, and, occasionally, lower limb deformities. This syndrome requires prompt diagnosis and treatment in the neonatal period because hematologic problems may cause central nervous system damage secondary to intraventricular bleeding. Platelet transfusion is often necessary.
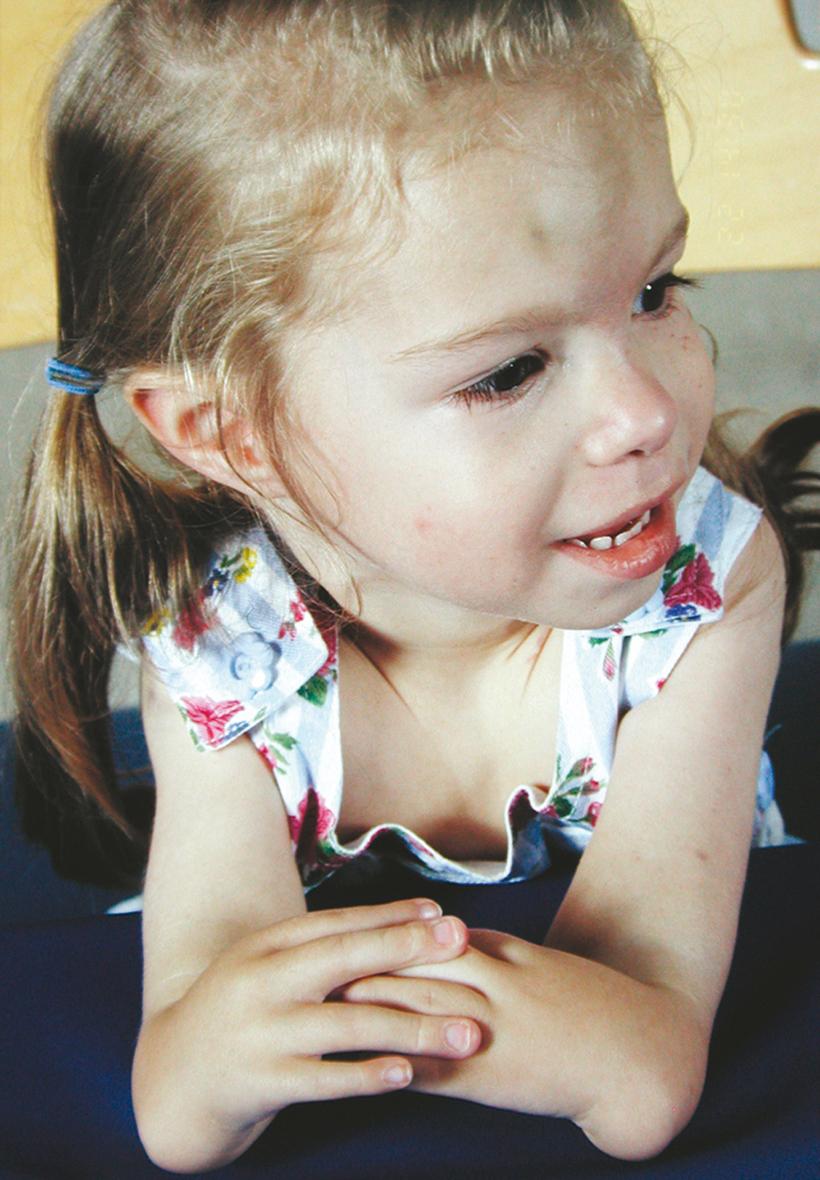
In Fanconi pancytopenia syndrome, dysmorphic and limb reduction defects vary considerably; skeletal abnormalities include absent or hypoplastic thumbs, hypoplastic radius, and developmental dysplasia of the hip. The patient is born with the limb defects, is relatively small at birth, and usually has patchy brown discoloration of the skin. Some children may have only hematologic disorders (e.g., bleeding, pallor, recurrent infections), which usually manifest between 5 and 10 years of age and can be treated with testosterone and hydrocortisone analogue therapy. Associated anomalies include cardiac, urogenital, and eye abnormalities, and a predisposition to leukemia. These patients have increased chromosomal breakage, which can be tested for by a diepoxybutane (DEB) assay, which some authors recommend obtaining in every patient with radial longitudinal deficiency.
Holt-Oram (hand-heart) syndrome results in upper limb deformities that may consist of partial or complete absence of the thumb or radial aplasia, a radially clubbed hand with or without elbow function, or severe hypoplasia of the forearm and defects of the humerus, clavicle, scapula, or sternum. Associated anomalies include cardiac defects (e.g., atrial septal defect, ventricular septal defect, tetralogy of Fallot) and vertebral defects (e.g., scoliosis, pectus excavatum). The syndrome is usually bilateral but asymmetric. It has an autosomal dominant inheritance pattern and has been mapped to a mutation in the TBX5 gene.
Anomalies associated with the VATER syndrome include vertebral defects, imperforate anus, tracheoesophageal fistula with esophageal atresia, and radial and renal dysplasia. The more current nomenclature, VACTERL, includes cardiac anomalies and separates renal and limb abnormalities. Patients may also have deficient prenatal growth, a single umbilical artery, and defects of the external genitalia. The cause of this condition is not known, and malformation patterns tend to be sporadic, but a greater frequency has been noted in children of diabetic mothers. However, recent publications have reported the possibility of genetic mutations in these patients.
Amelia has been associated with omphalocele and diaphragmatic hernia, and radial ray deficiencies have been associated with cardiac anomalies and imperforate anus. , ,
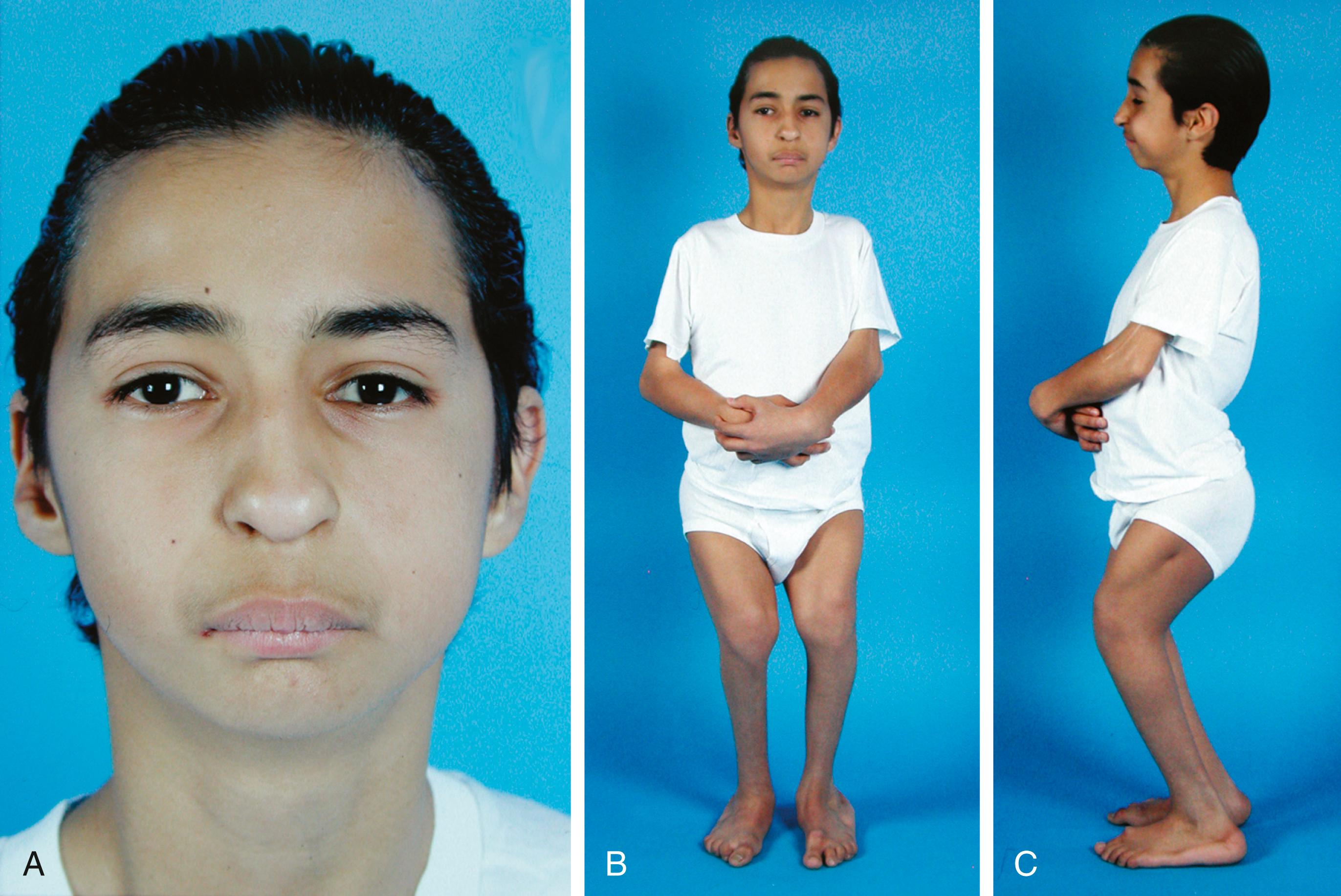
Tibial hemimelia is one of the few lower limb deficiencies in which a heritable pattern is often seen. , In femoral hypoplasia–unusual facies syndrome ( Fig. 21.9 ), an autosomal dominant trait, the limb deficiencies can range from a hypoplastic femur to an absent femur and fibula. The humerus may also be affected, therefore producing restricted elbow motion, and deformities may be seen in the lower spine and pelvis. Defects can be unilateral or bilateral. The distinct facial characteristics include a short nose with hypoplastic alae nasi, long philtrum and thin upper lip, micrognathia, cleft palate, and upward slanting of the palpebral fissures. The ulnar-femoral syndrome (a combination of femoral deficiency and ulnar abnormalities) may also be inherited. Some of the more common heritable limb deficiencies are listed in Box 21.4 .
Longitudinal deficiencies: preaxial, radial, and tibial
Fanconi pancytopenia syndrome (autosomal recessive): upper limb deficiencies of thumb and radius, with occasional developmental dysplasia of the hip; associated anomalies include cardiac, urogenital, and eye abnormalities and a predisposition to leukemia
Thrombocytopenia–absent radius syndrome (autosomal recessive): radial aplasia or hypoplasia with radially clubbed hand, deformed or absent thumb, and hypoplasia of the ulna; associated anomalies include short stature, congenital heart disease, foreshortened humeri and hypoplastic shoulder girdles, strabismus, micrognathia, dislocated hip, clubfoot, and occasional lower limb deficiency
Longitudinal deficiencies: postaxial, ulnar, and fibular
Isolated ectrodactyly (autosomal dominant): deficiency of central rays of both hands and feet; may be difficult to differentiate from autosomal recessive ectrodactyly because of incomplete penetrance
Longitudinal deficiencies: intercalary, sometimes phocomelic; middle segment
Holt-Oram syndrome (autosomal dominant): upper limb deficiencies ranging from partial or complete absence of the thumb, radial aplasia, and radially clubbed hand with or without elbow function to severe hypoplasia of the entire forearm and defects of the humerus, clavicle, scapula, or sternum; associated anomalies include cardiac and vertebral defects
Tibial aplasias
Tibial absence with polydactyly (autosomal dominant): tibial deficiency with duplications of radial ray; associated anomalies include cardiac defects
Whenever a child has a limb deficiency, the physician should carefully assess the craniofacial, cardiac, gastrointestinal, genitourinary, integumentary, and nervous systems. Peripheral blood cell counts, urinalysis, skeletal radiographs, hearing, visual acuity, and growth should be monitored regularly during infancy and early childhood until it is obvious that the limb deficiency is an isolated event. If the child has a family history of an abnormality and the disorder resembles one known to have a genetic cause, the parents should be provided with appropriate genetic counseling.
A common vascular cause has been proposed for patients with Poland syndrome, Klippel-Feil syndrome, Möbius syndrome, terminal transverse limb defects, and Sprengel anomaly. According to this hypothesis, these conditions are caused by interruption of the early embryonic blood supply in the subclavian or vertebral arteries or their branches during the fifth through eighth weeks of fetal development. Vascular disruption in the subclavian artery may be caused by internal obstruction of the vessel from edema, thrombi, or emboli, or by obstruction secondary to external pressure on the vessel from tissue edema, local hemorrhage, cervical rib, aberrant muscle, amniotic band, tumor, or embryonic intrauterine compression. Exogenous factors (e.g., drugs, chemicals, generalized hypoxia, and hyperthermia) may cause premature regression of vessels or a delay in vessel development.
The specific anomaly depends on the blockage site, the extent and timing of the blockage, and the duration of the interruption. Various combinations of obstructions of the subclavian and branch arteries may account for the overlapping clinical features seen in patients with Poland syndrome, Möbius syndrome, Klippel-Feil syndrome, terminal transverse limb defects, and Sprengel anomalies ( Fig. 21.10 ). , , Disruptions of specific arteries and their likely subsequent effects are listed in Table 21.4 .
| Obstructed Artery | Subsequent Anomaly |
|---|---|
| Internal thoracic artery | Ipsilateral absence of the costosternal heads of the pectoralis and hypoplasia or aplasia of the breast |
| Subclavian artery, distal to the origin of the internal thoracic artery | Isolated terminal transverse limb defects |
| Subclavian artery, proximal to the internal thoracic artery but distal to the vertebral artery | Poland syndrome |
| At the origin or any segment along the developing vertebral artery or radicular branches | Klippel-Feil syndrome |
| One or more early arteries of the brain, or the basilar or vertebral arteries | Möbius syndrome |
| Subclavian, internal thoracic, or supracapsular artery | Sprengel anomaly: hypoplasia of scapula and lack of development of the upper portion of the serratus anterior |
The degree of upper limb deficiency seen in patients with limb defects associated with Klippel-Feil, Poland, or Möbius syndromes varies from very mild, such as a slightly smaller hand with no disability, to severe, with a small arm and no functional hand. The severity of the defect depends on the timing of the vascular interruption—the earlier the occurrence, the more severe the abnormality.
Poland syndrome may affect either side but usually is not bilateral, which may lead to death in utero. When the left side is affected, the child has approximately a 10% likelihood of concomitant dextrocardia. This finding supports the premise that abnormal vascular development is the cause of Poland syndrome.
When a child is born with a significant abnormality, such as the absence of one or more limbs, the family experiences a sense of profound loss that normally requires a time of grieving. During this period the orthopaedist must deal sympathetically with family members and encourage them to work through their feelings of disappointment. , , , In many cases, these early “wounds” are best healed by the child who has the abnormality. Children with limb deficiencies are just as responsive and interactive as other children, and most have normal intellects. The degree of limb deficiency has not been found to be associated with depressive symptoms, anxiety, or behavioral problems in children or adolescents, , nor does the degree of limb loss affect the general self-esteem of children, , although it can adversely affect adolescents’ self-esteem. ,
The child’s achievement of early developmental milestones helps the family direct attention to the positive aspects of the child’s future. Children with congenital abnormalities do not have a feeling of loss and thus do not require an adjustment period. They are able to make extraordinary adaptations to achieve milestones and perform normal daily activities. Their development of gross motor skills may not follow the “normal” pattern, but novel adaptations based on the limb deficit should not be misconstrued as evidence of developmental delay. Even children with multiple congenital limb deficiencies may achieve nearly normal physical function by using whatever limb components they have in place of those that are missing ( Fig. 21.11 ).

It is important for the clinician to explain to the parents early on what can and cannot be done to improve their child’s situation. Often, the parents’ primary concerns are not the same as those of the medical team. Usually, the parents are most anxious about the child’s appearance and how others perceive the abnormality, whereas the medical team tends to focus on the functional capabilities of the patient.
The possibility of future medical breakthroughs that could benefit the child should be discussed with the parents. The physician needs to help parents distinguish between medical advances that hold promise and impossible hopes. Undoubtedly, nonoperative and operative treatment methods will improve dramatically over the child’s lifetime, but the fundamental biologic principles will likely remain the same. Will whole limb transplants become available during the child’s lifetime? Will prosthetic implants that can grow be developed for the child with a femoral deficiency? Although we cannot answer these questions with certainty, we can make a guess that some of these advances are not in the near future. In all cases. the long-term interests of the child must be the primary concern. Consideration of treatment choices must realistically assess the costs in time out of school and activities and the discomfort of each intervention relative to future function and cosmesis.
PFFD is one of several terms used to describe a deformity in which the femur is shorter than normal and an apparent discontinuity exists between the femoral neck and shaft. In many cases, the defect in the proximal femur ossifies as the child grows older. A congenitally short femur without radiographic evidence of an ossification defect may be a less severe form of femoral deficiency. In most cases, the cause of the femoral deficiency is unknown. The disorder does not have a genetic link, except for the form with femoral deficiency and abnormal facies (femoral hypoplasia–unusual facies syndrome), which is an autosomal dominant malformation.
The Aitken classification system has some clinical relevance and is the most widely used system for classifying femoral deficiencies. PFFDs are categorized as type A, B, C, or D ( Figs. 21.12–21.14 ).
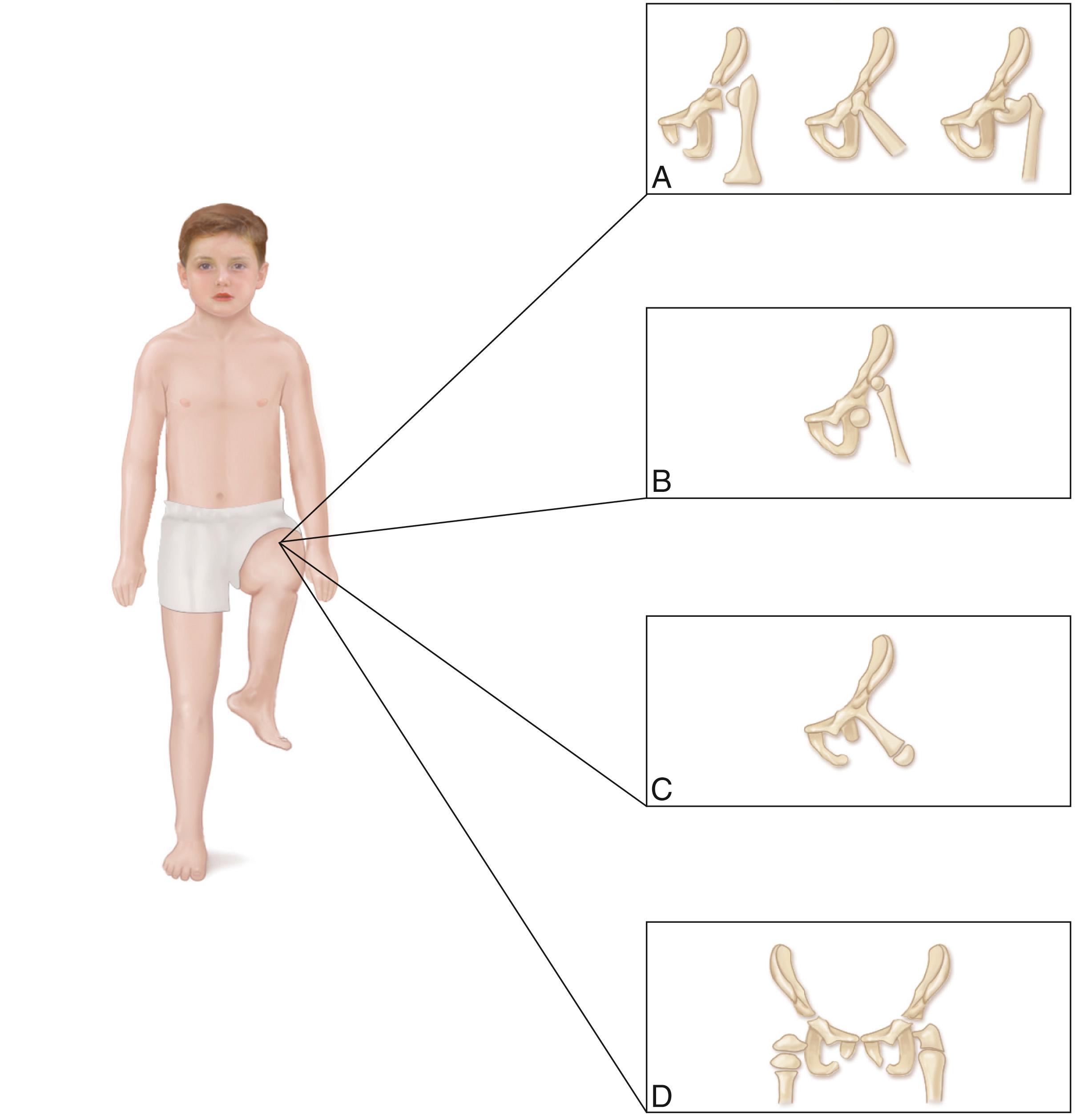
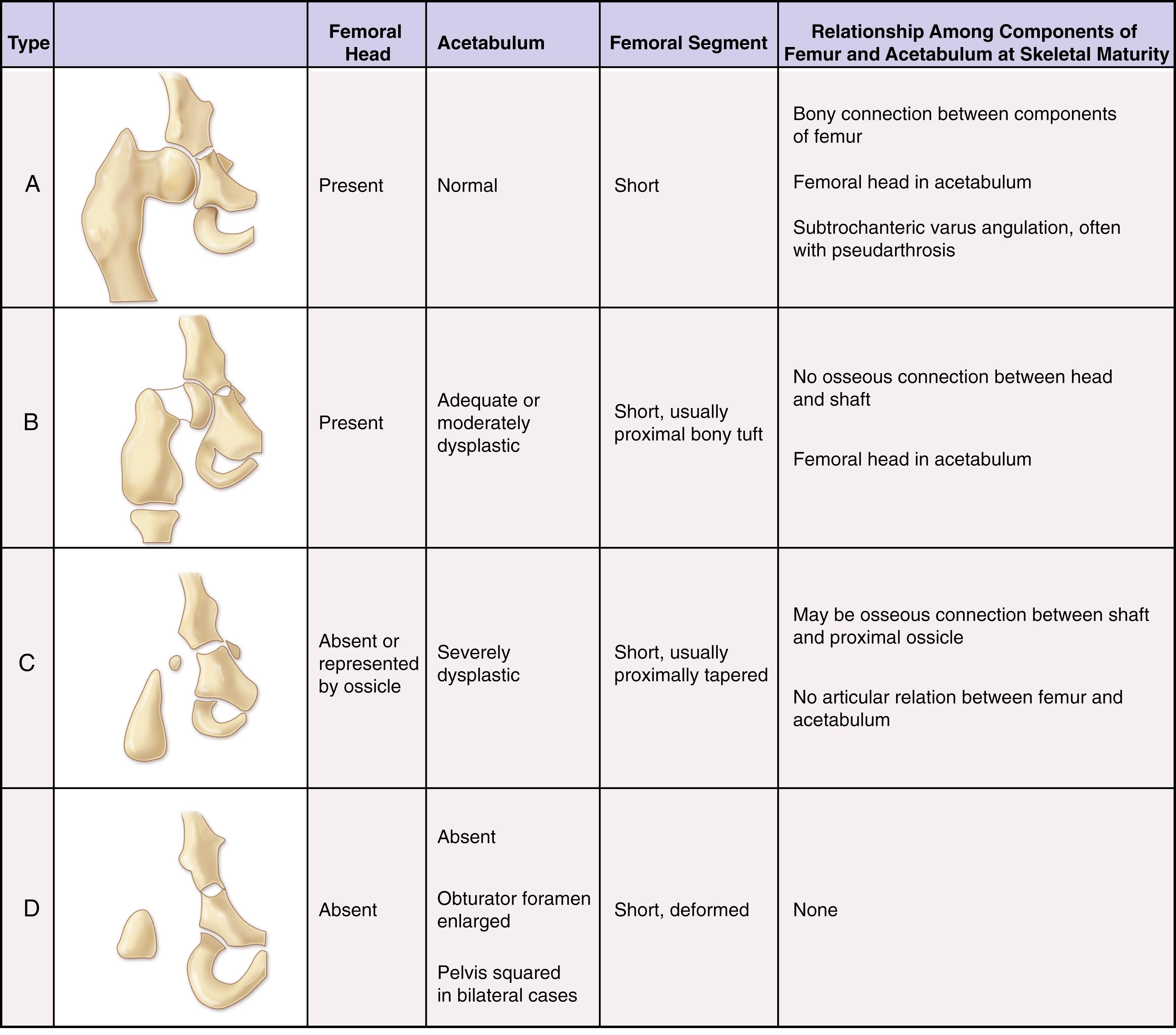
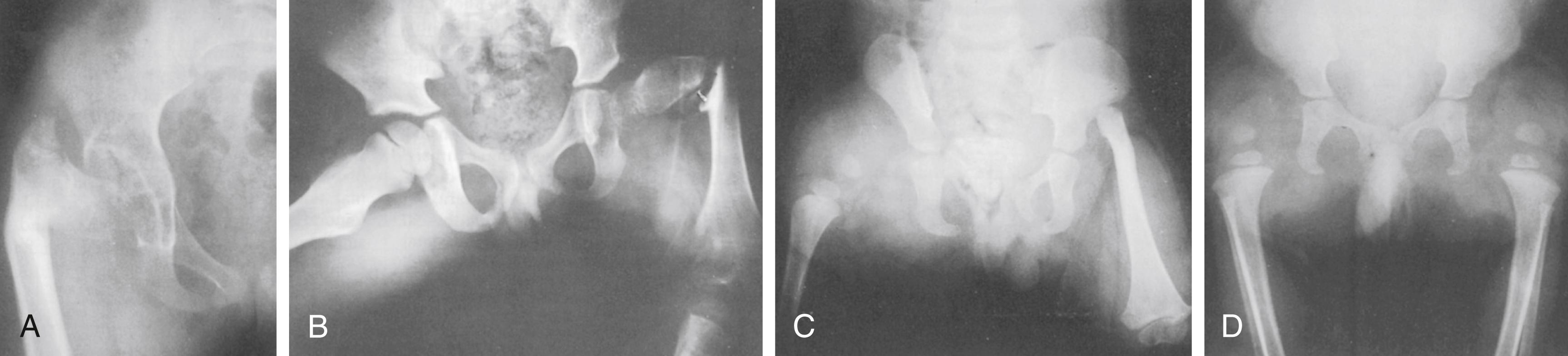
In type A PFFD, radiographs of the young child reveal a defect in the upper femur that ossifies as the child matures. The femoral head is present, and the acetabulum is well formed. Pseudarthrosis in the subtrochanteric area normally resolves by the time the patient reaches skeletal maturity. A varus deformity of the upper segment of the femur, which can vary in severity, is usually present, and the shaft of the femur may be positioned above the femoral head.
In type B deficiencies, the femoral portion of the limb is shorter than in type A, a tuft is often present at the proximal end of the femur, and the acetabulum is well formed. At birth, the upper portion of the femur may not be ossified, but as the child matures, the femoral head develops. The proximal end of the femur is usually positioned above the acetabulum. At maturity, no ossific continuity exists between the femoral shaft and head (a definitive feature of Aitken type B deformity).
In type C defects, the femoral segment is short and a tuft is present at the proximal end. There is no ossification of the upper portion of the femur and the femoral head is missing. The acetabulum is poorly developed or absent. In patients with no acetabulum, the flat, lateral segment of the pelvic wall is seen in its place.
In type D deficiencies, the shaft of the femur is extremely short or absent, no femoral head is present, and the acetabulum is either poorly developed or absent.
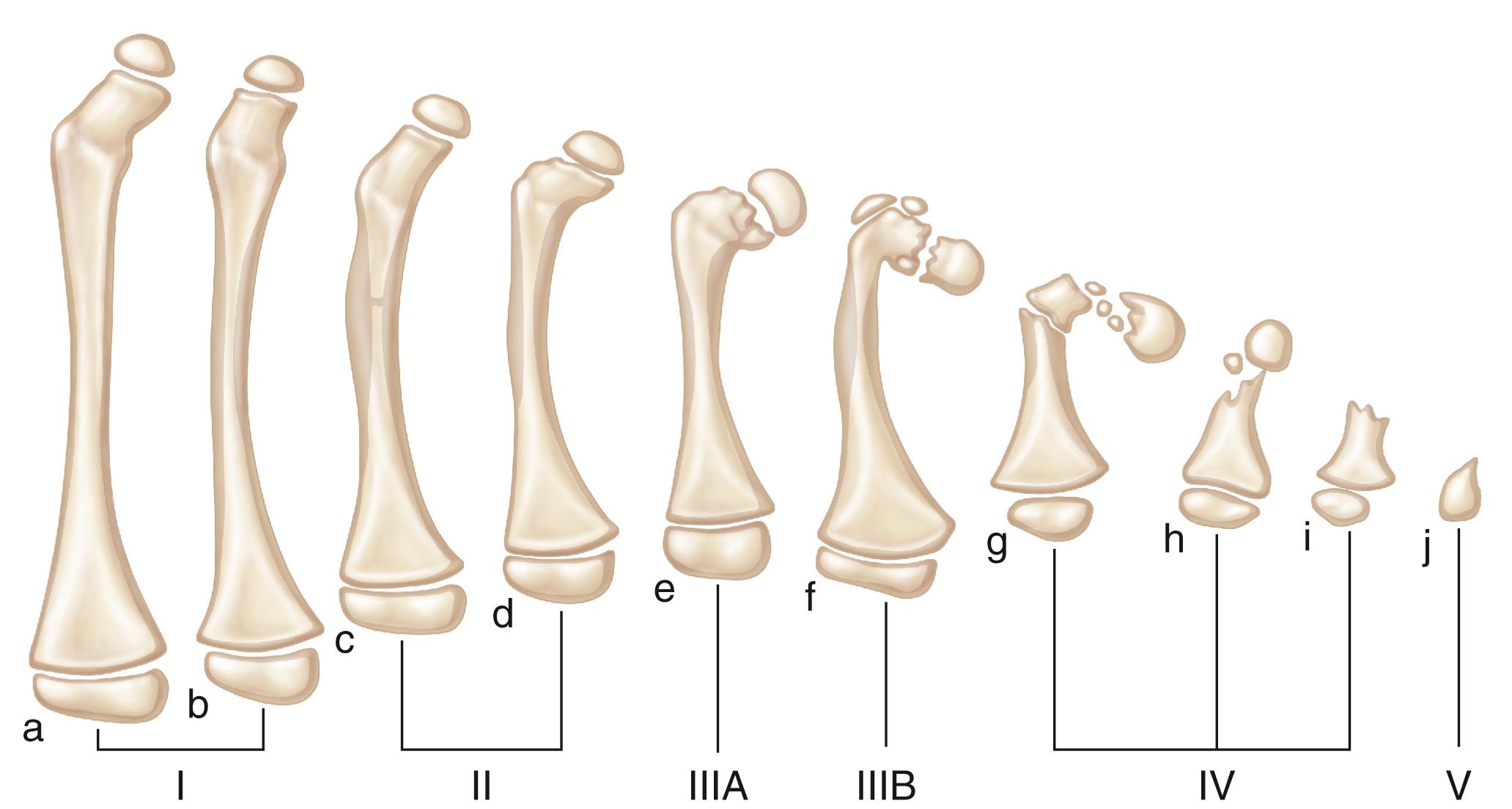
The Hamanishi classification of PFFDs is more comprehensive than the Aitken system. It comprises 6 primary groups and 10 subgroups of femoral malformation, with a category for almost every deformity. The mildest form is a shortened femur with no radiographic defect (grade Ia); the most severe is complete absence of the femur (grade V) ( Fig. 21.15 ). This system supports the premise that limb deficiency is a continuous spectrum representing varying degrees of response to an insult, rather than a group of distinct clinical entities. The severity of the malformation depends on the degree to which growth and development are inhibited. In this classification, isolated congenital coxa vara is considered a separate condition not associated with PFFD.
In the clinically based, treatment-oriented Gillespie classification, patients are placed in one of three groups. In group A, the femur is up to 50% shorter than the normal femur, and the hips and knees can be made functional. These cases have also been called congenital short femur. On examination, the foot on the affected side reaches at least to the midtibia on the normal side with the legs extended. These patients are considered suitable candidates for limb-lengthening procedures.
Group B comprises those patients with more severely shortened femora in which the foot on the affected side reaches above the midtibia on the normal side, often at the level of the normal knee. With the hips flexed the femur is noted to be at or less than half the length of the contralateral femur. These patients are usually treated with amputation or rotationplasty and prosthetic management ( Fig. 21.16 ).
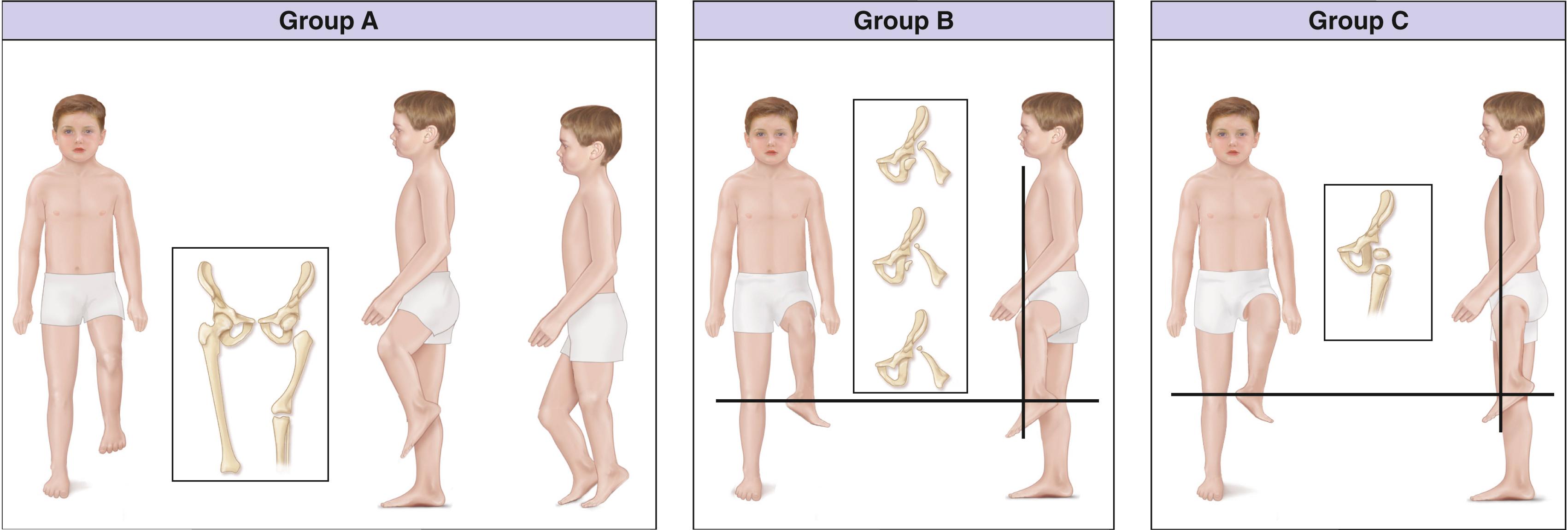
Patients in group C have a subtotal absence of the femur. Arthrodesis of the knee is not indicated in group C cases, and these patients will require prosthetic management.
The anatomy within these groups varies widely, and the surgeon should carefully analyze each child as a unique individual. This classification by Gillespie is, nonetheless, very useful to place the patient in a likely treatment category.
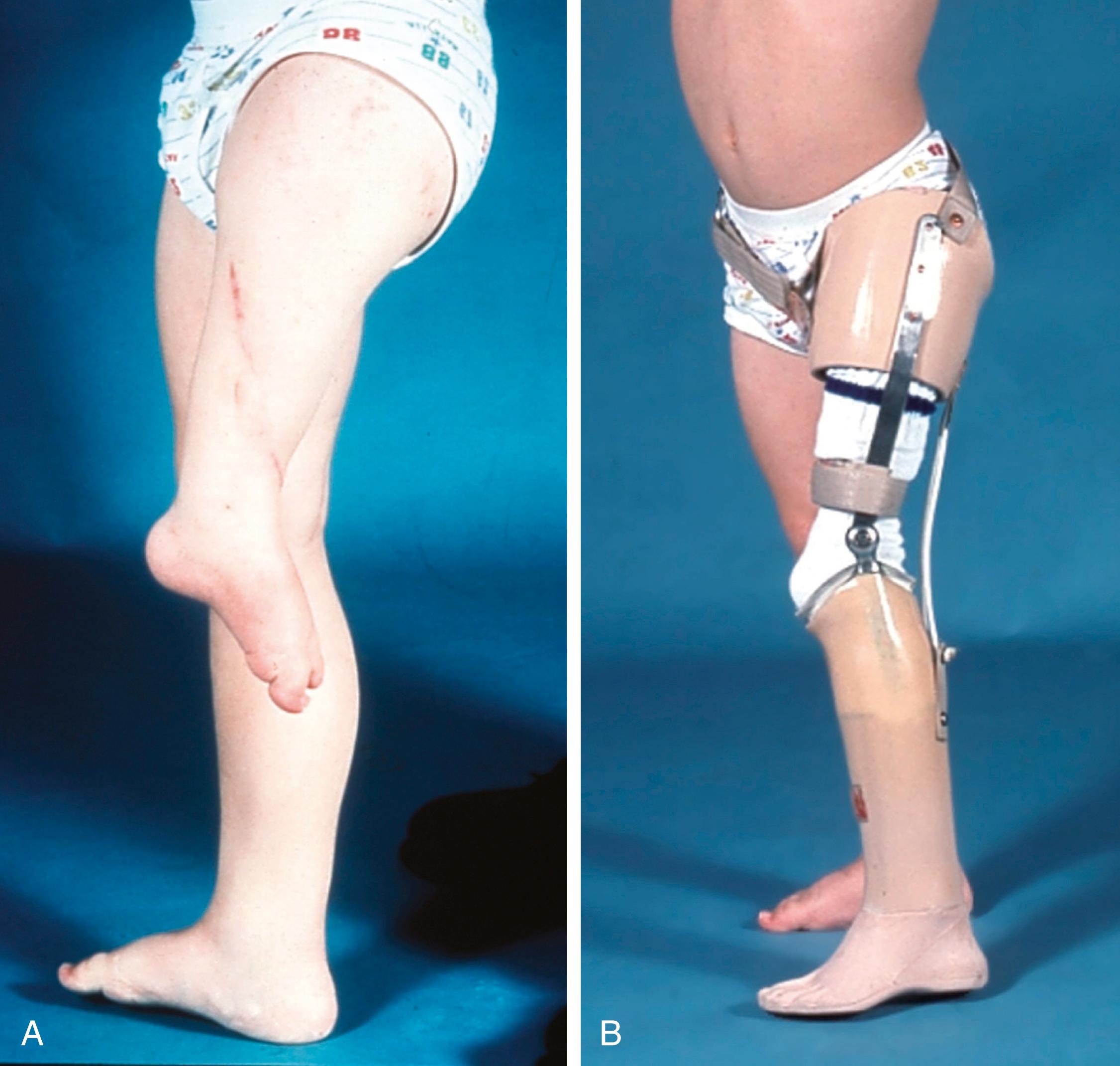
Patients with PFFD have a characteristic appearance. The affected thigh is extremely short, the hip is flexed and abducted, the limb is externally rotated, the knee often has a flexion contracture, and the foot is usually at the level of the contralateral knee ( Fig. 21.17 ). Flexion contractures of the hip and knee make the limb appear shorter than it actually is anatomically. The actual discrepancy can be better determined by comparing the length of the two limbs while the patient is sitting. Although the hip abductors and extensors are present, they are foreshortened and unable to function properly because of the abnormal anatomy of the proximal femur. The knee joint is positioned in the groin and acts as an unstable intercalary segment. In approximately 45% of cases, the patient also has ipsilateral fibular hemimelia of the affected limb, with a short tibia and an equinovalgus deformity of the foot. Lateral rays of the foot may be missing. The disorder may be accurately diagnosed prenatally with sonography.
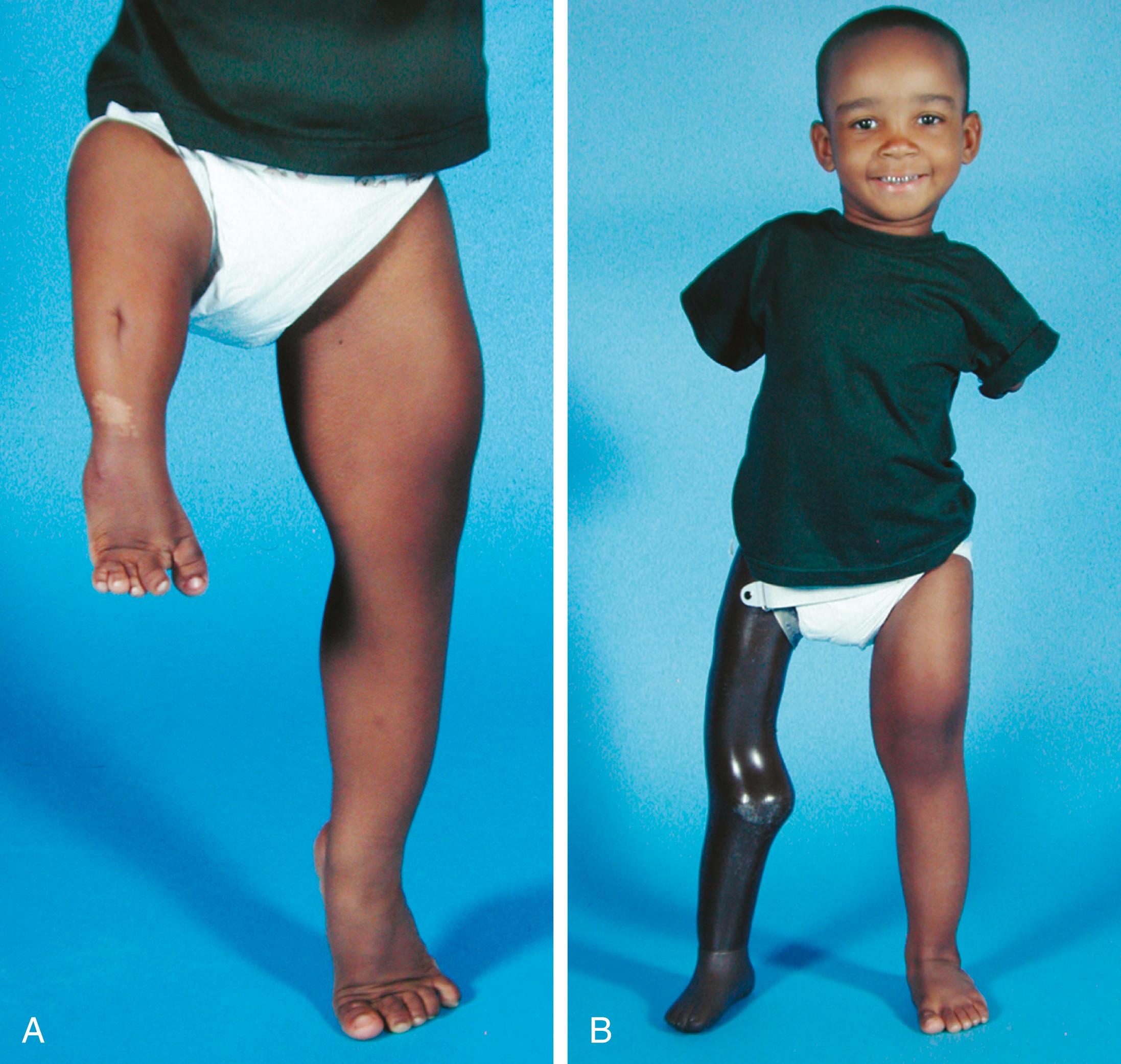
In patients with congenital shortening of the femur, a disorder related to PFFD, the clinical presentation is less severe. The affected thigh is shorter than the contralateral thigh, and the lower leg may also be shorter. There is an associated anterolateral femoral bow, along with valgus deformity and external rotation of the knee. Patients often lack the anterior cruciate ligament of the knee, and anteroposterior laxity of the joint results. Some patients have shortened hamstrings, with restricted straight-leg raising. Patients with congenital shortening of the femur also frequently have an associated ipsilateral fibular hemimelia.
Most young children with femoral deficiencies are able to compensate for their deformities without delay of developmental milestones. A child who has significant shortening of one lower limb walks by bearing weight on the knee of the normal limb and the foot of the affected leg to equalize the limb length discrepancy ( Fig. 21.18 ). A child with a congenitally short femur walks with the hip and knee of the normal limb flexed and with equinus on the shortened limb to accomplish the same goal. These children usually walk at the expected age.

To establish an appropriate treatment plan, one must first determine the current absolute limb length discrepancy by clinical examination and radiographs and calculate the percentage of overall limb shortening. Then, based on the assumption that the relative shortening of the limb will remain consistent throughout the child’s growth, the probable discrepancy at maturity is estimated by multiplying the average length of the adult femur by the percentage of the existing discrepancy. The final discrepancy can also be determined by using the multiplier method of Paley, the original Green and Anderson method, , , or a combination using the straight line graph by Moseley.
The first and most important decision is whether the ultimate goal is walking with the foot reaching the ground or walking with a prosthesis. The Gillespie classification is helpful. Different surgeons have varying opinions on the threshold for lengthening versus amputation. Ultimate discrepancy, overall deformity, and degree of anatomic deficits of the foot are the primary considerations. When the plan is to reconstruct for ambulation without a prosthesis, treatment focuses on achieving hip, knee, foot, and ankle stability and mobility, correction of deformities, and lengthening the short segments.
When the plan is for prosthetic management, stability of the hip and upper femur are first evaluated. When the hip is unstable, usually with acetabular deficiency and or proximal femoral varus or rarely valgus alignment, femoral and pelvic osteotomies may be indicated to produce a stable hip. Progressive upper femoral deformity through an osseous defect of the femoral neck is usually an indication for femoral osteotomy and osteosynthesis.
In the absence of a reconstructible hip, fusion of the femur to the pelvis may be indicated to reduce the abductor lurch of hip instability. This procedure is usually not done in very young children. Several techniques, such as the Steel and Brown procedures, may be used. Results with the Steel procedure have often been disappointing. Options for managing the remainder of the extremity include knee fusion, Syme amputation, or rotationplasty to replace knee function ( Box 21.5 ).
Predicted length of affected limb at maturity <50% of contralateral limb
Knee fusion and Syme amputation: indicated primarily when the hip is stable, the relationship between the greater trochanter and the femoral head is relatively normal, and the patient does not wish to have a rotated foot
Knee fusion and rotationplasty: indicated when the hip is stable with a good femoral head–to–greater trochanter relationship, and the patient appreciates the value of the rotated foot
Predicted length of affected limb at maturity >50% of contralateral limb
Limb lengthening: additional criteria for lengthening are a predicted discrepancy of <17–20 cm and a condition that can be corrected with three or fewer separate limb-length equalization procedures
Steel fusion and Syme amputation: indicated when the hip is unstable and the patient does not desire a rotationplasty
Steel fusion and rotationplasty: indicated when the hip is unstable and the patient desires the improved function of a rotationplasty
Brown fusion of femur to pelvis with rotationplasty: indicated as an alternative to the Steel procedure for an unstable hip and for improved knee control and proprioception
Knee fusion is performed to create a stable upper segment. As a child with a short femur bears weight, the knee is pushed upward and anteriorly and acts as an intercalated segment. After knee fusion, the lower extremity is aligned more directly beneath the acetabulum and the hip flexion contracture is reduced. The gait mechanics improve such that the tendency to lurch laterally and forward is reduced. When the femoral segment is absent or extremely short, the fusion is no longer necessary.
The Syme amputation has the advantage of being a single procedure that produces satisfactory cosmetic and functional results with prosthetic wear. Usually, multiple surgical procedures are not necessary, and this option is especially attractive when the foot is poorly formed. Alternatively, rotationplasty (surgical rotation of the foot 180 degrees) has significant functional advantages in that the patient has active motor control of the prosthetic knee. In addition, the patient will have proprioception of knee position within the prosthesis. A recent long-term study showed good levels of function and patient satisfaction. The disadvantages of rotationplasty are that the backward foot may be a significant cosmetic hurdle for the patient to overcome. Another problem is that the rotated segment tends to derotate as the patient grows, especially if the hip is unstable. Rotationplasty may be performed at the time of knee fusion or in conjunction with femoral-to-pelvis fusion.
In the Gillespie A group when final predicted discrepancy at maturity is less than 20 cm, the child may be a suitable candidate for limb-lengthening procedures. For femoral lengthening to be successful, the hip and upper femoral segment must be stabilized. The degree of shortening and the anatomy of the upper femur and hip, as well as the structure and function of the foot, are important considerations.
Treatment decisions are made by the parents and the medical team, and many factors must be considered. Modern prosthetics have made an equinus prosthesis a reasonable early choice and allow the child to walk at the usual developmental age. Our approach is to introduce parents to treatment options, and meeting other children and parents with similar conditions has proven to be very helpful. While the child is ambulating with an equinus prosthesis, the parents have time to reach an in-depth understanding of the available treatment options.
Bilateral PFFD is uncommon ( Fig. 21.19 ). The primary functional problems of patients with bilateral PFFD are short stature and a waddling gait. Surgical treatment such as amputation is contraindicated for this condition. Treatment is usually limited to the use of a pair of extension equinus prostheses to enhance the child’s height and enable him or her to participate in certain physical activities.
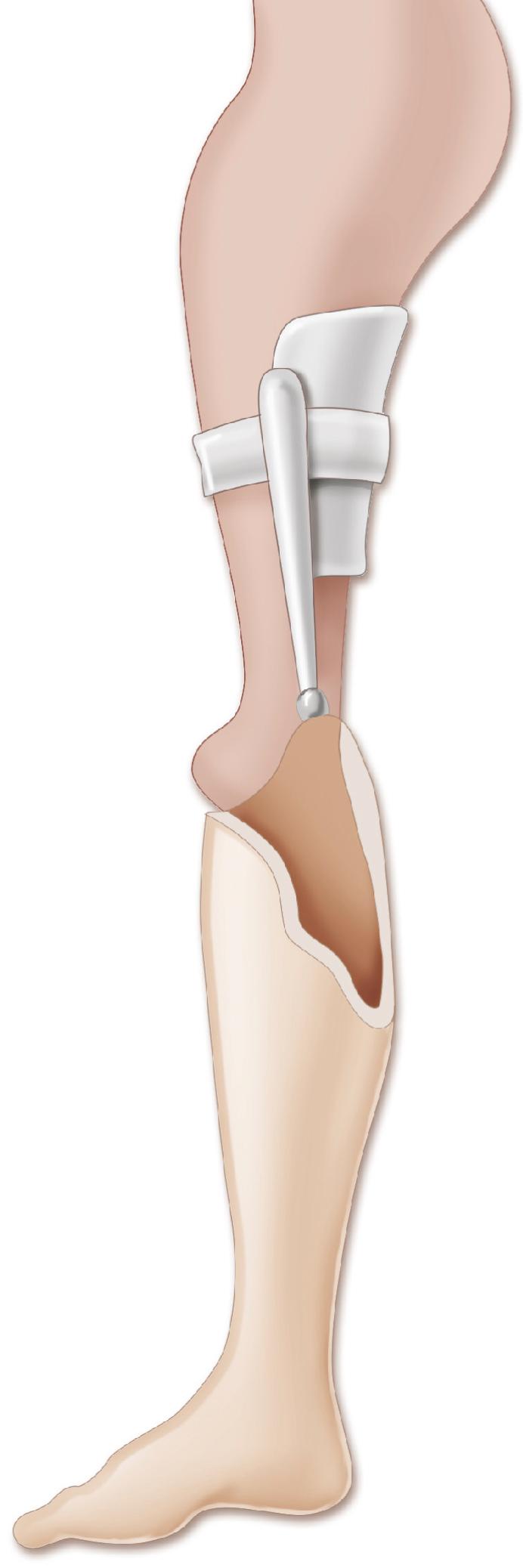
Amputation of the foot, combined with knee arthrodesis, is a well-documented means of treating significant femoral shortening ( Fig. 21.20 ). Before the knee is fused, the orthopaedist may consider fusing the femur to the pelvis to improve hip stability. Either a modified Syme amputation or a Boyd amputation can be performed; both techniques create a residual limb well suited to prosthetic management and weight bearing ( ![]() ). , , , However, because migration of the heel pad is rarely a problem following a Syme amputation, the Boyd technique is usually not necessary. After the procedure, the child is able to walk either by using a Syme-type prosthesis or by bearing weight on the end of the amputated limb and the contralateral knee (i.e., “knee-walking”), therefore equalizing the limb-length discrepancy without using a prosthesis.
). , , , However, because migration of the heel pad is rarely a problem following a Syme amputation, the Boyd technique is usually not necessary. After the procedure, the child is able to walk either by using a Syme-type prosthesis or by bearing weight on the end of the amputated limb and the contralateral knee (i.e., “knee-walking”), therefore equalizing the limb-length discrepancy without using a prosthesis.
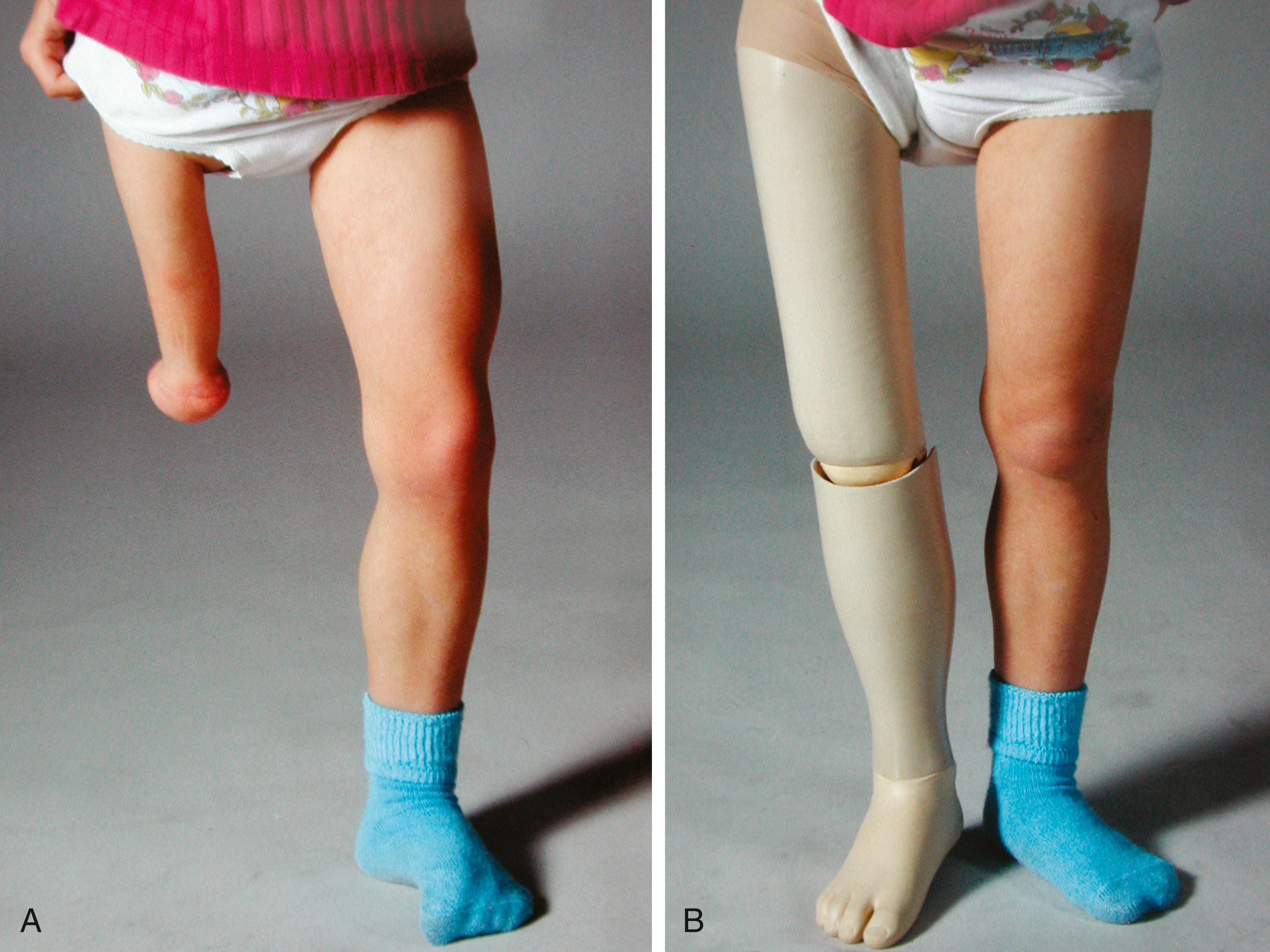
As the child grows, the knee (which is at the upper brim of the prosthesis) flexes with weight bearing and allows the prosthesis to displace proximally and anteriorly. This instability causes the child to lurch forward and to the side. This problem can be addressed by performing a knee arthrodesis so that the residual limb is straight and the prosthesis is positioned directly below the acetabulum. This surgical approach converts the affected limb to a functional above-knee amputation, usually with the additional advantage of distal loading tolerance. Opinion varies on when the procedures should be done. Some orthopaedists elect to perform the amputation just before the child starts walking and wait to perform the knee arthrodesis until the patient is 3 to 4 years of age. Other orthopaedists perform both procedures simultaneously when the child is 2 to 3 years of age and use an equinus prosthesis before surgical treatment. The patella may be excised because reports have noted patellofemoral pain when this structure is retained.
Ideally, after a Syme amputation and knee arthrodesis, the proximal end of the residual limb lies at least 5 cm (2 inches) above the contralateral knee at skeletal maturity to allow for proper placement of the prosthetic knee. Often, however, the end of the residual limb is at or below the level of the contralateral knee, and the tibial part of the prosthesis must be shortened to accommodate the mechanism of the prosthetic knee, therefore resulting in a discrepancy in knee heights. This length discrepancy can be minimized by excising the epiphysis of the distal femur at the time of the knee arthrodesis, followed by fusion of the proximal tibial epiphysis to the distal femoral metaphysis (see Plate 21.1 on page 926). If this procedure is performed when the child is 3 to 4 years of age, an acceptable final length discrepancy can usually be achieved. The final discrepancy can be estimated from established growth charts; however, hip and knee flexion contractures before fusion reduce the accuracy of these predictions. FLOAT NOT FOUND
Rotationplasty was first described in 1930 by Borggreve, who used the procedure to treat a knee severely damaged by tuberculosis. In 1950, van Nes described his technique of treating congenital femoral deficiencies by rotating the foot of the affected limb 180 degrees so that the toes pointed posteriorly and the motion of the foot and ankle controlled the prosthetic knee ( Fig. 21.21 ).
The goal of the van Nes rotationplasty is to convert the affected limb to a functional “below-knee” amputation in which the rotated foot serves as a knee joint. For optimal prosthetic function, it is essential that the ankle joint of the affected limb be normal and be capable of at least a 60-degree arc of motion postoperatively. The ankle should be at the level of the knee of the contralateral limb, and the ankle joint should be rotated a full 180 degrees. After rotationplasty, the gastrocsoleus muscle provides primary motor control to the ankle, which, in essence, is now the “knee” extensor. Sensory feedback from the ankle also allows the patient better proprioceptive control of the prosthetic knee ( Fig. 21.22 ). , , ,
In a study comparing the gait mechanics of patients who had undergone van Nes procedures with those who had undergone Syme amputations, the patients treated with rotationplasty demonstrated better prosthetic limb function and fewer compensations with the contralateral normal limb. Data from the rotated group were closer to normative data for ground reaction forces, forward propulsion, and active knee control. Oxygen cost was lower, and walking speed was higher in this group as well. The difference in oxygen cost (0.12 mL/kg/min) was comparable to the differences reported between below-knee and above-knee amputees.
Despite good initial functional results with the original van Nes procedure, the foot often gradually derotates as the limb grows, therefore making repeated rotationplasties necessary. ,
In a technique described by Gillespie and Torode, the tibia is rotated on the femur at the same time that the knee arthrodesis is performed to achieve most of the rotation (approximately 120 degrees); then, a tibial osteotomy is performed to gain additional rotation. Krajbich further modified the Gillespie-Torode approach such that all the rotation is obtained through the knee, with simultaneous knee arthrodesis. , , To prevent late derotation, all the muscles crossing the knee joint are detached (i.e., the medial hamstrings, gracilis, and sartorius from their insertion on the tibia and the medial head of the gastrocnemius from its origin on the distal femur) and moved into a new position so that they all pull in a straight line across the rotated, arthrodesed knee. The popliteus is divided at the level of the knee joint. Osteotomy is performed approximately 0.5 to 1.0 cm proximal to the distal femoral growth plate, with the length of the removed segment based on the goal of making the limb equal to or slightly longer than the contralateral thigh. The distal femoral growth plate is removed in almost every case to prevent the operated limb from growing too long and to obtain adequate shortening so that rotation can be performed without excessive tension on the neurovascular bundle. Adductor tendon insertion division is recommended so that the popliteal vessels can move freely medially and anteriorly. Appropriate prosthetic management follows the surgical procedure. The major drawback to these rotationplasties is gradual derotation as the child grows. When rotational malalignment occurs, overall function deteriorates, and prosthetic management is particularly difficult. Ackman and co-workers have reported satisfactory outcome at more than 20-year follow-up.
Brown devised a rotational procedure that eliminates the tendency to derotation. In this variant of a limb salvage procedure the femur is rotated externally 180 degrees and fused to the pelvis. The muscles of the thigh are excised, isolating the neurovascular structures to allow free rotation of the limb. The femoral segment is fused to the pelvis in a reversed, extended position, with neutral rotation and abduction. The medial hamstrings are attached to the anterior or lateral muscles of the rotated limb. The gastrocnemius is sutured to the iliopsoas and acts as both a hip flexor and a knee extensor, with the reversed knee joint functioning as a hinged hip joint. As in the van Nes rotationplasty, the retained ankle and foot serve as a knee joint. The procedure is complex and has serious potential complications, including vascular compromise.
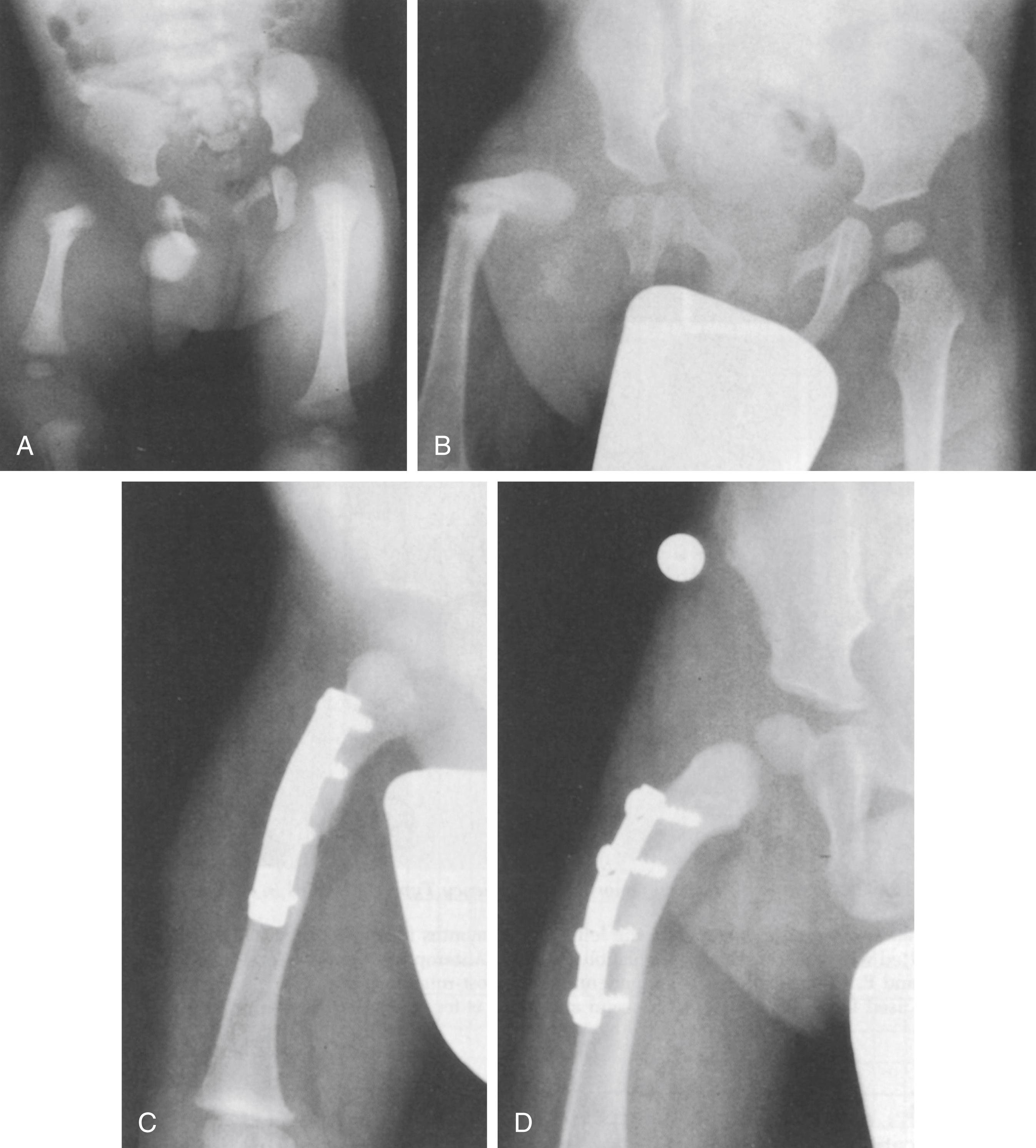
The need to stabilize the upper femoral defect is controversial. Paley and others recommended osteosynthesis of the defect with realignment of the soft tissues at an early age. , Other authors have recommended leaving the defect alone unless the patient has progressive deformity of the upper femur. Many upper femoral defects are stable and do not require surgery. Progressive deformity, usually into varus, is a definite indication for stabilization ( Fig. 21.23 ).
Children with major femoral deficiencies who are managed with either amputation or rotationplasty usually function well and, in most cases, are able to enjoy an active lifestyle. They walk without aids and, when running, tend to hop twice on the unaffected limb. The primary problem encountered with gait is hip instability. Because of the deformities of the proximal femur (i.e., a short, varus femoral neck combined with abnormalities of the acetabulum), the abductor muscles are unable to support the patient’s body weight during the stance phase of gait, and the result is an abductor lurch. In a young child, this condition is often barely discernible and is not a major inconvenience. As the child grows older, however, the abductor lurch becomes much more evident relative both to cosmesis and function.
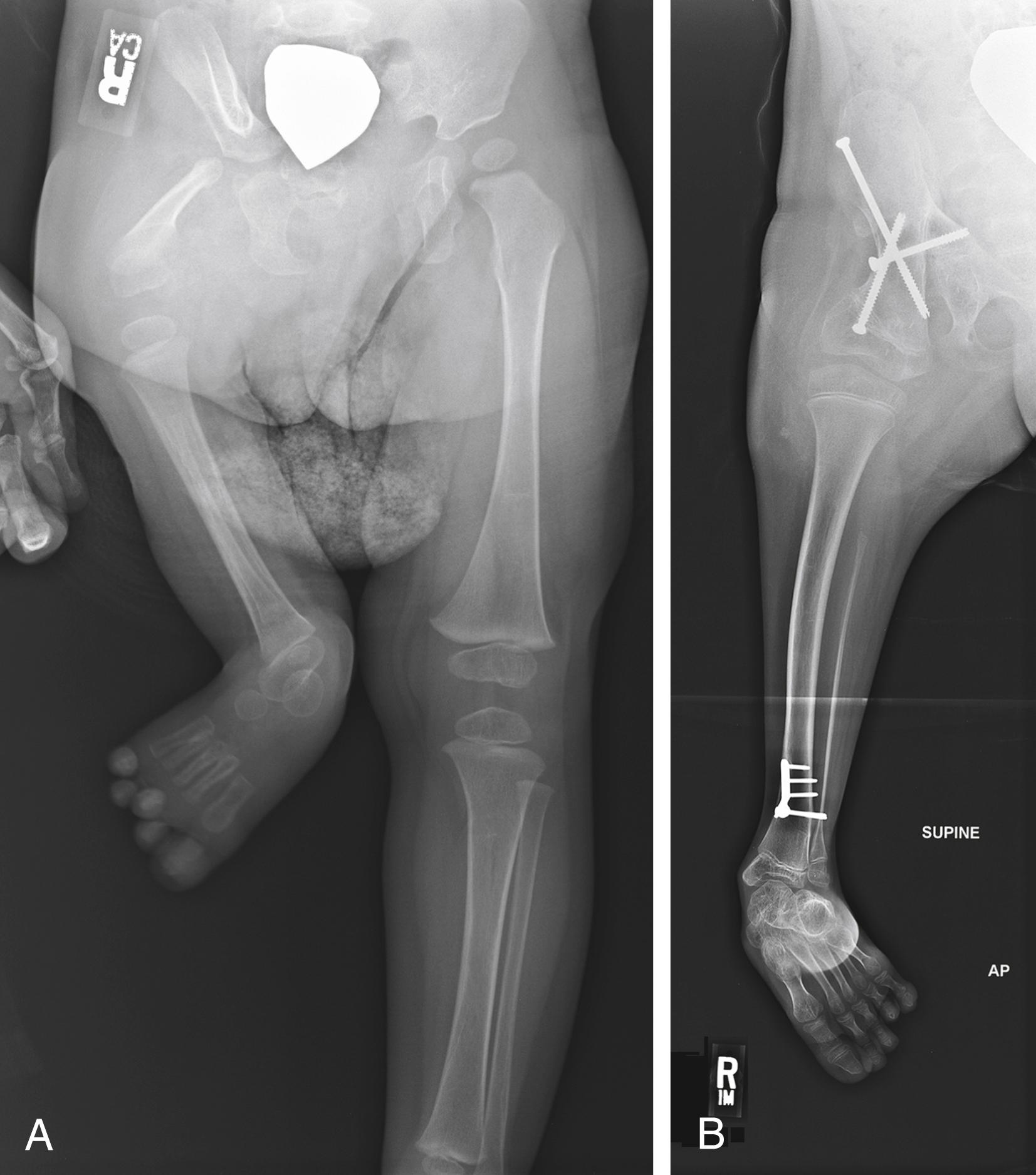
Numerous different surgical techniques have been developed to try to eliminate the lurch. In the Steel procedure the femoral segment is fused to the pelvis (iliofemoral fusion) in a flexed position, and the knee functions as a hip joint. , The distal femoral physis should be fused to prevent overgrowth. This operation may be done at the same time as a Syme amputation or a rotationplasty ( Fig. 21.24 ).
The Steel procedure was technically demanding and has largely been replaced by an operation designed by Brown. In this variant of a limb salvage procedure used with tumor resection, the femur is rotated externally 180 degrees and is brought as far proximal as possible for fusion to the pelvis. Excess soft tissue is excised before the femoral segment is fused in a reversed, extended position. The rotated leg is attached in neutral to slight abduction and neutral to slight external rotation. The medial hamstrings are attached to the anterior or lateral muscles of the rotated limb. The gastrocnemius acts as both a hip flexor and a knee extensor, with the reversed knee joint functioning as a hinged hip joint. As in the van Nes rotationplasty, the retained ankle and foot serve as a knee joint. In Brown’s series, at 7-month follow-up, passive range of hip motion was 90 degrees, and active hip flexion was 60 degrees. Longer follow-up is required to establish the outcome of this approach.
In some patients with PFFD, and in most patients with congenital shortening of the femur, the projected final discrepancy is less than 20 cm. The affected femur is at least 40% to 60% of the length of the contralateral femur, and patients are classified in group I of the Gillespie-Torode system or in group A of Gillespie’s modified classification system. The foot of the shortened limb is usually at the level of the mid-tibia of the opposite limb. In these cases, limb lengthening is often the ideal treatment.
The goals of limb-lengthening procedures are to correct the existing deformity and to eliminate the length discrepancy. Some patients need only a single lengthening of the femur combined with an epiphysiodesis of the opposite limb. , Other patients may need to undergo two or more femoral lengthening procedures. In some cases, it is also necessary to perform tibial lengthening. Because limb lengthening procedures are technically demanding and subject to multiple complications, they should not be undertaken lightly. , , , , Current indications for limb lengthening of the congenital short femur include a limb that is predicted to be at least 50% as long as the normal limb at maturity, a predicted discrepancy of less than 17 to 20 cm, and a condition that can be corrected in three or fewer separate limb-length-equalization procedures.
The anatomy of the child’s hip must be almost normal before limb lengthening can be performed. Preexisting deformities that compromise stability should be corrected well before lengthening is begun. Mild dysplasia of the acetabulum is often present. If the dysplasia is not corrected, the muscle forces created by femoral lengthening will cause the dysplastic hip to dislocate. To prevent this complication, a pelvic osteotomy may be performed, with the specific type of procedure depending on the nature of the acetabular deficiency. A Salter osteotomy is done if the deficiency is anterolateral, and a Dega osteotomy is performed if the patient has posterior acetabular insufficiency.
Deformity of the proximal femur, which is often in varus and retroverted, must also be treated before limb lengthening is performed. The femoral neck–shaft angle should be corrected to normal (approximately 135 degrees); however, the femur should not be positioned in valgus because this predisposes the hip to dislocation. , This osteotomy should be well healed and hardware should be removed before lengthening is begun. The iliotibial band is usually contracted, and it should be released before lengthening.
Price and Noonan recommended the use of a monolateral fixator for limb lengthening when the congenital short femur has a projected discrepancy of less than 17 cm and the patient has a stable hip and knee and an intact posterior cruciate ligament. If the projected discrepancy is greater than 17 cm, multiple staged lengthening procedures are planned, with the goal of achieving a 15% to 20% gain at each stage ( Fig. 21.25 ). Spanning the knee joint prophylactically may prevent articular cartilage damage during major lengthening. , However, Price and Noonan preferred to avoid spanning the knee by staging the lengthening with a maximum gain of 20% per stage. Although bilevel lengthening may reduce the total time for osteogenesis, the authors advised against this approach because muscles and nerves appear to respond better to slower rates of distraction. , , , ,
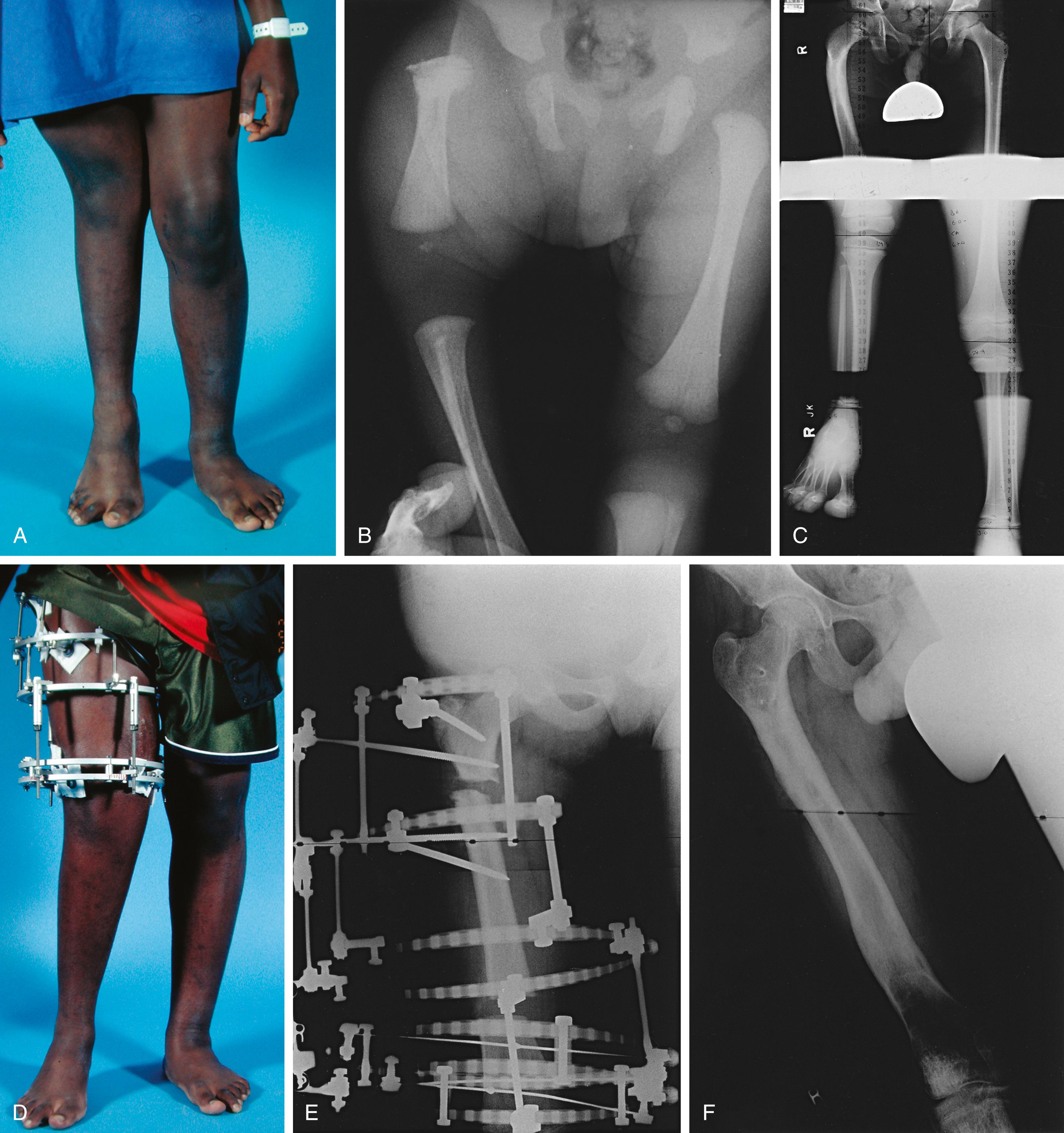
For the same reason, ipsilateral lengthening of the femur and tibia is discouraged in most cases. To prevent translation during lengthening, fixator alignment should be parallel to the mechanical axis. Osteotomies are usually made distal to the midshaft of the femur because of better bone healing at that level. The iliotibial band is released distally when the distal pin is inserted. If contractures start to develop, additional soft tissue releases can be performed. If the adductor magnus is tight, it can be released at the adductor hiatus. The chances of subsequent knee subluxation are minimized by distal pin placement, avoidance of knee flexion movements, and extension splinting for at least 12 hours each day. If knee subluxation occurs, it should be treated promptly by hamstring lengthening or traction and immobilization in extension. Hip subluxation or dislocation should be treated by appropriate soft tissue releases, femoral shortening, extension of fixation across the hip joint, open reduction, or a combination of these procedures. Hip or knee subluxation represents a serious complication and must be corrected before further lengthening is done.
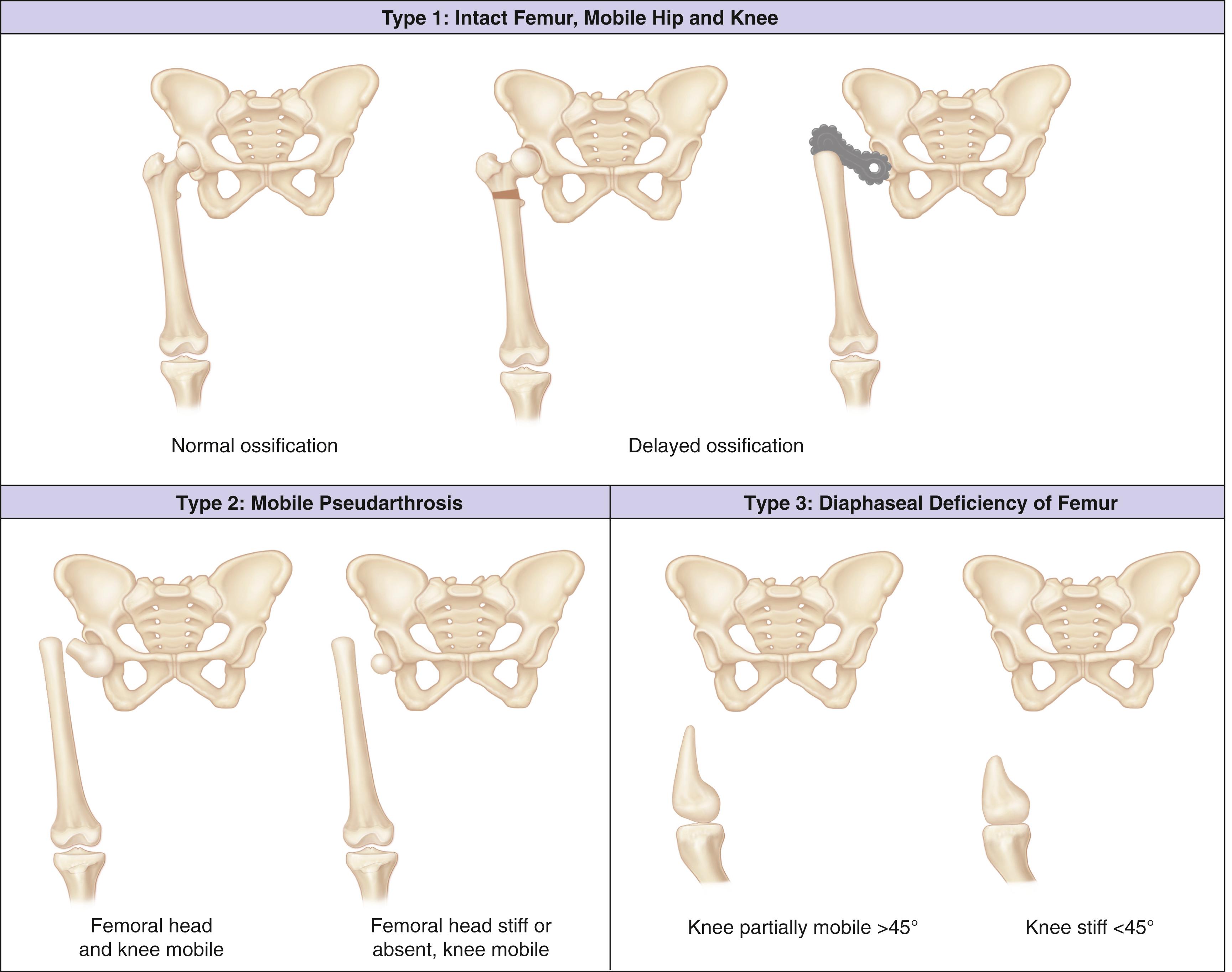
Paley proposed a classification of femoral deficiencies to aid in planning reconstructive lengthening procedures ( Fig. 21.26 ). His group 1, which corresponds to congenitally short femur in other classification systems, is the best group for lengthening. He recommended correction of femoral neck varus and acetabular dysplasia before lengthening. He also outlined reconstructive approaches for patellar instability and instability of the knee joint. Paley’s group 2a is defined by a mobile pseudarthrosis of the upper femur and a femoral head that is mobile in the acetabulum. The first step in reconstructing these hips is obtaining union of the pseudarthrosis, with subsequent lengthening. Group 2b is defined by a located, immobile, femoral head or a dislocated hip; these hips require a pelvic support osteotomy before lengthening. Patients with these more typical femoral deficiencies must undergo multiple staged lengthenings. Specific indications for reconstruction await further studies that consider both function and length, as well as the hardships involved.
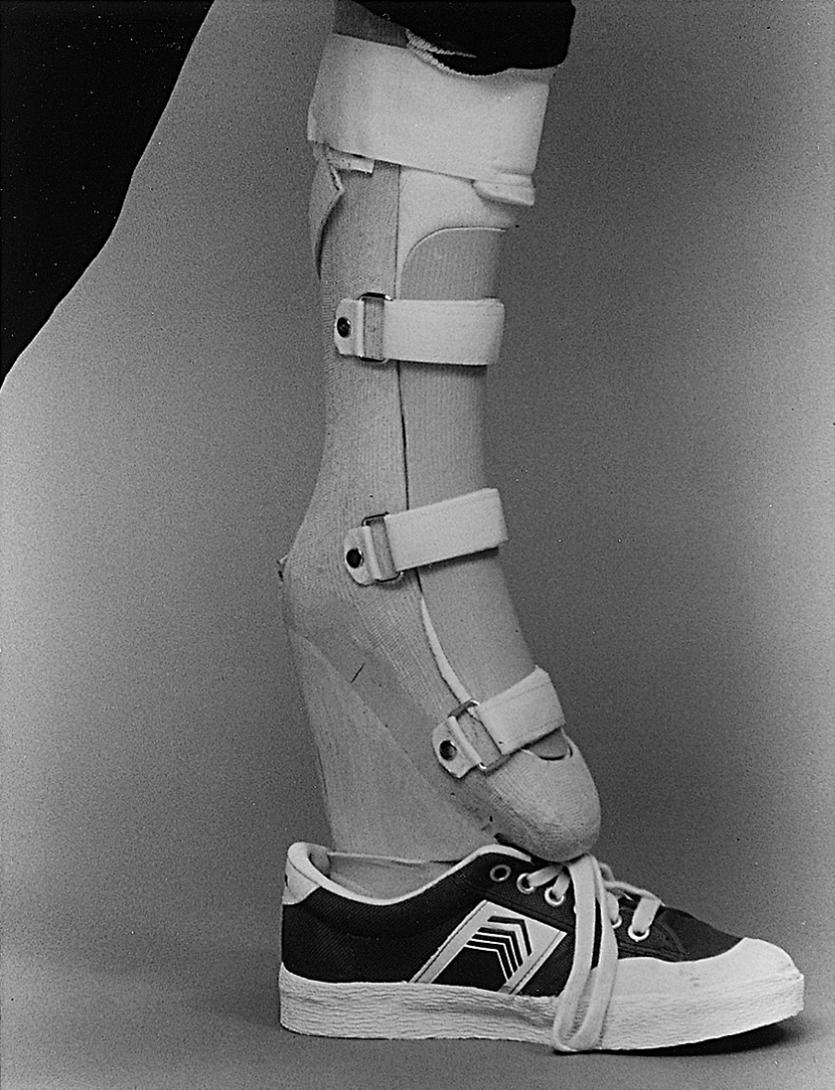
Normally, femoral lengthening is not performed when the child is young. This means that the orthopaedist must manage the patient so that he or she can participate in normal, everyday childhood activities. In very young children, the shorter limb can be treated with simple extension prostheses or large shoe lifts. Older children find these devices unattractive; for them, a more cosmetically pleasing equinus prosthesis can effectively equalize limb lengths and allow full function ( Fig. 21.27 ). For milder discrepancies, small-to-moderate shoe lifts can be used.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here