Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Atherosclerotic disease is the leading cause of mortality in developed countries. Better understanding of the pathophysiology of atherosclerosis has led to successful therapies and preventative strategies; however, treatment of conventional risk factors for atherosclerotic disease may only prevent 50% of cardiovascular events. A more complete understanding of atherosclerotic risk factors and the atherosclerotic process has the potential to yield improved therapies and preventative strategies.
Several less commonly considered areas of investigation have added insights into the development and progression of atherosclerosis. First, the atherosclerotic lesion itself appears to be more complex than previously described, and the roles of macrophages, vascular smooth muscle cells (VSMCs) and endothelial cells are not as distinct and static as once thought. Second, the effect of hyperlipidemia on atherosclerosis formation appears to involve more than just low- and high-density lipoprotein (HDL) levels. Finally, less commonly considered environmental exposures as well as the interaction between diet and the gut microbiome seem to be associated with atherosclerosis and conventional atherosclerotic risk factors such as diabetes mellitus (DM; Table 16.1 ).
| Residual Hyperlipidemia |
| Lipoprotein a |
| Diet |
| Trimethylamine oxide (TMAO) |
| Human Gut Microbiome |
| Infection |
|---|
|
| Environmental |
|
The prevailing model for the progression of the atherosclerotic lesion from fatty streak to mature plaque has been well described. , In short, injury to the vascular endothelium allows for adherence and transmigration of bone marrow-derived monocytes into the arterial wall. Monocytes within the arterial wall differentiate into macrophages that phagocytose oxidized LDL (ox-LDL), and become lipid-rich foam cells. VSMCs within the arterial media migrate toward the luminal surface of the plaque to form a fibrous cap. The degree of inflammation, lipid content, and fibrous cap thickness within the lesion affect the stability of the plaque.
Recent evidence suggests that the roles of endothelial cells, VSMCs, and macrophages may not be as static as suggested by this model. Under atherogenic conditions, endothelial cells can transition into fibroblast-like cells, while VSMCs can transition to an inflammatory phenotype resembling macrophages. , It is difficult to determine the degree to which foam cells are derived from macrophages versus VSMCs in vivo , as the two cells are difficult to distinguish based on traditional cell surface markers. VSMC-derived foam cells express cell surface markers typically seen on macrophages, such as CD68 and Mac-2 antigen, while downregulating typical VSMC markers, such as alpha-smooth muscle cell actin. These altered VSMCs can phagocytose ox-LDL to become lipid-rich foam cells, and contribute to the progression of atherosclerotic lesions through increased cellular apoptosis, and dysfunctional reverse cholesterol transport. Therefore it seems that VSMCs can undergo both compensatory and pathologic changes during the atherosclerotic process, by both forming the fibrous cap of the atherosclerotic lesion and contributing overall plaque stability while also contributing to the foam cell population of the atherosclerotic lesion. The varying effects of VSMCs in atherosclerotic lesions may be partially explained by the fact that VSMCs are not a homogenous population derived only from the arterial media. Rather, VSMCs can be derived from precursor cells in the bone marrow, adventitia, or arterial media, with VSMCs of different origins producing different effects within the atherosclerotic lesion. A better understanding of the role of various VSMC populations in the formation of atherosclerosis may potentially lead to new therapies that can enhance the protective effects of VSMCs while decreasing the harmful effects.
Hyperlipidemia is one of the most well-known risk factors for atherosclerotic disease and a primary target of pharmacologic intervention for preventing or slowing the atherosclerotic process. High LDL cholesterol and low HDL cholesterol have been the most-studied lipid components related to atherosclerotic disease. Total cholesterol and LDL reduction has been the cornerstone of medical treatment and prevention of ischemic heart disease with an abundance of randomized control trials that attest to efficacy of cholesterol lowering therapy in preventing ischemic heart disease. However, additional lipid variables have emerged as risk factors for atherosclerotic heart disease and potential routes of residual risk reduction.
Lipoprotein a, or Lp(a), consists of an LDL-like molecule linked to apolipoprotein (a), or Apo(a). Oxidized phospholipids of the LDL-like moiety are proatherogenic and proinflammatory, while the Apo(a) moiety is a plasminogen-like glycoprotein with prothrombotic effects. There are several different isoforms of Apo(a) and therefore of Lp(a) as well. Lp(a) levels correlate with both primary and secondary cardiovascular events independent of LDL and HDL levels. Statin medications and niacin can reduce Lp(a) levels modestly. Further reductions in Lp(a) can be achieved through apheresis, which can be used to remove both LDL and Lp(a) particles or Lp(a) particles alone. Lp(a)-specific apheresis may be able to prevent progression of angiographically measured coronary atherosclerosis and decrease major adverse cardiovascular (MACE) events when added to maximal medical therapy. The more recent development of subcutaneously-administered antisense oligonucleotides capable of reducing Lp(A) levels may provide a more practical and widely-applicable means of Lp(a) reduction compared to apheresis. Given that conventional therapy with statins may reduce only half of cardiac adverse events, there seems to be a potential role for the consideration of lipid parameters beyond HDL and LDL and additional lipid-based interventions.
A diet low in meats and animal products is a component of a low-fat, low-cholesterol diet. However, there may be a link between diet and atherosclerosis beyond dietary cholesterol intake. Trimethylamine-N-oxide (TMAO) is a proatherogenic molecule that is derived from carnitine, which is predominantly found in red meats, and phosphaditylcholine (PC), which is found in most animal products including dairy and eggs. Intestinal bacteria synthesize trimethylamine (TMA) from carnitine and PC. TMA is transported to the liver via the portal circulation, where it is converted to the proatherogenic molecule TMAO by a family of enzymes known as the flavin mono-oxygenases (FMO), particularly FMO3. The FMOs also oxidize a wide range of drugs and environmental toxins, such as persistent organic pollutants (POPs) in pesticides, and there may be a synergistic effect of diet and environmental toxins on atherosclerosis formation ( Fig. 16.1 ).
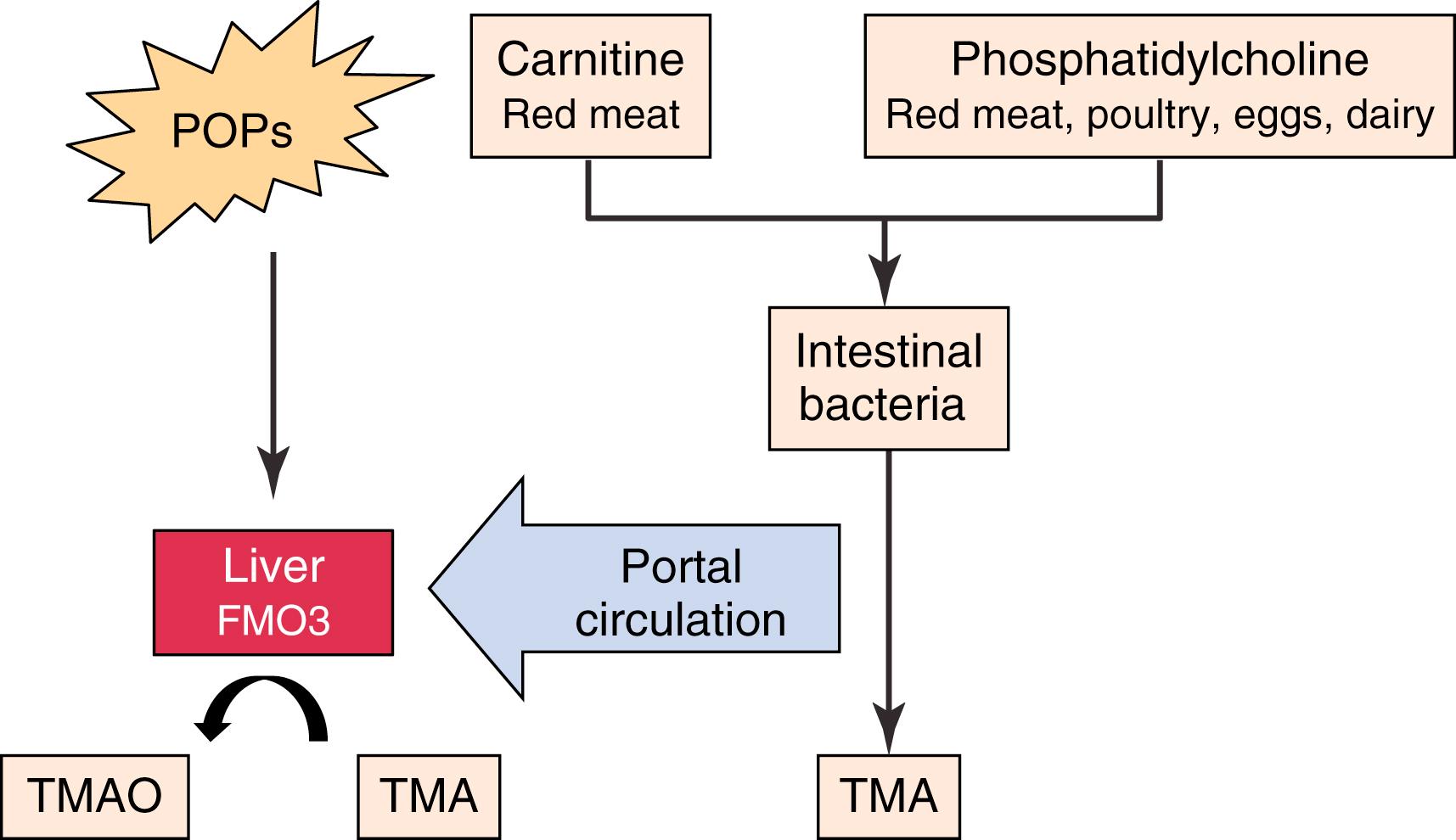
The production of TMAO from dietary precursors is dependent on intestinal bacterial metabolism and can be suppressed by antibiotic administration. The amount of animal products in the diet affects the intestinal microbiome and the amount of TMAO produced. Vegetarians and vegans produce significantly less TMAO than omnivores, and even short-term changes in diet can alter intestinal microbial populations. , Therefore, there appears to be a positive feedback loop whereby the consumption of animal products leads to TMAO production and causes an increase in the bacterial populations responsible for TMAO production.
Dietary TMA and choline supplementation also induce atherosclerosis in animal models, and blood TMAO levels are associated with increased rates of PAD, MI, MACE, CAD, and overall mortality in human studies. , Increased consumption of TMAO precursors, PC and L-carnitine, is also associated with increased atherosclerotic diseases and cardiovascular-specific mortality in animal models and several large human studies.
On the whole, the observational and animal data are highly suggestive of a role for PC, carnitine, and TMAO in atherosclerosis formation. However, there is some seemingly contradictory evidence regarding the association between TMAO and atherosclerosis. L-carnitine supplementation, while increasing TMAO production may prevent atherosclerosis in animal models, and L-carnitine supplementation in patients with acute MIs decreases all-cause mortality. Some studies of meat consumption have also suggested that an increased risk of cardiovascular disease and diabetes is not associated with fresh or frozen meat consumption, but is limited to consumption of processed meats. These studies did not account for the effect of non-meat animal products that lead to increased TMAO levels, and therefore, may not have been able to fully determine the effect of TMAO production on cardiovascular risk. Further studies including randomized, interventional trials that assess TMAO as a risk factor for MACEs are needed to further clarify the link between diet, TMAO levels, and atherosclerotic disease.
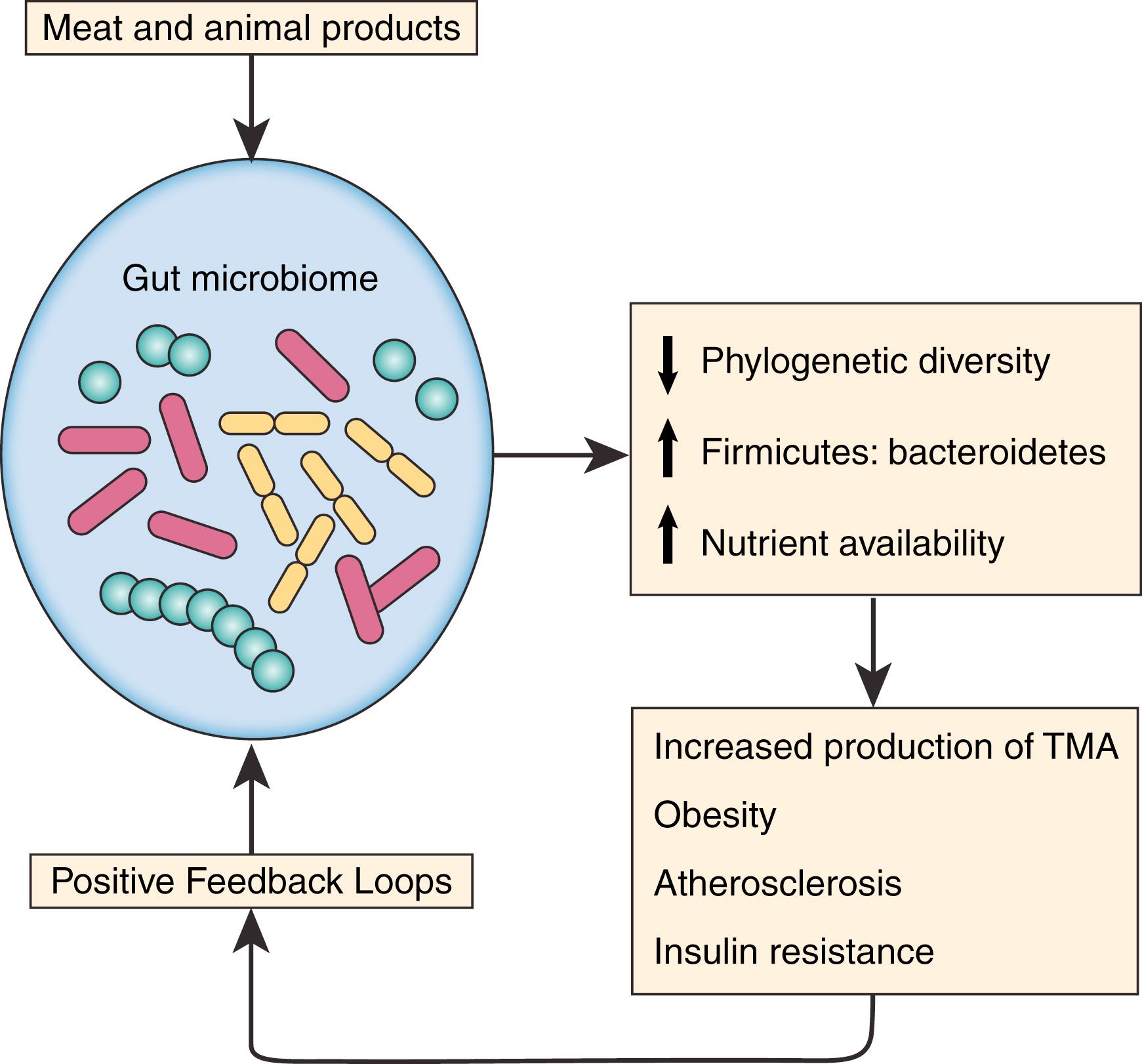
The interaction between diet and the human gut microbiome in atherosclerotic disease is an area of fervent investigation. As discussed in the section on diet, the gut microbiome is responsible for the production of the pro-atherogenic compound TMA from consumed animal products. Additionally, the human gut microbiome also affects the amount and type of absorbable nutrients derived from the diet. Understanding of variations in human gut microbiome populations is in its initial stages; however, it does seem that certain bacterial phyla are overrepresented in patients with atherosclerotic disease and risk factors compared to patients without atherosclerotic disease. Specifically, there is decreased phylogenetic diversity of gut bacteria and an increased prevalence of Firmicutes relative to Bacteroidetes in obese individuals and those with type-2 diabetes compared to controls. , Individuals with increased prevalence of Firmicutes are able to produce more absorbable calories for an identical meal than individuals with decreased prevalence of Firmicutes. Additionally, increased prevalence of Firmicutes relative to Bacteroidetes is associated with decreased production of the short-chain fatty acid butyrate and increased production of acetate resulting in inflammation, decreased insulin sensitivity, and obesity. The relationship between obesity, DM, and microbiome profile seems to be bidirectional in that dietary changes and weight loss alter microbiome profiles towards a more “healthy” state consisting of less Firmicutes and alteration of microbiome profile also causes weight loss and increased insulin sensitivity in animal and human studies respectively ( Fig. 16.2 ). , Larger, prospective and randomized studies with meaningful clinical endpoints are needed before these promising initial investigations into the gut human microbiome can be adopted in the clinical practice setting.
Efforts to link atherosclerosis with infectious etiologies have spanned decades. Although enthusiasm for an infectious etiology of atherosclerotic disease may have waned after several negative trials of antibiotics, the association and potential causal link between infectious pathogens and atherosclerosis remains an active area of investigation. Numerous viral and bacterial pathogens have been examined as potential causes of atherosclerosis.
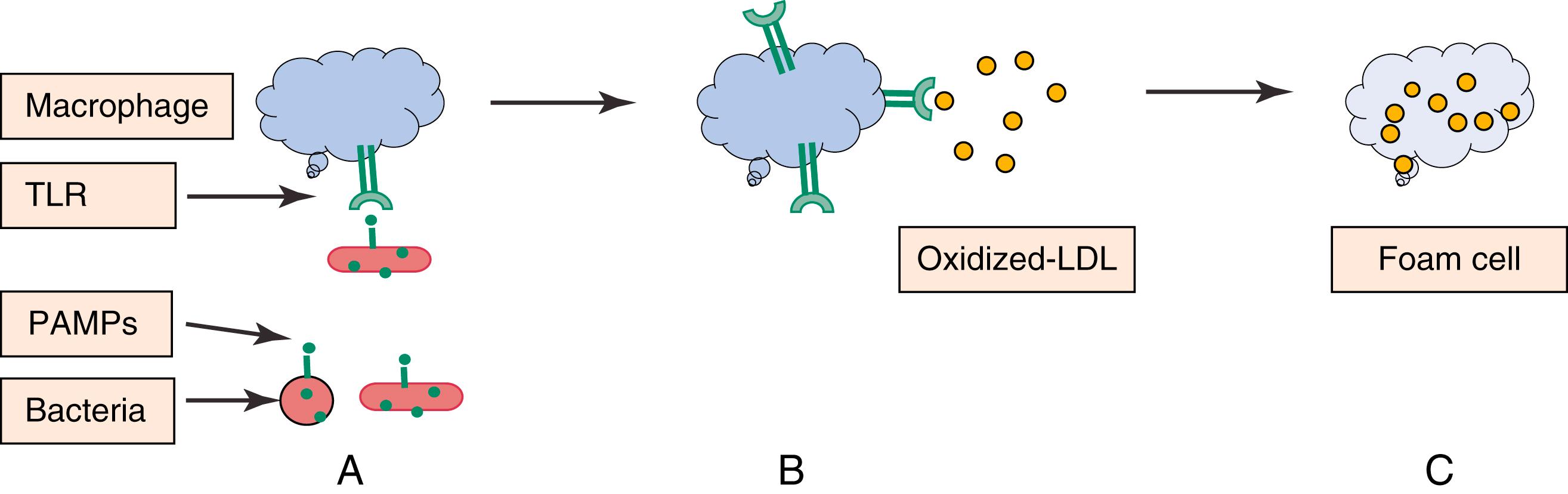
Potential mechanisms by which bacteria and viruses may cause atherosclerosis development include upregulation of adhesion molecules in vascular endothelium and foam cell formation. , The effect of many pathogens on foam cell formation seems partially dependent on Toll-like receptors (TLRs), which are present on inflammatory cells and bind to bacterial and viral pathogen-associated molecular patterns (PAMPs) as part of the innate immune system. However, they also bind endogenous compounds, such as ox-LDL, and cause macrophages to transform into foam cells ( Fig. 16.3 ).
Although the association between atherosclerosis and any single pathogen has been inconsistent, the concept of an increased pathogen burden may better explain the potential link between infection and atherosclerosis. The pathogen burden hypothesis postulates that exposure to an increased number of pathogens creates an increased systemic inflammatory response, which promotes the development of atherosclerosis. This hypothesis is supported by studies demonstrating a higher risk of CAD in patients with antibodies to a larger number of pathogens.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here