Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lasers and light sources are the treatments of choice for a variety of congenital and acquired cutaneous vascular lesions.
Based on the principles of selective photothermolysis, lasers induce photocoagulation of vessels by using wavelengths well absorbed by hemoglobin.
Appropriate pulse durations, equal to or shorter than the thermal relaxation time of the vessels, are chosen to confine the thermal damage.
Lights with longer wavelengths have increased optic penetration and are employed to target deeper dermal vessels.
Epidermal cooling techniques can protect against epidermal injury and improve treatment efficacy by allowing use of higher fluences.
Light-based treatments can be used effectively in skin of color, but special precautions should be made to prevent complications.
Fractional photothermolysis has a potential role in treating hypertrophic portwine stains and involuted hemangiomas.
Photodynamic therapy works by activation of photosensitizer by light sources and can be used to treat recalcitrant or nodular portwine stains.
Lesions treated with light sources include portwine stains, infantile hemangiomas, telangiectasia, facial erythema, poikiloderma, hypertrophic scars and leg veins.
The development and refinement of laser technology has provided an effective means to treat a wide variety of congenital and acquired cutaneous vascular disorders. Newer generation lasers with high vascular selectivity and good safety profiles have been pursued over the past few decades. The concept of selective photothermolysis was founded in the 1980s and revolutionized the light-based treatment of cutaneous vascular lesions. It describes the use of light with specific wavelengths to produce highly selective thermal injury of a targeted structure in the skin, while avoiding unnecessary damage to nearby tissues. Pulsed-dye laser, developed based on the concept, was proven effective in treatment of a variety of vascular disorders and emerged as the gold standard of treatment for portwine stains, hemangiomas, and telangiectasia. Today, light-based treatments are capable of producing excellent outcomes with an incidence of scarring of less than 1%, even after multiple treatments.
Currently, a wide variety of light-based systems is used to the treat cutaneous vascular lesions ( Table 35.1 ). Selection of a light system depends on several factors, such as the type, thickness, and depth of lesional involvement. Emergence of skin cooling techniques has been another major breakthrough in modern laser development. Appropriate epidermal cooling allows the use of high laser fluences and hence improves the overall efficacy, while minimizing the most undesirable complication of scarring.
| Laser/light source | Wavelength (nm) | Pulse duration (ms) |
|---|---|---|
| Pulsed-KTP | 532 | 1–200 |
| Pulsed-dye | 585 | 0.45 |
| Long-pulsed dye | 585, 590, 595, 600 | 1.5–40 |
| Long-pulsed alexandrite | 755 | 3–20 |
| Diodes | 800, 810, 940 | 1–250 |
| Long-pulsed Nd:YAG | 1064 | 1–100 |
| Intense pulsed light source | 515–1200 | 0.5–30 |
The term “LASER” is an acronym for Light Amplification by Stimulated Emission of Radiation. In the early twentieth century, Einstein revolutionized the modern theory of light by describing the peculiar wave-particle duality of electromagnetic radiation. Light interacts with matter in the form of a photon. Photon, a quantum of electromagnetic radiation, can stimulate the emission of another photon from an excited atom. This unique principle of stimulated emission allows light amplification and production of laser light.
The interaction between laser light and skin depends on the optical properties of the skin constituents and the wavelength of incident light. As a rule, laser light interacts with skin in four principle ways: (1) reflection; (2) scattering; (3) absorption; and (4) transmission. In general, skin reflects 4–7% of light approaching its surface. Reflection increases with angle of incidence. It is therefore lowest when the light is delivered to the skin perpendicularly. Scattering refers to the spreading of incoming light by skin particles in all directions and is related to the non-uniformities within the medium. Electromagnetic energy is lost with scattering. In the skin, scattering of light is primarily due to dermal collagens. Electromagnetic radiation is absorbed by chromophores (light-absorption molecules). When a photon is absorbed by a chromophore, all the energy it carries will be transferred to that molecule. Different chromophores absorb lights of specific wavelengths. This feature constitutes the basis for the theory of selective photothermolysis. Light that is not reflected, absorbed, or scattered will be transmitted to deeper tissue.
Anderson and Parrish founded the concept of selective photothermolysis in 1983, and inaugurated the new era of modern laser application. According to the principles, a target structure can be destroyed preferentially with minimal injury to surrounding tissues, given the use of specific laser light wavelength. The success of selective photothermolysis depends on three major factors: (1) wavelength of the light source; (2) duration of light pulse; and (3) amount of energy (fluence) delivered.
To achieve desirable photothermolysis, the wavelength of the light source must be able to penetrate adequately into the skin (to reach the target structure) and be absorbed preferentially by the target chromophore. Optic penetration varies with wavelengths: light with shorter wavelengths (300–400 nm) is largely scattered by dermal collagens and is only able to penetrate less than 0.1 mm into the skin. In contrast, longer wavelengths (600–1200 nm) penetrate deeper, with less scattering.
The major target chromophores in skin are hemoglobin, melanin, and water. The absorption spectra of important skin chromophores are shown in Figure 35.1 . Hemoglobin has a few absorption peaks, whereas melanin has a broad spectrum of absorption, which gradually diminishes with longer wavelengths. Water absorption dominates in the far-infrared range while protein absorption dominates in the far-ultraviolet range. Such properties render wavelengths in the visible light spectrum the choice for vascular lasers.
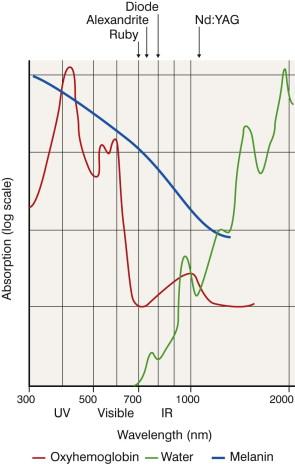
To target a vascular lesion, the wavelength chosen must be well absorbed by oxyhemoglobin and poorly absorbed by epidermal melanin. Oxyhemoglobin has major absorption peaks at 418, 542, and 577 nm, corresponding to blue, green, and yellow wavelengths, respectively ( Fig. 35.1 ). Melanin normally presents in the epidermis and hair follicles, and absorbs broadly across the visible light spectrum. Despite strong absorption by blood in the blue band (418 nm), wavelengths in this region are less desirable because of limited penetration and interference by high melanin absorption. The yellow band (577 nm) of oxyhemoglobin absorption was chosen initially for targeting superficial vessels using the flashlamp-pumped pulsed-dye laser. The wavelength of this laser was subsequently increased to 585 nm and later 595 nm, to provide deeper penetration. Pulsed potassium titanyl phosphate (KTP) lasers emit at 532 nm (green light spectrum). Green light is absorbed by hemoglobin nearly as well as yellow light, and has roughly the equivalent optic penetration through fair skin.
There is also a broad oxyhemoglobin band at around 900 nm, which features advantages of preferential hemoglobin absorption, minimal interference from melanin absorption and good optic penetration. Lasers in this near-infrared region have been applied to treat cutaneous vascular lesions. They include the alexandrite (755 nm), diode (800 nm), and neodymium-doped yttrium aluminum garnet (Nd:YAG) (1064 nm) lasers. Intense pulsed light (IPL) sources use high-intensity pulsed flashlamps that produce broadband emission from 500–1100 nm. The broad emission spectrum provides selective absorption by hemoglobin and deep penetration for large, deep-seated vessels.
The pulse duration (or exposure time) of laser or light sources determines heat confinement and hence extent of thermal injury produced. An important concept underlying selective photothermolysis is thermal relaxation time (TRT). TRT is the time required for the peak temperature in a heated region of the tissue to decrease to half of the total rise. Thermal confinement occurs when the pulse duration of a light source is less than the TRT.
TRT is proportional to the square of object size. It is approximated by the formula TRT = d 2 /4κ, where “d” is the diameter of the object (or thickness of the tissue) and “κ” is the thermal diffusivity. Thermal diffusivity is a material property that describes the ability of heat to diffuse. Given the same material and shape, an object one-half the size cools in one-quarter of the time, while an object one-tenth the size cools in one-hundredth of the time.
Energy of electromagnetic radiation is measured in units of joules (J). The amount of energy delivered per unit area is the fluence, which is measured in J/cm 2 . Power is the rate at which light energy is delivered and is measured in watts (W). By definition, 1 Watt is 1 Joule per second (W = J/s). In practice, sufficient fluence must be supplied such that the light beam is able to reach (adequate optic penetration) and produce irreversible damage to the target. In addition to wavelength, optical penetration is also determined by spot size. With smaller spots, a greater fraction of photons scatter outside the beam and may render the treatment ineffective. Larger spots allow more photons to remain within the diameter of incident beam and hence offer greater optic penetration. The ratio of dermal to epidermal damage increases with spot size. Lower treatment fluence is required with larger spots when compared to smaller ones. For instance, 7- and 10-mm spots require only one-half to two-thirds of the fluences required for a 5-mm spot with the 585-nm pulsed-dye laser.
Although higher fluence produces more thermal injury, it can also lead to more adverse events. Absorption by epidermal melanin and heat transfer from dermal targets can result in serious epidermal injury. Ideally, the epidermis should remain unaffected during light treatments. Effective skin cooling methods were developed over the past decade to minimize tissue injury by extracting heat from the epidermis.
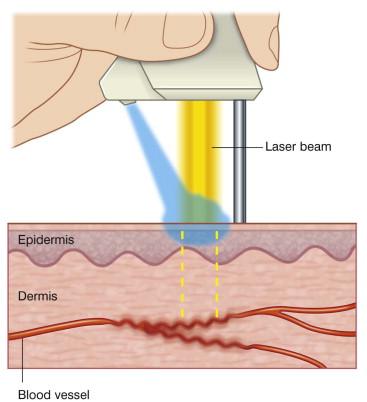
Skin cooling can be achieved before , during , and after successful laser pulse impacts. Skin cooling, before and after laser exposure, with ice can reduce pain and swelling, which in turn enhances patient tolerability. Cooling the skin during laser treatment (dynamic skin cooling) effectively decreases epidermal heating by the laser pulses. Skin cooling devices extract heat from the epidermis by means of conduction in various ways, which can be brought about by application of a cold gel, a liquid medium, or a liquid cryogen spray during treatments ( Fig. 35.2 ).
Effects of light on skin begin with absorption of the electromagnetic radiation and its conversion into heat. Photobiological effects of laser on skin result when essential cellular structures (DNA, RNA, and cell membranes) denature with heat. Denaturation results in loss of cellular function via unfolding and coagulation of macromolecules. Meanwhile, lasers also cause photomechanical effects . Rapid heating can lead to sudden thermal expansion, causing rupture or increased permeability of cell membranes. Photomechanical disruption can also be achieved by a process called cavitation. Heating and evaporation of water can lead to the appearance, expansion and violent collapse of vapor bubbles. Cavitation is the major mechanism of vessel rupture caused by pulsed-dye lasers.
Pulsed-dye laser, originally designed in the 1980s, was the first laser device to treat vascular lesions, i.e., portwine stain (PWS). With improved knowledge of laser–tissue interactions and technology, a wide variety of laser and light systems are now available to use for different vascular lesions ( Table 35.1 ).
The theory of selective photothermolysis predicted that a laser with a 577-nm wavelength and 1-ms pulse duration was ideal for treating immature PWS. For vessels 10–50 µm in diameter, the thermal relaxation time would be 0.1–10 ms, with an average of 1.2 ms. For larger vessels 100 µm in diameter and with a wall thickness of 3–6 µm, an exposure time of 0.1–0.3 ms is required to produce irreversible damage to them. Pulsed-dye laser (PDL) (577 nm) with shorter pulse durations (1–20 µs) has been evaluated in animal and normal human skin. Although selective vascular injury was achieved at such short exposure time, the rapid heating led to vessel rupture and significant dermal hemorrhage. This was due to the mechanical rupture of blood vessels as a result of erythrocyte explosion and vaporization of blood. The pulse duration of the PDL was then lengthened to maximize thermal coagulation of vessels while minimizing vaporization injury. Longer pulse duration can minimize dermal hemorrhage (purpura), but requires higher fluence to achieve the required thermal coagulation.
The wavelength of PDL was subsequently increased to 585 nm, which provided similar vascular selectivity with doubled depth of penetration in tissue. The 585-nm, 400-µs PDL demonstrated excellent clearing of PWS with a high safety profile in infants, children, and adults. Clinically, this laser produces transient blue–black purpura after treatment. Histologically, there was selective intravascular and perivascular coagulation necrosis with epidermal injury. In 1995, Dierickx and colleagues performed a landmark in-vivo study, which demonstrated that the thermal relaxation time for PWS vessels (approximately 60 µm in diameter) was between 1 and 10 ms for a 585-nm PDL. Since the 1990s, newer generation PDL systems with longer pulse durations greater than 1 ms have been developed. They offer the additional advantage of reduced incidence of prolonged, unsightly purpuras.
Millisecond-duration pulsed potassium titanyl phosphate (KTP) laser was introduced in the 1990s. Pulsed-KTP lasers, with a wavelength of 532 nm, emits in the green light spectrum. The 532-nm laser light is absorbed by hemoglobin approximately as well as the 585 nm, and has roughly equivalent optic penetration through fair skin. Most of these systems use grouped, nanosecond-domain Q-switched pulses to produce millisecond exposure times. Thus, purpura is precluded by the more uniform heating of vessels.
Longer pulse durations minimize photomechanical injury and hence reduce post-treatment purpura. Pulse durations greater than 1 ms produce gentle vaporization of blood rather than explosive vessel rupture. Controlled evacuation of blood produces an empty, thermally coagulated blood vessel. Lengthening the pulse duration of PDL to 1.5 ms further reduces the intensity and duration of purpura significantly. Kauvar and associates demonstrated that in treating adult PWS, a 595-nm, 1.5-ms PDL resulted in reduced purpura when compared with the 585-nm, 450-µs system. Moreover, lengthening the pulse duration also minimizes epidermal damage, as melanosomes are less effectively heated with longer pulses. They cool significantly during laser pulse delivery due to small size.
As mentioned above, longer wavelengths provide deeper optic penetration. They are often used to treat deep-seated vessels with large calibres. The absorption coefficient of blood decreases with wavelengths beyond 577 nm. However, highly selective microvascular injury can still be achieved by increasing the fluences in compensation, to produce the same thermal injury. PDL with a longer wavelength (595 nm) works well in treating PWS and telangiectasia with high fluences. Pigment cell injury can be minimized by using long pulse durations (1.5 ms) and skin cooling techniques.
IPL systems are high-intensity flashlamps that emit a broad spectrum of polychromatic lights from 500 to 1200 nm. Cut-off filters are used to match the spectrum of lights that are capable of penetrating to the desirable depth in skin and to maximize absorption by target chromophores. By filtering the shorter wavelengths, deeper or larger-diameter blood vessels can be targeted effectively. Energy is delivered by single or multiple synchronized pulses with exposure times of 0.5–80 ms. The pulse duration is also matched to the thermal relaxation time of the vessels being treated. The inter-pulse delays allow cooling of the epidermis and smaller vessels, whereas heat is retained in larger vessels.
More recently, the near-infrared wavelengths have been used to treat vascular lesions. Alexandrite (755 nm), diode (800, 940 nm), and Nd:YAG (1064 nm) lasers use the broad oxyhemoglobin absorption band in the 800–1000-nm spectrum, and features the advantage of greater optic penetration. These longer wavelength lasers are being used to treat larger vessel vascular anomalies, as well as larger leg veins (1–5 mm). Newer techniques involve hybrid laser systems, which emit a dual band of wavelengths (e.g., Nd:YAG/KTP, 1064/532 nm; Nd:YAG/PDL, 1064/595 nm) in a sequential manner. They can be used to treat resistant PWS with lower laser fluences.
Photodynamic therapy (PDT) is another effective modality in treating vascular lesions. PDL uses intravenous photosensitizer and, upon activation by the appropriate light source, generates reactive oxygen species and causes desirable vessel damage. PDT alone or in combination with PDL has been proven effective in blanching PWS.
Fractional photothermolysis (FP) is a new technology developed over the past decade. By using focused, high-energy laser microbeams, distinct columns of thermal injury are created. Skin tightening effects are achieved via tissue healing and collagen remodeling. In contrast to the traditional ablative laser, FP allows a rapid healing time and has much better patient tolerance. Ablative and non-ablative FP were reported to be successful in treating the unsightly fibrofatty residuum of involuted hemangiomas. Based on the success of FP in improving cosmetic outcome of hypertrophic scars, there is a potential role of FP in treatment of hypertrophic PWS. Destruction of redundant vascular tissues and subsequent volume reduction could be achieved by FP. However, well-designed, large-scale studies are needed to prove the theory.
The applications for vascular lasers and light sources are listed in Box 35.1 . Important concepts are highlighted and discussed in detail below.
Capillary malformations
Infantile PWS
Macular PWS in adults
Hypertrophic PWS in adults
Hemangiomas
Superficial hemangiomas
Mixed-type hemangiomas
Involuting hemangiomas
Venous malformations
Telangiectasias
Spider angiomata
Actinic telangiectasia
CREST syndrome
Essential telangiectasia
Hereditary hemorrhagic telangiectasia
Radiation dermatitis
Rosacea
Facial erythema
Associated with rosacea
Flushing and blushing
Cherry angiomas
Venous lakes
Poikiloderma of Civatte
Other lesions treated with vascular lasers
Angiokeratomas
Glomus tumors
Pyogenic granulomas
Adenoma sebaceum
Blue rubber bleb nevi
Hypertrophic and erythematous scars
Striae distensae
Warts
PWS are congenital capillary malformations that present in 0.3–0.5% of live births. The lesions grow commensurately with the patient and never regress spontaneously. Most if not all PWS are congenital. Nevertheless, antecedent trauma has been reported leading to PWS. PWS lesions have a tendency to undergo hypertrophy with time, particularly in middle age. It is associated with various physical and psychological complications including bleeding, ulceration, functional impairment and depression.
Histologically, there is an increased number of ectatic vessels in the dermis with no abnormal turnover of endothelial cells. The etiology of PWS is so far unknown, but has been postulated to be associated with altered or even absent neural modulation of the vascular plexus. The abnormal neural modulation is possibly due to both decreased sympathetic and sensory innervation of the papillary vascular plexus. By applying confocal microscopy, Chang and colleagues demonstrated a decrease in nerve density in facial PWS. Overexpression of vascular endothelial growth factor (VEGF) and its receptor has also been observed in PWS lesions.
With the advance in molecular techniques, the genetic basis of PWS has been under active investigation. Shirley and co-workers demonstrated that PWS and associated Sturge–Weber syndrome (SWS) were caused by an activating somatic mutation in the guanine nucleotide-binding protein G(q) subunit alpha (GNAQ) gene. They identified a non-synonymous single-nucleotide variant ( c .548G→A, p.Arg183Gln) in the GNAQ and hypothesized that such a mutation would cause activation of the extracellular signal-regulated kinase (ERK). The subsequent selective downstream signaling would possibly lead to non-oncogenic proliferation in PWS and SWS.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here