Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Humoral immune tests assess production of specific antibody responses to past or recent infections, and cellular immune assays measure current immune responses.
The immune system changes with age and nutritional status. Differences in immune responses of test subjects associated with immaturity, immunosenescence, or malnutrition should be taken into account when evaluating the results of specific tests.
Primary immunodeficiency may be associated with an increased incidence of malignancy. Malignancy, chemotherapy, and radiotherapy can significantly suppress the immune response and alter cellular immunoassay results.
Evaluation of the cellular immune response is undertaken in a graduated sequence of stages that may include both in vitro and in vivo testing to identify areas of immunodeficiency.
Measurement of lymphocyte activation may be accomplished in vitro by flow cytometry using activation-specific fluorescent-labeled monoclonal antibodies and vital dyes. The usefulness of this approach has increased significantly with the development of fluorochromes with different excitation spectra.
The understanding of the immune system is greatly enhanced by the detection of specific abnormalities in patients with suspected immunodeficiency. These advances have come from studies of immune cell differentiation and function, experimental gene deletion, and detailed analysis of human immunodeficiency syndromes. New experimental approaches have helped to elucidate the mechanisms and functional basis of immune dysregulation in patients with primary (congenital) genetic mutations of the immune system or secondary (acquired) infections. Because some immunodeficiencies cannot be accurately diagnosed by the combination of clinical symptoms and appropriate immune function assays, genetic information is becoming an increasingly important component of diagnostic testing and interpretation. The mission of the clinical immunology laboratory is to translate new research leads into highly standardized and clinically relevant tests for the individual patient.
Studies of the human cellular immune system have principally focused on three areas: (1) primary immunodeficiency, which reveals the impact of congenital immune defects on host defense; (2) acquired immunodeficiency, such as human immunodeficiency virus (HIV) infection, in which infection damages the immune system directly; and (3) autoimmune diseases, in which the effect of excessive or inappropriate immune activity is evident. In addition, cellular immune defects in patients with diseases with immune dysfunctional features—such as chronic infection, cancer, malnutrition, or traumatic injury—provide crucial insight into immune-mediated host defense.
The general concept of immunity is often equated with humoral immunity because antibodies to infectious agents introduced by natural infection or by immunization have been studied for more than a century. Cellular immunology, as currently practiced in the clinical and research laboratory, is a relatively new science ( ; ; ; ; Hamilton, 2006). The modern science of cellular immunology developed during the 1980s through a series of independent events and major research discoveries, including the use of monoclonal antibodies to identify immune cells, the development of analytic and sorting capabilities of the flow cytometer, the discovery of cytokine regulation of immune response, the birth of molecular immunology, and, above all, the tremendous need to understand and control the emerging HIV epidemic ( ). The appearance of HIV occurred virtually in parallel with the potential to identify CD4 + T cells. The first analyses of cellular immune functional deficiency in the acquired immunodeficiency syndrome (AIDS) were based on analysis of lymphocyte proliferative response ( ; ) and have since evolved into a range of functional approaches, with the ultimate goal of developing a vaccine against the HIV virus ( ; ; ; ; ). During the past decade, further developments in cellular immunology, genetics, and other sciences resulted in the emerging field of cellular immunotherapy, also called adoptive cell therapy, in which engineered immune effector cells are used in the treatment of cancer, autoimmune diseases, viral infection, and other diseases. Immunotherapy using chimeric antigen receptor T cells (CAR-T cells) is currently the most promising form of cellular immunotherapy ( ; ).
In contrast to humoral immunity, cellular immune function is both fundamentally complex and difficult to measure. Basic humoral immune tests measure the specific antibody product of a past response to a specific virus or microbe; by contrast, most cellular immunoassays measure current responses. Because a majority of peripheral blood lymphocytes are resting cells, the cellular immune reaction must be re-created or generated freshly within the test system. The system must be capable of triggering the response, supporting the reaction by providing all needed elements available in vivo, and having a measurable endpoint.
This chapter presents current cellular immunologic tests in light of future trends. Cellular immune assessment is moving away from single assays and single-number fixed endpoints toward an integrated analysis of cell function at several levels that reflect cellular interactions as a dynamic process.
Two main immune cell types are known: (1) T lymphocytes (T cells), and (2) B lymphocytes (B cells) ( ; ; ; ; ; ; ). T lymphocytes are defined by expression of the T-lymphocyte receptor, which binds to antigen and CD3, a surface determinant associated with the T-cell receptor that is essential for activation. T lymphocytes have different, clonally variable receptors for a large range of antigens, require thymic maturation for normal function, and mediate cellular immunity ( ). B lymphocytes are identified by surface immunoglobulin (detected by monoclonal antibodies such as CD19 or CD20) and, upon appropriate activation, develop into plasma cells secreting specific antibody and, thus, mediating humoral immunity ( ; ). Loss of the normal thymus will compromise T-lymphocyte function and affect T-dependent B-lymphocyte activation. Failure at the bone marrow level can affect both T-lymphocyte and B-lymphocyte immune responses, although specific linkages may be involved.
The distinction between a specific and nonspecific immune response is a fundamental necessity because the system must be able to distinguish between self and nonself ( ; ). In general, self-recognition is accomplished by incorporating the molecular major histocompatibility complex (MHC) self-antigen system into the antigen recognition phase. Antigen must be processed and presented in the context of self-MHC to be recognized and to lead to response and development of immune memory. The antigen-processing function is carried out by antigen-presenting cells (APCs); the best studied is the monocyte. This response triggers lymphocyte activation and proliferation, which may include production of effector cells and triggering of B lymphocytes to produce antibody. This type of immunity, often termed adaptive immunity , is retained as “memory” and typically is elicited following immunization or natural infection ( ; ; ; ). Lack of expression of MHC class II antigen can be detected on lymphocytes by flow cytometry using monoclonal antibodies against human leukocyte antigen (HLA)-DR or HLA-DQ and is a hallmark of MHC class II deficiency.
A second fundamental type of immunity can be described as innate immunity. This type of immunity is an ancient host response to infectious agents or self cells with absent or altered self-recognition molecules. It is encoded within the genome, does not have memory, and is not improved by repeated contact ( ; ; ). The innate immune system consists of (1) external barriers (i.e., skin, mucosal surfaces) to prevent microorganisms from entering the body; and (2) a programmed, coordinated series of events to destroy microorganisms that penetrate the external barriers or molecules that arise within the host from disease processes. These events include both chemically mediated (cytokines, complement, interferon) and cellular components (phagocytes, natural killer [NK] cells). The modern era of discovery about innate immunity began in 1989, when the late Charles Janeway, an immunologist at Yale University, predicted that sentinel cells (i.e., macrophages, dendritic cells) have nonclonal, germline pattern recognition receptors (PRRs) that directly recognize some invariant molecular signatures of microbes (pathogen-associated molecular patterns [PAMPs]) not found in the host ( ). Lipopolysaccharide, a lipoglycan component of the Gram-negative bacteria cell wall, is the best characterized PAMP. Additional PAMPs include other lipoglycans, such as peptidoglycans and 1,3-β-glucans proteins such as bacterial flagellin, and microbial nucleic acids ( ). More recently, it was found that certain substances released from host tumor cells or dead or dying cells resulting from hypoxia, infection, or other disease processes also activate PRRs. These host molecules have been termed damage-associated molecular patterns , or DAMPs ( ; ). In 1997, studies of Drosophila melanogaster (fruit fly) identified the Toll-like receptor (TLR) as the major effector of the innate immune response in the fly, which has no adaptive immune system. Since that time, multiple TLRs have been found in humans, largely on dendritic cell, macrophage and neutrophil membranes, as well as on epithelial cells lining the respiratory and gastrointestinal systems. TLRs can be thought of as a primitive, highly conserved alarm system that recognizes bacterial pathogens and stimulates the expression of molecules that initiate the local inflammatory response and phagocytosis ( ; ). TLRs are highly conserved molecules characterized by an extracellular domain of leucine-rich repeats and an intracellular signaling domain with homology to that of the interleukin (IL)-1 and IL-18 receptor family. TLR signaling initiates the transcriptional expression of genes that constitute a core inflammatory response, including proinflammatory cytokines such as IL-1α, IL-β, tumor necrosis factor (TNF)-α, and IL-6, as well as numerous chemokines and cell surface receptors that regulate T- and B-cell immune responses ( ; ; ).
Subsequent to the discovery of the TLRs, a large number of other PRRs have been characterized on the cell surface, cytoplasm, and nucleus of mammalian cells. These include C-type lectin receptors, NOD-like receptors (NLRs), RIG-I-Like receptors (intracellular retinoic acid-inducible gene-I-like receptors [RLRs]), leucine-rich repeats (LRLs), and cytosolic DNA sensors (CDS) ( ). The cascade of transcriptional changes and posttranslational modifications results from the binding of a ligand to a PRR lead to the secretion of cytokines and chemokines, the recruitment of phagocytic cells, the induction of antimicrobial peptides, pyroptotic cell death, and many other changes that constitute the innate immune response. The inflammasome, restriction factors, the proteasome, C-reactive protein, the endosomal/phagocytic system, and transcription factors such as NFκB play an essential role in this response ( ).
Unlike phagocytic cells, NK cells are not functionally developed at birth, probably because the key cytokine, interferon (IFN)-γ, which is needed for development and maturation of this system, is also downregulated at birth. The NK cell, once called the K cell , null cell , or third population , has neither surface immunoglobulin nor a rearranged T-cell receptor (Bonavida et al., 2009). The NK system is constitutively active and does not have to be primed by antigen to kill ( ; ). NK cells make up a diverse population that has eluded conventional classification by cell lineage analysis. However, CD56 is currently considered the most definitive immunophenotypic marker for the NK cell ( ). NK cells have been best known as cells that can kill nonspecifically (naturally) virus-infected cells and bacteria and can prevent tumor cell metastasis. However, NK cells also regulate T- and B-cell functions as well as hematopoiesis ( ). These functions of NK cells are probably dependent on their ability to produce lymphokines, particularly IFN-γ. NK cells are important for antigen-independent activation of phagocytic cells early in infection and for favoring the development of antigen-specific T-helper type 1 (Th1) cells. When armed with specific antibody, however, these cells can kill specifically.
Although the immune system is classically divided into humoral and cellular components, this separation is in no way absolute in that considerable interdependence is seen between B and T cells. The most commonly measured functional cellular immune parameter is lymphocyte proliferation ( ). Measurement of lymphocyte activation/proliferation has evolved substantially since the late 1950s and early 1960s, when cell division was determined by counting the number of lymphocytes that had transformed into blasts. The latter method was later replaced by the quantitation of incorporated radiolabeled nucleic acid precursors (tritiated thymidine) into newly synthesized deoxyribonucleic acid (DNA). Although this “bulk assay” remains the most commonly used laboratory procedure for measuring cellular proliferation, new reagents and new procedures have recently become available to assess lymphocyte activation and proliferation. These include commercially available cell surface proliferation markers, the ability to measure the percentages of cells in specific phases of the cell cycle, the quantitation of cell-associated and secreted cytokines/cytokine receptors, and the ability to assess the number of cell divisions in lymphocytes labeled with tracking dyes. In this section, we review the molecular events involved in T-lymphocyte activation and proliferation plus review some of the new methods that have been developed to assess the functions of the cellular immune system.
Specific interaction of mitogen or antigen/MHC with the appropriate lymphocyte receptors leads to a cascade of cellular processes that include changes in membrane transport, rearrangement of the cytoskeletal system (polarizing the lymphocyte toward the APC), and activation of several signaling pathways ( ; ; ; ). These changes ultimately lead to a number of outcomes, including T-cell differentiation, cytokine secretion, proliferation, anergy, or apoptosis. Ongoing investigations are unraveling the complex molecular and biochemical pathways that drive the activated T cell down these pathways. Specific abnormalities in these pathways are constantly being discovered, which underlie many of the primary immunodeficiency diseases. Unfortunately, abnormalities in a bulk proliferation assay indicate only that there is limited or no cell division and provide no information regarding the underlying abnormality in lymphocyte activation. Therefore, more sophisticated assays are required to investigate underlying T-cell abnormalities.
Antigen/MHC-induced activation of T lymphocytes involves a series of complex and defined events that differ slightly between the activation of a naïve T cell versus the activation of a memory T cell. Antigen is processed by B cells or monocytes, leading to the assembly of immunogenic peptides into class I or class II products of MHC genes ( ). The peptide–MHC complex is presented to T cells bearing the appropriate T-cell receptor ( ). In addition, the APC expresses a series of adhesion and costimulatory molecules that interact with appropriate ligand/counter-receptors on the T-cell surface. Ligation of the T-cell receptor alone is not sufficient for activation of the T cell, which has led to the development of the two-signal model for T-cell activation ( ; ). The first signal delivered via the T-cell antigen receptor (TCR)/CD4/CD8 modulates transition of the T cell from the early stages of activation (i.e., G 0 to G 1 ). Signal two is delivered via the costimulatory pathways, most notably CD28, and to lesser extents, LFA-3, CD2, CD5, and CD7, and leads to the induction of IL-2 and other cytokine genes required for T-cell proliferation and differentiation to effector cells.
Lymphocytes are unique in that they express surface receptors able to identify virtually any molecule or foreign substance (antigen). Structural diversity within these receptors is created by the differential rearrangement of T-cell receptor genes. In general, only a limited number of circulating lymphocytes are able to recognize any single antigen. When a lymphocyte recognizes a foreign antigen in vivo, cells proliferate rapidly in a clonal manner to generate a large number of both effector and memory cells ( ).
The TCR complex is composed of both a heterodimeric antigen recognition structure (i.e., the TCR) and a noncovalently bound transducing complex referred to as CD3 ( ; ; ). The TCR cannot be expressed on the cell surface without CD3 and has no inherent signaling capabilities of its own. The antigen recognition structure is composed of structurally divergent α and β chains (or, less frequently, γ and δ chains), and the CD3-transducing complex is composed of five invariant polypeptide chains: α, β, ε, η, and a ζ chain dimer. Each of the CD3 proteins contains a motif called the immune tyrosine activation motif ( ITAM ), which binds the SH2 domains of protein tyrosine kinases. The ζ chain (which exists as a ζ homodimer, a ζ with an η, or a ζ with an Fc ε RI γ chain) contains three ITAMs and is the most significant component of the TCR complex involved in signal transduction from the TCR ( ; ). Originally described by Reth, these motifs play an essential role in early events following T-cell activation ( ; ; ). CD4 and CD8 molecules on the surfaces of T cells are also noncovalently attached to the TCR complex. They bind to HLA class II and class I molecules, respectively, on the APC and are also involved in transduction of activation signals ( Fig. 46.1 ). Processed antigen is presented to T cells in the context of the MHC antigens. In general, CD4 + T cells respond to exogenously processed antigens presented in the context of MHC class II, and CD8 + T cells respond to endogenously processed antigens presented in the context of MHC class I. CD4 and CD8 are also associated with tyrosine kinases involved in the early events following T-cell activation. In addition to these interactions and the costimulatory molecular interactions, another group of molecules present (adhesion molecules) on both the APC and the responding T cell bind to each other and serve to increase the avidity of the binding.
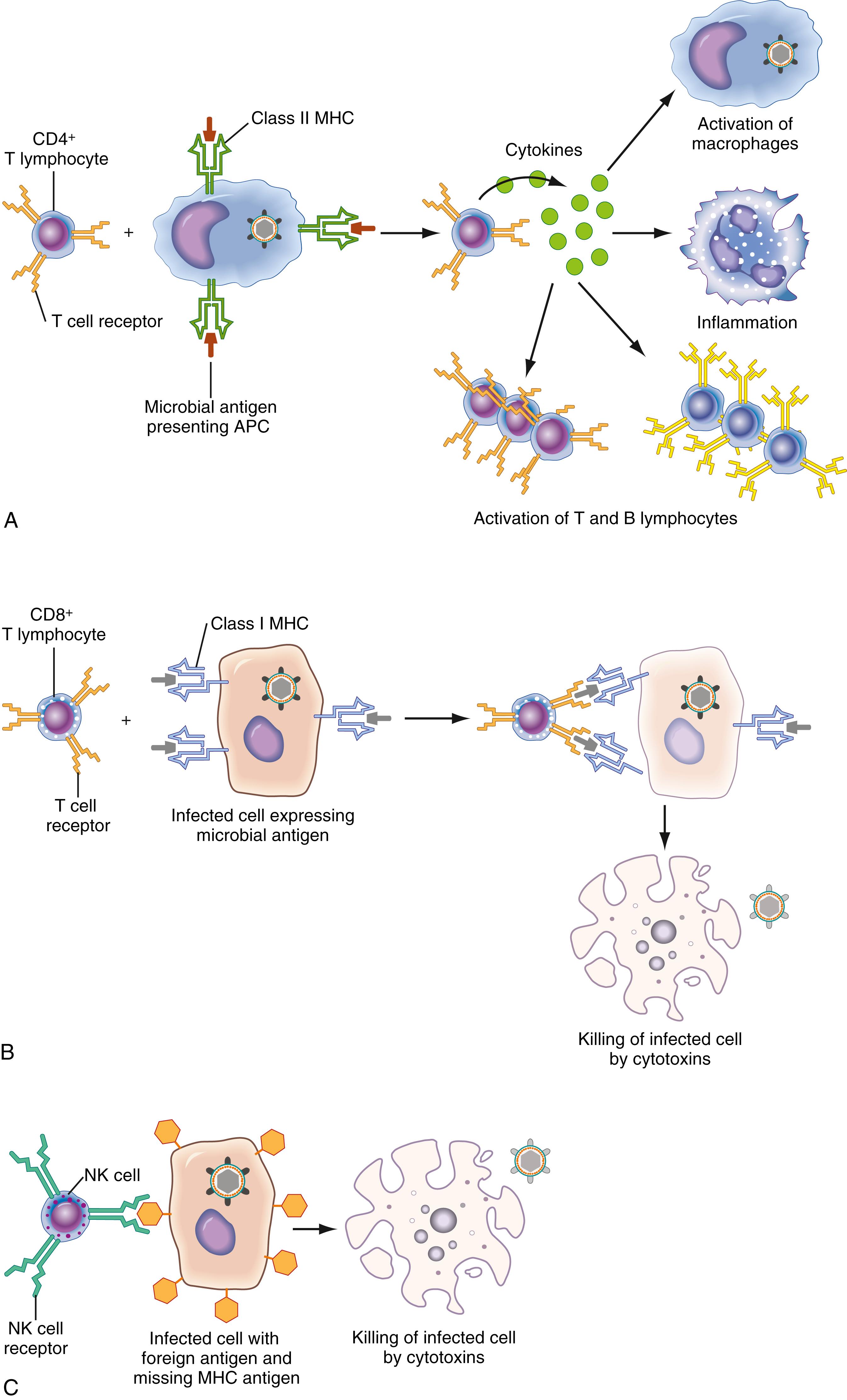
Costimulatory molecules identified on APCs include B7 (CD80) ( ), B7.2 ( ), and heat-stable antigen (HSA) ( ), among others ( ; ). On T cells, CD28 is the primary costimulatory molecule and binds B7; CTLA-4, on the other hand, binds both B7 and B7.2 and is involved in downmodulating T-cell activation ( ). The receptor on T cells for HSA has not been identified. Antigen presentation in the presence of reagents that block the costimulatory molecules leads to anergic response (tolerance) on subsequent exposure to that specific antigen but does not affect the responses to other antigens ( ). The ability to make nonimmunogenic transplantable tumors immunogenic by transfecting them with the B7 gene ( ; ; ; ) suggests that costimulatory molecules play an important role in T-cell activation in vivo.
The presentation of antigen to T cells leads to aggregation of the TCR–CD3 complexes and activation of protein tyrosine kinases (PTKs). The TCR itself has a small cytoplasmic tail with no known transducing activity. It is the associated ζ chains of the CD3 complex that contain the ITAMs and that have been shown to coprecipitate PTK activity. Two well-known classes of cytoplasmic PTK families are involved in the very early events following T-cell receptor aggregation: Src and Syk/ZAP-70. Signaling cascades downstream from the TCR–CD3 complex and the CD28 costimulatory pathway are fairly well understood ( ; ). Activation of TCR-associated tyrosine kinases ZAP70, p59 fyn , and p56 lck leads to activation of three pathways: p21 ras , calcium/calcineurin, and protein kinase C (PKC). These pathways are discussed further in Chapters 24 ( Fig. 24.15 ) and 77 ( Fig. 77.1 ). Activation of p21 ras activates mitogen-activated protein kinases, which, in turn, phosphorylate several transcription factors, thereby regulating gene expression. Activation of the PTKs also activates phospholipase C, which hydrolyzes phosphatidyl inositol and leads to generation of the second messengers diacyl glycerol (DAG) and inositol triphosphate (IP 3 ). DAG activates PKC, and IP 3 leads to rapid and sustained increases in cytoplasmic calcium. The increase in free calcium activates the calmodulin-dependent phosphatase calcineurin. These events also lead to the induction of DNA-binding proteins and the transcription of numerous genes, including IL-2 and the IL-2 receptor required for T-cell proliferation.
Understanding the pathways leading to T-cell activation has led to the discovery of molecular defects in several acquired immunodeficiency diseases and may ultimately help provide therapeutic strategies to correct these deficiencies ( ; ; ). For example, mutations in the PTK ZAP-70 have been reported and are associated with the autosomal form of severe combined immunodeficiency (SCID) syndrome in humans ( ). Mutations in the common γ chain of the interleukin receptors IL-2, IL-4, IL-7, IL-9, and IL-15 lead to transduction abnormalities and are associated with the X-linked form of SCID ( ). It is interesting to note that another form of autosomally inherited SCID is associated with mutations in the downstream Janus family protein tyrosine Jak3, the only signaling molecule associated with the common γ chain ( ). As an increasing number of the underlying abnormalities leading to T-cell immunodeficiency are discovered, including at least 10 different molecular defects for SCID alone ( ; ), it has been proposed that these disorders be classified using a comprehensive system that would identify the disorders according to abnormalities in differentiation, maturation, and function ( ). These designations would begin to focus on the actual physiologic or biochemical defect and may ultimately provide new options for therapy, including gene therapy ( ; ; ; ).
Recently, the designation of T-cell type I versus type II cytokine responses has been preferred for those T-cell responses that lead to cytokine secretion patterns known to be involved in cellular immunity versus cytokine secretion patterns observed in humoral immunity, respectively. Type I responses are characterized by the secretion of cytokines known to enhance inflammation (proinflammatory) and induce activation and proliferation of T cells and monocytes, namely, IL-2, IFN-γ, and IL-12. Type II responses are characterized by the secretion of cytokines that suppress inflammation (anti-inflammatory) and stimulate B cells to divide and differentiate into immunoglobulin-secreting cells (i.e., IL-4, IL-5, IL-10, and IL-13). Evidence suggests that secretion of type I cytokines regulates the secretion of type II cytokines and vice versa ( ). For example, in the presence of IL-4 both in vivo ( ) and in vitro ( ; ), T cells will not develop into IFN-γ–secreting cells (i.e., this environment favors the development of a humoral immune response). It has been suggested that the relative amounts of IL-4 and IL-12 that are present during stimulation of naïve T cells will shift the response one way or the other ( ).
Several factors are involved in regulating the type of T-cell response that ensues following antigenic stimulation. In addition to the cytokine environment, evidence suggests that the dose of antigen influences the type of response ( ; ). The predominant response that develops following T-cell activation has significant clinical implications. It has been postulated that the development of a type I response to HIV infection may lead to protective immunity ( ). Clearly, a type II response is not protective, as most infected persons seroconvert and eventually succumb to profound immunosuppression. , argue that repeated exposure to low-dose HIV-1 may lead to protective type I cellular immunity. Results reported by this group indicate that between 39% and 75% of peripheral blood mononuclear cells of HIV-1–seronegative and polymerase chain reaction–negative high-risk individuals (i.e., men who have sex with men, intravenous drug users, and infants born to HIV-1–positive mothers) secreted IL-2 in response to the env protein in vitro. These scientists propose that seronegative high-risk individuals develop protective cell-mediated immunity as a result of low-dose immunization or infection.
Antibody-producing B cells govern the humoral immune response to contain and eliminate primarily extracellular pathogens and, to a lesser extent, intracellular threats. Initiation of B-cell activation involves initial antigen binding and requires costimulatory signals. These signals, in some cases—as in nonpeptide antigens, for example—may be delivered by the antigen itself, by TLRs, by surface immunoglobulin cross-linking, or by other mechanisms ( ). Alternatively, the Th2 subset of CD4 + helper T cells provides important costimulatory triggers to activate B cells in response to peptide antigens ( Fig. 46.2 ).
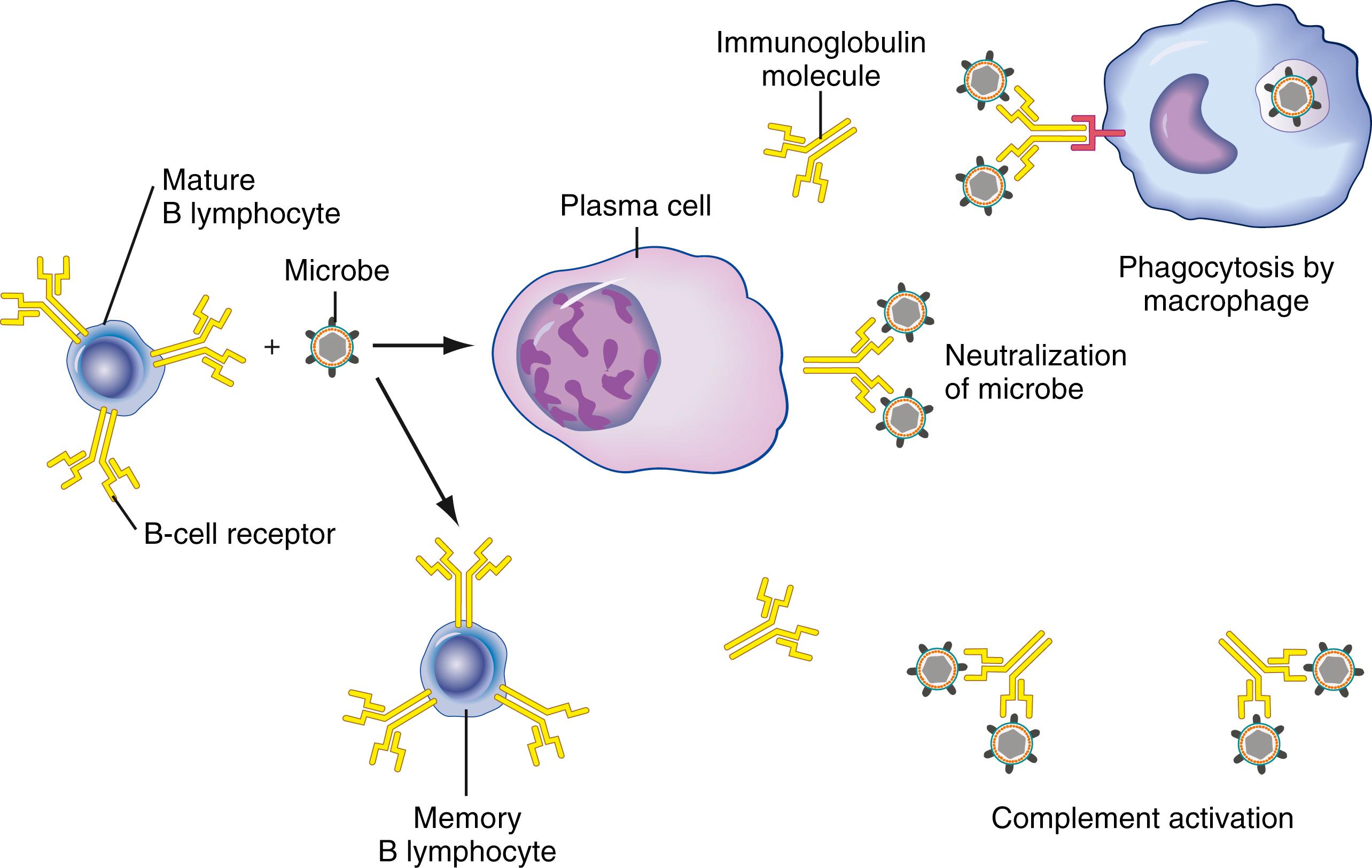
Surface immunoglobulin on naïve B cells binds antigen and transmits intracellular signals. It also delivers antigen into the cell, where it is processed into peptide fragments and then distributed and bound to MHC class II surface receptors. Epitopes of these fragments are recognized by specific Th2 class helper T cells primed by related antigen exposure, and costimulatory signals are transmitted between CD40-ligand (CD154) on T cells and CD40 receptor molecules on B cells ( ). These interactions generate T-cell cytoskeletal changes and subsequent release of cytokines, including IL-4, which binds to B cells to favor cell cycle progression and, hence, clonal expansion, and also promotes isotype switching to immunoglobulin (Ig) G1 and IgE. These engaged T cells also release transforming growth factor (TGF)-β, which has been shown to induce isotype switching to IgG2B and IgA, along with IL-5 and IL-6, which promote B-cell differentiation into plasma cells ( ). These cellular interactions occur in the T cell/B cell zone borders of primary lymphoid organs. Differentiation into effectors/plasma cells with antibody secretion appears to be sentinel for the primary/early focus of humoral immune response. Some activated B cells migrate into germinal centers and undergo further enhancement—including affinity maturation, IgH variable gene alteration, and isotype switching—to provide what is thought of as a more durable, prolonged response. A subset of memory cells is also produced that provide low-level surveillance with an associated rapid amnestic response upon antigen reexposure.
Since its development 3 decades ago, laser-based single-cell analysis (flow and image cytometry) has become an essential tool for the medical research laboratory and the standard of laboratory clinical practice in the study of the cellular immune response ( ; ; ; ; ; ; ; ). Measures of immune competence and immune modulation of specific surface markers and receptors, characterization of lineage by the immunophenotyping of hematolymphoid neoplasms, detection of minimal residual disease, monitoring patients receiving organ transplantation, and immunotherapy are analyses that are commonly performed in many laboratories, as detailed by many authors ( ; ; ; ; ). Classification of cell types by these means enables the definition of biological and effector functions on a molecular basis and allows these measures to be related to disease process and definition. These are but a few of the applications that are possible when laser-based technologies are applied.
Two major technologies are used. The first is termed flow-through , in which the particles that are being counted and their physical and chemical characteristics are measured by particles passing in a fluid stream in a single-cell suspension ( ; ; ). In the second, known as static analysis , the particles are stationary and the stage or the laser moves as in image analysis ( ). Image analysis technology is slowly making its mark in the laboratory in the evaluation of touch preparations or cytospins, chromosome preparations, and tissue sections for certain applications, such as DNA and fluorescent in situ hybridization (FISH). Advances in recent years in the availability of fluorescent probes for use in FISH and chromosome painting will make these analyses more relevant and useful tools in the clinical laboratory ( ; ). As with many techniques, including cell cycle analysis in DNA, each of the new markers and new techniques must be defined, evaluated, and correlated with patient outcomes before agreeing on absolute clinical utility. There is great interest in instrumentation that combines the strengths of flow cytometry and the static advantages of image analysis. These instruments, known as scanning laser cytometers, use the traditional flow cytometry measures of forward scatter, side scatter, and fluorescence on cells in suspension or fixed onto a glass slide. Experience with these systems will reveal whether this approach offers measurement advantages not available with the current flow cytometric and image system configurations ( ; ; ; ). Scanning image cytometers and image analysis are beyond the scope of this chapter.
Combined advances in electronic pulse processing, optics, and data storage, along with advances in computer technology and software, have allowed flow cytometry technology to become a routine tool in the clinical and research laboratory. Furthermore, the wide availability of workshop-clustered monoclonal antibodies now numbering 371 (Engel, 2015), labeled in multicolor, directly conjugated, and in premixed formats, has allowed the simultaneous detection of multiple surface antigens, as well as cytoplasmic and nuclear constituents. The ability to perform multiparameter analysis is the greatest strength of flow cytometry ( ; ). Measurement of both phenotypic and intracellular markers is now done routinely in many laboratories. The major manufacturers have turned the art of flow cytometry into a routine laboratory measurement—a “black box” science—much to the dismay of many ( ). As described, this black box approach phenomenon is largely the result of the use of flow cytometry in the phenotyping of T-cell subsets in monitoring patients with HIV ( ; ). Before the onset of the HIV epidemic, flow cytometry was used in the clinical laboratory primarily for the characterization of leukemias and other hematologic malignancies, and for DNA analysis of tumors for synthesis phase (S phase) and DNA index (DI). Although flow cytometry technology may be trending more toward a black box application, the HIV epidemic brought the power of the flow cytometer to a much larger number of institutions and laboratories. This has allowed the technology to become an integral part of many diagnoses and to be used as an important adjunct in the treatment of patients ( ). Despite its simplification, many issues with regard to U.S. Food and Drug Administration (FDA) regulation, proficiency testing, data management, and reproducibility of data remain. Although all current flow cytometers can adequately perform routine immunophenotyping and other assays, most laboratory problems are related to the nonavailability of standard quality control reagents and calibrators, and the lack of reliable, rapid methods for data transfer and storage that are compatible with laboratory information systems. More important for clinicians is an understanding of the technology, along with its strengths and pitfalls, and how it can be used for quality control and quality assurance, specimen preparation, and data interpretation. Some of these issues are discussed later, although it is not the purview of this chapter to describe all nuances of flow cytometric analysis.
See Chapter 35 for a complete description of flow cytometry.
Today’s clinical flow cytometer is rarely used for cell separation (i.e., cell sorting). This property continues to be associated with research instruments in the highly specialized laboratory. Most multifaceted clinical flow cytometers incorporate 1 to 3 lasers with 4 or more photomultiplier tubes to perform 5- to 10-color immunophenotypic analysis ( Fig. 46.3 ) ( ; ). The most advanced research flow cytometers on the market currently have 5 or 6 diode or solid-state lasers, including ultraviolet (355 nm), near-UV (372 nm), violet (405 nm), blue (488 nm), green (532 nm), olive (552), yellow-green (561 nm), and red (633 nm) and infrared (805 nm), with up to 64 fluorescence detectors and the ability to resolve up to 40 colors. Commercial manufacturers of flow cytometers and cell sorters include ACEA Biosciences, Inc. (San Diego, CA), Apogee Flow Systems (Hertfordshire, UK), Becton Dickinson (San Jose, CA), Beckman Coulter (Fullerton, CA), Bio-Rad Laboratories (Hercules, CA), CompuCyte Corp. (Westwood, MA), Cytek (Fremont, CA), MilliporeSigma (Billerica, MA), Fluid Imaging Technologies, Inc. (Yarmouth, ME), Luminex Corporation (Austin, TX), Miltenyi Biotec (Bergisch Gladbach, Germany), ORFLO Technologies, LLC (Ketchum, ID), Sony Biotechnology, Inc. (Champlain, IL), Stratedigm Corporation (San Jose, CA), Sysmex Partec GmbH (Munster, Germany), ThermoFisher Scientific (Waltham, MA), and Union Biometrica, Inc. (Holliston, MA). Analytic software is provided by the instrument manufacturers, as well as by several independent software companies, including Applied Cytometry (Sheffield, UK), CyFlo Ltd. (Turku, Finland), Cytognos S.L. (Santa Marta de Tormes, Spain), De Novo Software (Los Angeles, CA), eBioscience, Inc. (San Diego, CA), Tree Star, Inc. (Ashland, OR), Verity Software House (Topsham, ME), and Walter and Eliza Hall Institute of Medical Research (Melbourne, Australia).
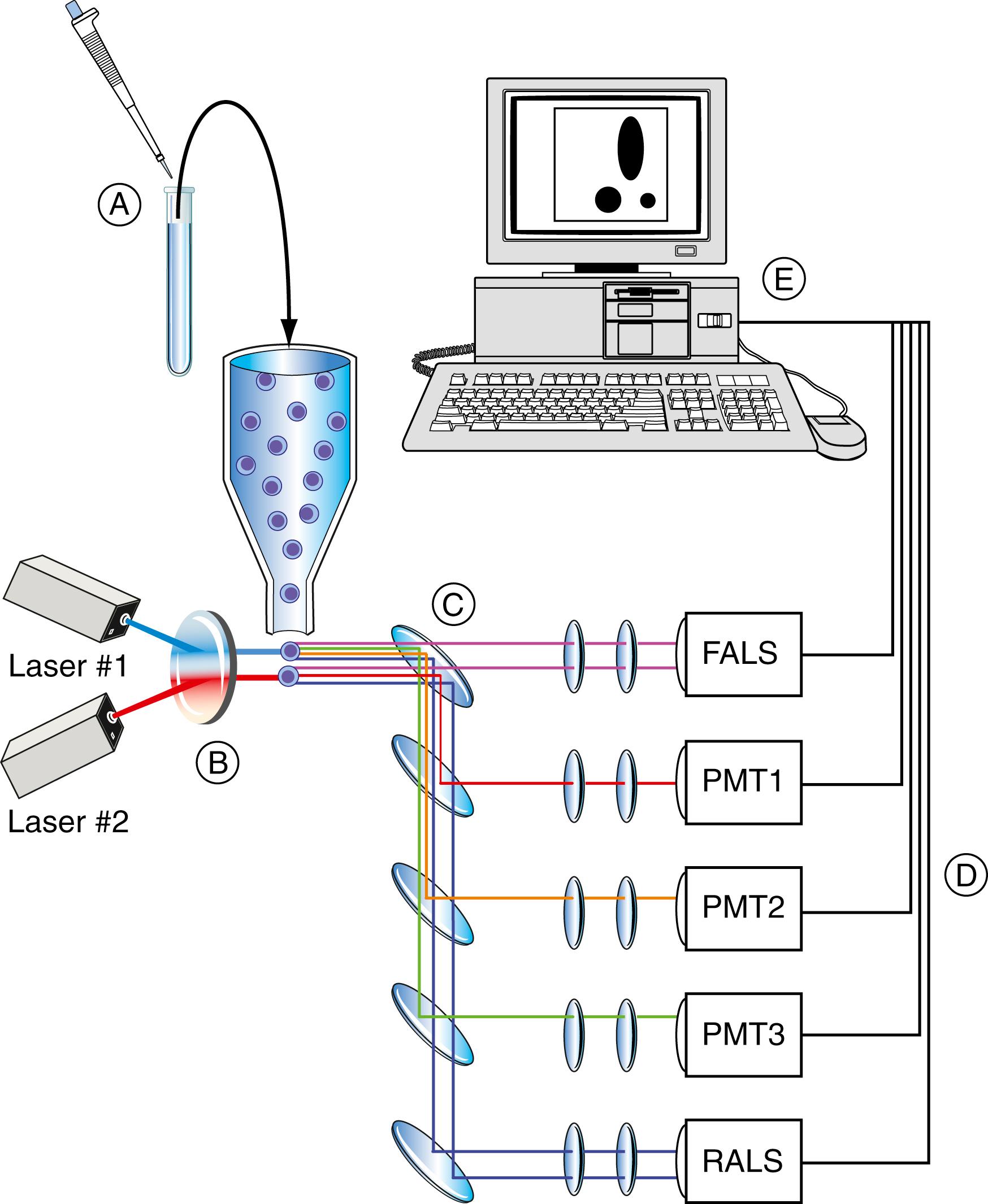
Two common flow cells are used in the clinical laboratory, but because most clinical instruments are used in the measurement of CD4 cells in the monitoring of HIV disease, a closed system is used as a biohazard precaution ( ; ; ). The first, known as a stream-in-air or flow-in-air flow cell , allows the optical measurement point to be directly on the sample stream. This type of flow cell minimizes the distance between the flow chamber and the sample injector tip, thus minimizing carryover between specimens and the sample wash time necessary between samples. This chamber allows greater sample flow rate variability than a closed system. Other advantages of the stream-in-air tips are important in cell sorting and are not considered here. With the closed system, often referred to as a quartz tip flow cell , the focal point is within the chamber. Disadvantages of these quartz systems include the thickness of the quartz and, thus, the diffraction of the laser beam or the scattering of the signal. Additionally, the relatively large cross-section (200 μm 2 ) makes the flow rate more difficult to control. The success of these quartz flow cells in the clinical system depends on illumination and collection optics. Flow cytometric manufacturers have made many advances in these systems regarding both safety and maximum sensitivity with the provision of low-power, laser-based systems currently using diode or solid-state lasers ( ; ; ).
Many terms are used in the definition of flow cytometry systems, which include flow rate , sheath pressure , core size , resulting particle velocity , resulting coefficients of variation ( CVs ), and so forth. However, the most important factor for a laboratory worker to understand is that in DNA analysis, the cells are analyzed at a slow flow rate to increase the time a particle spends in the beam, allowing greater sensitivity and better CVs. In immunophenotyping, sensitivity typically is not an issue, and the particle flow rate can be increased. Most clinical systems were developed with compromises to accommodate the most common application of immunophenotypic analysis ( ). Research flow cytometers offer much greater flexibility and operator control over sample flow rate, differential pressure, and time.
Fluorescent probes (fluorochromes) are sensitive and versatile compounds that have been extensively utilized in the biological sciences since their introduction by Weber and Lawrence in the early 1950s. There are many techniques for their measurement, and some fluorescent probes can be coupled to other excited states or chemical species by energy transfer or chemical reactions. Information derived from fluorochromes includes the intensity and/or polarization of the emitted light, the fluorescence emission and excitation spectrum, the time dependence of the emitted light, and energy transfer between fluorescent probes.
Fluorochromes are most commonly used clinically for the detection and quantitation of cell surface and intracellular antigens ( Table 46.1 ). However, fluorescence emission can also reveal much information about the cellular environment since the chemical and biological properties of a fluorochrome are not only influenced by their chemical composition but also by the physical and chemical environment of the fluorochrome, along with a variety of other factors.
| Fluorochrome | Ex, nm | Em, nm | Excitation laser lines, nm | Comment |
|---|---|---|---|---|
| Hoechst 33342 | 343 | 461 | 355 | Nucleic acid probe, AT selective |
| DAPI | 359 | 461 | 355 | Nucleic acid probe, AT selective |
| Pacific Blue | 410 | 455 | 360, 405, 407 | |
| Pacific Orange | 400 | 551 | 360, 405, 407 | |
| CFSE | 517 | 488 | ||
| Fluorescein isothiocyanate (FITC) | 493 | 525 | 488 | pH sensitive |
| Alexa Fluor 488 | 495 | 519 | 488 | Good photostability |
| Acridine Orange (AO) | 510 | 530 | 488 | Nucleic acid probe |
| R-Phycoerythrin (PE) | 496, 565 | 575 | 488, 532, 561 | High quantum yield, poor photostability |
| PE/Texas Red (Red 613) | 496, 565 | 613 | 488, 532, 561 | |
| Propidium iodide (PI) | 305, 540 | 620 | 325, 360, 488 | Nucleic acid probe, DNA intercalating, used as viability dye |
| Thiazole orange (TO) | 510 | 530 | 488 | Nucleic acid probe |
| 7-Amino actinomycin D (7-ADD) | 546 | 647 | 488 | Nucleic acid probe, GC selective, used as viability dye |
| PE/Cy5 conjugates | 496, 565 | 670 | 488 | Tandem dyes, Cychrome, R670, Tri-Color, Quantum Red |
| Peridinin chlorophyll protein (PerCP) | 482 | 675 | 488 | |
| PerCP/Cy 5.5 | 482 | 690 | 488 | Tandem dye |
| PE/Cy 5.5 | 496, 565 | 695 | 488 | Tandem dye |
| PE/Cy 7 | 496, 565 | 774 | 488 | Tandem dye |
| Texas Red | 589 | 615 | 595, 633 | Sulfonyl chloride |
| Allophycocyanin (APC) | 645 | 660 | 595, 633, 635, 647 | |
| Alexa Fluor 647 | 650 | 668 | 595, 633, 635, 647 | |
| APC/Cy7 (PharRed) | 650, 755 | 785 | 595, 633, 635, 647 |
There are no perfect fluorochromes. However, the properties of an “ideal” fluorochrome include a large extinction coefficient in the excitation wavelength, a high quantum yield, an optimal excitation wavelength that avoids autofluorescence from naturally occurring compounds, photostability, biological inertness, minimal “quenching,” and minimal pH sensitivity.
The influence of their chemical structure on the properties of chromophores has led to studies of many organic compounds as fluorochromes and even to the synthesis of specific fluorochromes for some applications. The existing biological dyes and even dyes utilized in the textile or other industries have been targets of these investigations. Although most commonly used as labels for monoclonal antibodies in the clinical laboratory, chromophores are also useful in research studies for the quantitation of cellular properties or biological activities, since they can be affected by the intracellular environment or act as substrates in intracellular biochemical reactions. The utilization of a compound as a fluorogenic substrate requires that it undergo a change in fluorescent properties with a change in the biological property or substance being studied. The present fluorochromes used in clinical applications can be classified as single, tandem, and protein.
Single fluorescent dyes are chemical substances with fluorescence properties that can be complexed to CD antigens and other biological molecules. Fluorescein isothiocyanate (FITC) was the first fluorescent dye, synthesized by Robert Seiwald and Joseph Burckhalter in 1958. FITC is a derivative of fluorescein, with an isothiocyanate reactive group (−N = C = S), replacing a hydrogen atom. FITC is reactive toward nucleophiles, including amine and sulfhydryl groups on proteins, and has achieved wide-ranging applications in the biological and medical sciences, including flow cytometry. FITC has peak excitation and emission wavelengths of approximately 495 nm and 519 nm, giving the emission a green color. Although FITC has a tendency toward photobleaching or losing its fluorescent properties when continually exposed to light, modern fluorescein derivatives, including Alexa 488 and DyLight 488, have greater photostability and higher fluorescence intensity.
Phycoerythrin (PE) and allophycocyanin (APC) are members of the phycobiliprotein family present in organisms such as algae and cyanobacteria, where they are a component of the photosynthetic system. Structurally, phycobiliproteins consist of a protein complex covalently bound to photosynthetic pigments termed phycobilins . PE is maximally excited at 565 nm and emits light in the yellow-orange region of the visible spectrum (573 nm). PE is among the brightest fluorophores available for flow cytometry. APC is maximally excited at 652 nm and emits red light at 658 nm. APC has a lower extinction coefficient and quantum yield and is not as bright as PE. Other single dyes include Alexa Fluor 405, Pacific Blue, Texas Red, and PerCP.
A tandem dye consists of covalently attached fluorescent molecules. One of these molecules, the donor, is a protein-based (i.e., PE, APC) or synthetic dye with a large extinction coefficient, while the acceptor fluorochrome is a smaller synthetic dye, such as Cy5, Cy5.5, Cy7, Alexa Fluor 647, and Alexa Fluor 705. The acceptor fluorochrome is activated by energy received from the donor, a process termed Förster resonance energy transfer ( FRET ). The use of multiple different tandem dyes in combination with single fluorochromes permit many labeled monoclonal antibodies to be analyzed with a single laser wavelength. Most tandem dyes are designed for multicolor flow cytometry using the 488-nm and 640-nm lasers available in nearly all clinical flow cytometers. Peridinin chlorophyll protein (PerCP)-Cy5.5 and PE-Texas Red are common tandem dyes that use the 488-nm wavelength.
Fluorescent proteins, including green fluorescent protein (GFP), mCherry, and yellow fluorescent protein, enter cells without permeabilization of the cell membrane, and are used to study intracellular substances.
The use of multicolor fluorescence has allowed the concept of multiparameter analysis to become a reality in most laboratories ( Fig. 46.4 ). For example, parameters can evaluate (1) different functional subsets of a particular cell population using an intracellular fluorescent probe; (2) use of multiple colors to identify small clusters of otherwise unidentifiable events (as in detection of minimal residual disease [MRD]); (3) the activation status of cells at a particular disease stage (e.g., use of HLA-DR and CD38 on CD4 and CD8s in HIV staging using an anchor gate approach); and (4) cell surface expression and the DI or S phase of a particular cell population, as in defining the CD19 S phase in acute leukemia. Obviously, the possible combinations are nearly infinite and their sophistication is enhanced by the specific dyes and DNA/ribonucleic acid (RNA) probes that are now available. A discussion of some of these approaches and techniques follows.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here