Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Five different classes of antibody are known—immunoglobulin (Ig) M, IgG, IgA, IgD, and IgE—each with a distinct heavy chain.
Two different light-chain types have been identified: κ and λ.
Antigens react with antibodies at the Fab region, which contains variable regions of both heavy and light chains.
The Fc region on the heavy chains determines what other proteins will bind to the antibody (e.g., complement).
IgG is prevalent in blood and tissue fluids; IgM is found mainly in the blood; secretory IgA is found primarily on epithelial surfaces; IgD is bound mostly to B cells; and IgE is bound largely to basophils and mast cells.
Polyclonal increases in immunoglobulins occur as part of the immune response and may be found in chronic disease.
Monoclonal increases in immunoglobulins suggest a plasma cell dyscrasia.
Diagnosis of disorders of immunoglobulins is aided by their quantitative measurement, in addition to qualitative measures of their clonality by immunofixation electrophoresis and by determination of free light-chain concentrations.
Cryoglobulins can arise as excess monoclonal immunoglobulin (type I), which causes hyperviscosity, or as an IgM autoantibody against IgG (types II and III), which forms circulating immune complexes and causes vasculitis.
Oligoclonal bands of immunoglobulin in cerebrospinal fluid suggest autoimmune disorders, such as demyelinating disease or infection.
Antibodies are the effector molecules of humoral (B cell–mediated) immunity. They are immunoglobulins that react specifically and bind with the antigens that stimulated their production. By mass, immunoglobulins make up about 20% of plasma proteins in healthy individuals. Antibody activity is associated with the slowest migrating proteins on electrophoresis: the γ-globulins. The focus of this chapter is to discuss the general structural and functional properties of immunoglobulins, their laboratory evaluation, and their clinical significance.
The molecular structure of antibodies has been well elucidated ( ; ; ). An immunoglobulin (Ig) molecule is a Y-shaped glycoprotein. It has two identical antigen-binding sites at the tips of the Y (Fab region) and binding sites for complement components and/or various cell surface receptors on the tail of the Y (Fc region). Each immunoglobulin molecule is composed of two identical heavy (H) chains and two identical light (L) chains. These polypeptide chains are held together by noncovalent interactions that are stabilized by disulfide bonds. Parts of both the H and L chains form the antigen-binding sites. Each immunoglobulin L and H chain consists of a variable region of about 110 amino acid residues at its amino-terminal end (the tips of the Y), which forms the antigen-binding site, linked to a constant region. The H chain constant region is three or four times larger than that of the L chain. Each chain is composed of repeating, similarly folded domains: An L chain has one variable region (VL) and one constant region (CL) domain, whereas an H chain has one variable region (VH) and three or four constant region (CH) domains ( Fig. 47.1 ). The amino acid sequence variation in the variable regions of both L and H chains is, for the most part, confined to three small hypervariable regions that come together at the amino-terminal end of the molecule to form the antigen-binding site. Each antigen-binding site is only large enough to bind an antigenic determinant the size of five or six sugar residues.
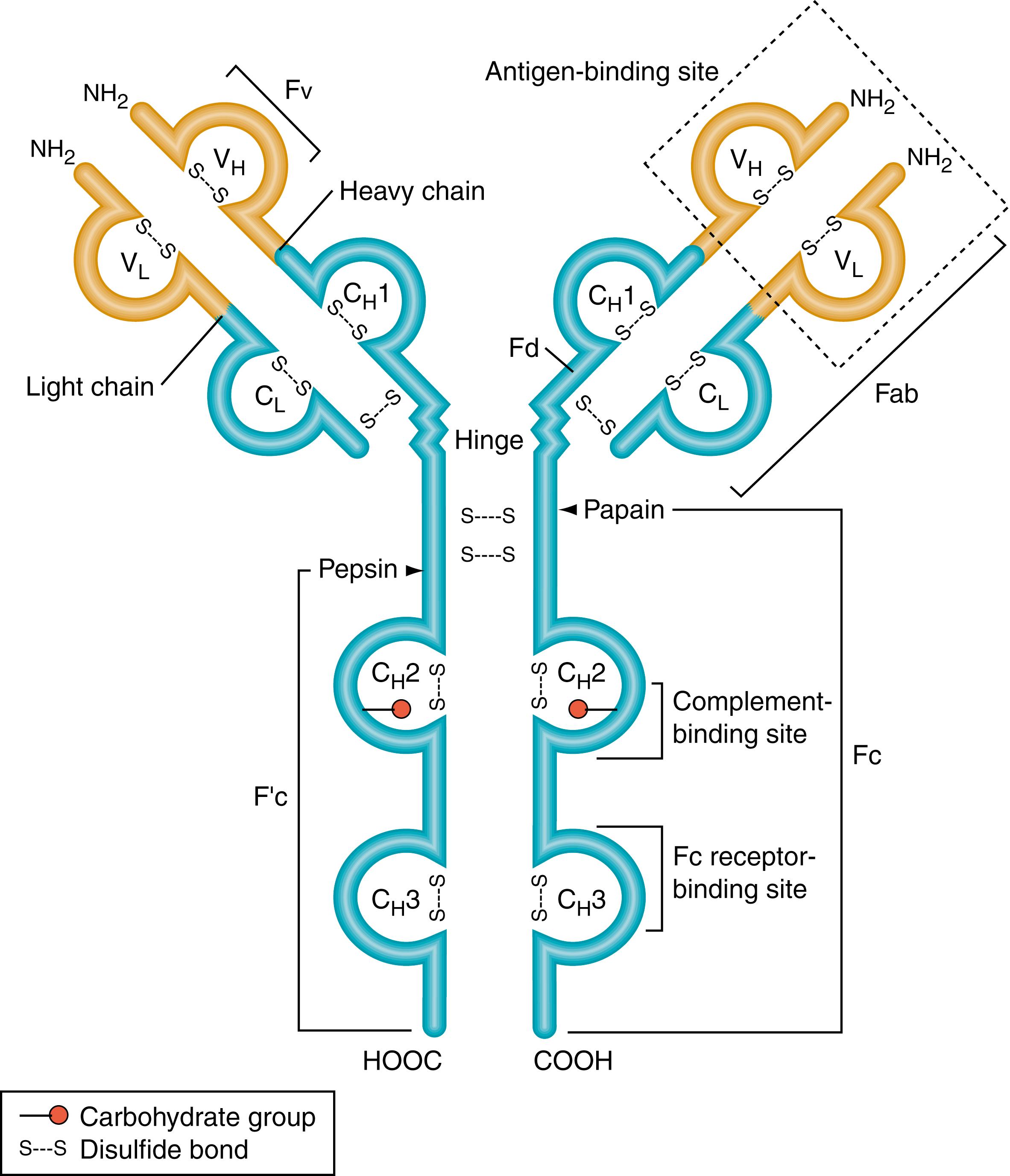
Five different heavy-chain isotypes (γ, α, μ, δ, ε) and two light-chain isotypes (κ and λ) have been identified, with some of the isotypes having further subtypes. For example, γ has subtypes γ1, γ2, γ3, and γ4. The isotypes are formed as a result of variations in the constant regions of the heavy and light chains. These isotypic designations are the basis for the nomenclature of antibodies. Because the heavy chain alone determines effector functions, immunoglobulins are conveniently referred to by their heavy-chain isotype (class) using an English letter terminology (IgG, IgA, IgM, IgD, and IgE).
Antibodies have two identical antigen-binding sites. An antigen-binding site is made up of amino acids from one H chain class and one L chain class. Thus, the four-chain Y monomer molecules (see Fig. 47.1 ) possess two identical antigen-combining sites and are said to be bivalent . Such antibody molecules can cross-link antigen molecules into a large lattice if the antigen molecules each have three or more antigenic determinants. Once the lattice reaches a certain size, it precipitates out of solution ( ). This cross-linking is physiologically important because it enhances the engulfment of antigen, such as that expressed by bacteria and by phagocytic leukocytes. It is also involved in activation of the complement system. In addition, cross-linking may be required for the triggering of antibody-producing cells (B lymphocytes) by antigen. The efficiency of antigen-binding and cross-linking reactions by antibodies is greatly increased by a flexible hinge region, where the arms of the Y join the tail, allowing the distance between the two antigen-binding sites to vary.
Multivalence affects the avidity with which an antibody can bind certain types of antigens. A particulate antigen (such as a bacterium or virus) has repeating antigenic determinants on its surface. An antibody molecule may interact with a single particle in such a manner that both of its antigen-combining sites are bound to antigenic determinants on that particle rather than to antigenic determinants on two adjacent particles. When this type of binding occurs, the effective energy of interaction or avidity is greatly increased compared with that associated with monovalent attachments to two particles. This mechanism has been shown to be physiologically important in the neutralization of viruses by antibodies of relatively low binding affinity.
The protective effect of immunoglobulins is not due simply to their ability to bind antigen. They engage in a variety of biological activities mediated by the tail of the Y, the Fc region of the molecule. This part of the antibody molecule determines what will happen to the antigen once it is bound. Immunoglobulins with the same antigen-binding capacity can have a variety of different Fc regions and, therefore, different functional properties, such as activating the complement system and attaching to Fc receptors on macrophages, thereby aiding phagocytosis of antigens.
Because of its exposed location and loosely folded structure, the hinge region is readily attacked by various proteolytic enzymes. As the name suggests, the hinge region confers a certain amount of flexibility on the molecule, allowing it to assume the Y-shaped structure. This permits an antibody to become attached to a single particle (e.g., a bacterium) through both of its antigen-combining sites or to stretch out to its full length to join two particles. The unique properties conferred by the hinge region on Ig molecules are related to its rich content of proline and hydrophilic amino acid residues. The inter–H chain disulfide bonds of IgG, IgA, and IgD molecules are located in the hinge region (see Fig. 47.1 ).
The proteolytic enzymes papain and pepsin cleave antibody molecules into different characteristic fragments that lead to an understanding of the structure–function relationship of the protein. Papain cleavage produces two separate and identical Fab (fragment antigen-binding) fragments, each with one antigen-binding site, and one Fc fragment (so-called because in nonhuman primates, it readily crystallizes). On the other hand, pepsin cleavage produces one F(ab′)2 fragment, so-called because it consists of two covalently linked F(ab′) fragments (each slightly larger than a Fab fragment). The rest of the molecule is broken down into smaller fragments of various sizes (see Fig. 47.1 ). Because F(ab′)2 fragments are bivalent, they can still cross-link antigens and form precipitates. This is not true of the univalent Fab fragments. Neither of these fragments (subunits of antibodies) has the other biological properties of intact antibody molecules because they lack the tail (the Fc region) that mediates these properties. However, monovalent Fab is the form of postimmunization monospecific, affinity-purified ovine antibody that is used therapeutically in patients for such applications as removing toxic levels of digoxin (Digibind) or neutralizing crotalid snake venoms (CroFab).
Each B-cell clone makes antibody molecules with a unique antigen-binding site. Initially, the molecules are inserted into the plasma membrane, where they serve as cell surface receptors for antigen. When antigen binds to the membrane-bound antibodies, B cells are activated to multiply, differentiate to a plasma cell, and synthesize a large amount of soluble antibody with the same antigen-binding site, which is secreted into the blood. Humoral antibodies defend humans and animals against infection by inactivating viruses and bacterial toxins via VH/VL domains and by recruiting complement and various cells to kill and ingest invading microorganisms via CL domains in the Fc region of the molecule. The biological properties of the various immunoglobulin domains are summarized in Table 47.1 .
| Domain | Known or Probable Function |
|---|---|
| CH3 |
|
| CH2 |
|
|
|
|
The binding of an antigen to antibody is reversible. The affinity and the number of binding sites contribute to the strength of an antibody–antigen interaction. This reversible binding is a result of many relatively weak noncovalent forces, including hydrophobic and hydrogen bonds, van der Waals forces, and ionic interactions. These weak forces are effective only when the antigen molecule is close enough to allow some of its atoms to fit into complementary recesses (regions) on the antibody surface. The complementary regions of a four-chain antibody unit are its two identical antigen-binding sites, whereas the corresponding region on the antigen is an antigenic determinant. Most antigenic macromolecules have many different antigenic determinants; if two or more of them are identical, the antigen is said to be multivalent .
The affinity of an antibody molecule reflects the tightness of fit of an antigenic determinant to a single antigen-binding site, and it is independent of the number of antigenic sites. However, the total avidity of an antibody for a multivalent antigen, such as a polymer with repeating subunits, is defined as the total binding strength of all of its binding sites together. A typical IgG molecule binds at least 10,000 times more strongly to a multivalent antigen if both antigen-binding sites are engaged than if only one site is involved.
For the same reason, if the affinity of the antigen-binding sites in an IgG and an IgM molecule is the same, the IgM molecule (because it is a pentamer and thus has 10 binding sites) will have much greater avidity for a multivalent antigen than an IgG molecule (which has two binding sites). This difference in avidity is important in view of the fact that antibodies produced early in an immune response usually have much lower affinities than those produced later. The increase in the average affinity of antibodies produced as time passes after immunization is called affinity maturation . This occurs because the antibody response to an antigen is heterogeneous—that is, antibodies with different antigen-combining sites are elicited against the antigen by the responding clones of B lymphocytes. Because of its high total avidity, IgM (the major Ig class produced early in immune responses) can function even when each of its binding sites has only low affinity.
Avidity of an antibody can be assessed by introducing a chaotropic agent such as urea into the assay mixture to break apart low-energy molecular interactions. Under these conditions, high-avidity antibodies remain bound to their antigens, whereas those of low avidity dissociate from their antigens and are not detected. Measurement of IgG antibody avidity is applied clinically for differentiating recent infections from previous infections in cases in which the IgM response is prolonged and, therefore, not useful for this purpose. As an example, West Nile virus infection shows low-avidity IgG antibodies in recent infections (2–43 days after onset of symptoms) but high-avidity IgG antibodies at 6 months or more after onset ( ). Similar use of antibody avidity measurements to distinguish recent/acute from longer-term/chronic infections has been applied to the human immunodeficiency virus (HIV) ( ); hepatitis C virus ( ); toxoplasmosis ( ); human cytomegalovirus ( ); measles, mumps, and rubella ( ); and other infectious agents.
The size of the antigen–antibody complex is determined by the valence of the antigen and the relative concentrations of the antigen and antibody. The antigen–antibody precipitation reaction is based on cross-linking of multivalent antigens by bivalent antibodies. If only one species of antibody (a monoclonal response) is present, molecules with only one antigenic determinant cannot be cross-linked. If an antigen is bivalent, it can form small cyclic complexes or linear chains with antibody, whereas an antigen with three or more antigenic determinants can form large three-dimensional lattices that readily precipitate. However, most antisera elicited against an antigen contain a variety of different antibodies (a polyclonal response) that react with different determinants on the antigen and can cooperate in cross-linking the antigen. By contrast, homogeneous (monoclonal) antibodies can precipitate molecules only if they contain repeating identical antigenic determinants.
Given valence conditions that allow the formation of large aggregates, the size of the antigen–antibody complexes that form depends critically on the relative molar concentrations of the two reactants. If an excess of antigen or antibody is present, large complexes are unlikely to form ( Fig. 47.2 ). This property is crucial to understanding seemingly paradoxical reactions in some immunoassays that depend on antigen–antibody complex formation. For example, a vast excess of antigen can completely saturate all antibody-binding sites, leading to a negative signal (i.e., no agglutination). This phenomenon is sometimes referred to as the prozone effect . The prozone effect remains a potential problem for modern assay systems, including syphilis testing, in which extremely high-titer antibodies can be missed unless the specimen is tested at dilutions ( ) and even in immunofixation electrophoresis (see later discussion, Fig. 47.6E ).
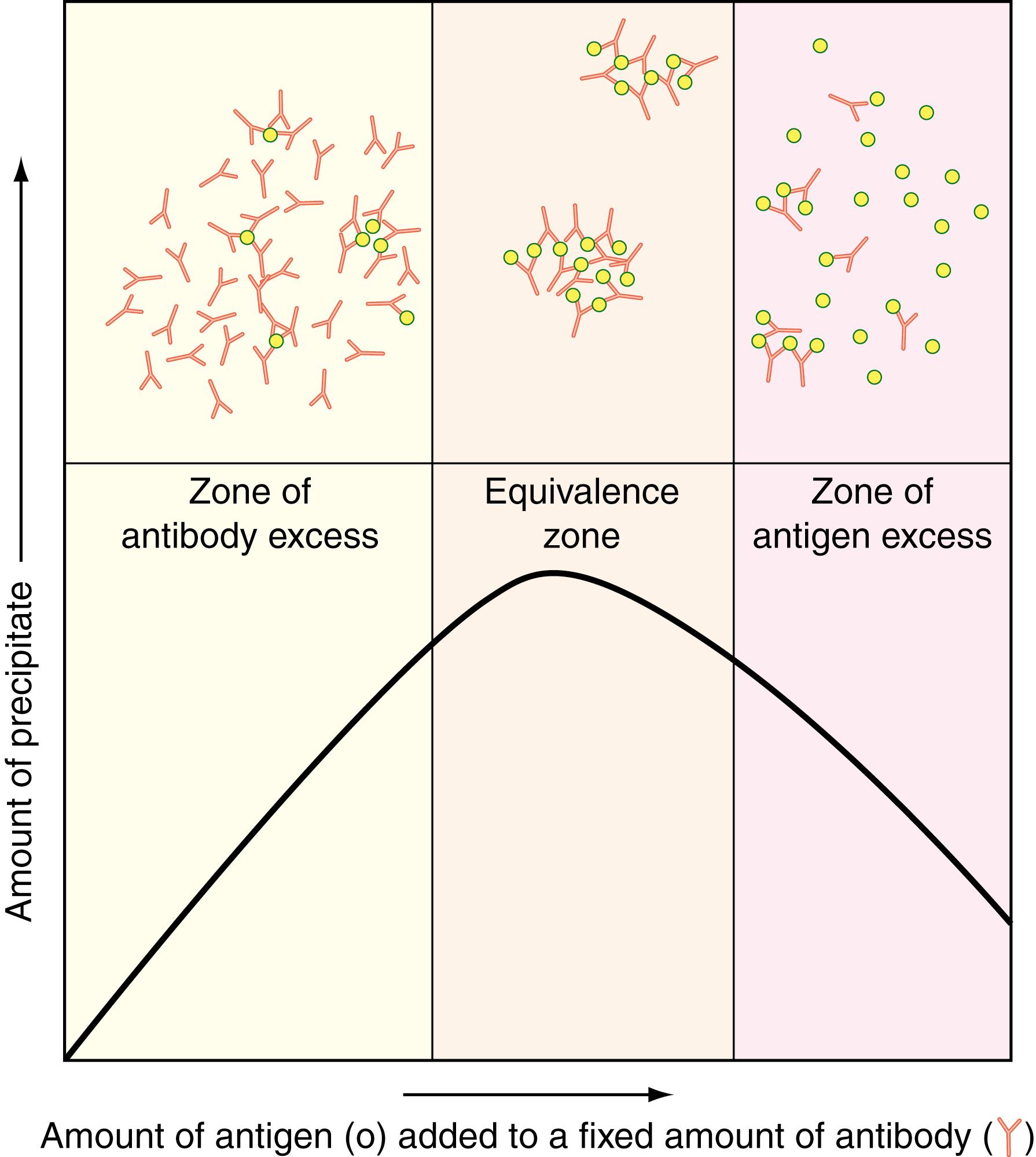
The prozone effect, or hook effect , occurs in many different immunoassays, particularly those based on sandwich formation involving a capture antibody (usually solid phase) that binds antigen plus a second reporter antibody (typically labeled with an enzyme, fluorescent or chemiluminescent dye, or radioactivity) also directed against the antigen. When antigen is present in excess, it can saturate both capture and reporter antibodies, thereby preventing sandwich formation. This situation is analogous in part to the right side of Figure 47.2 , where signal strength diminishes at high antigen concentration. Assay results can be inappropriately low or even nonreactive with exceedingly high antigen concentrations. As the specimen is further diluted and reassayed, the measured value paradoxically increases as antigen concentration falls back into the assay range. This manifestation of the prozone is termed the hook effect after the shape of the dose-response curve, which is directly proportional to antigen concentration in most specimens but reverses and falls off at much higher antigen concentrations. The hook effect occurs in immunoassays in which the antigen concentration can vary over several orders of magnitude. The assay may be configured to measure antigens over only two or three orders of magnitude, but when antigen concentration ranges over 4, 5, or more orders of magnitude, the hook effect will likely occur, giving rise to falsely low results. Immunoassays in which the hook effect can be prominent include those for human chorionic gonadotropin, IgA (especially in IgA myeloma), and immunoglobulin light chains.
The size and composition of antibody–antigen complexes are not only important in influencing precipitation reactions in vitro, but they are crucial in determining the fate of the complexes in the body. Complexes formed at equivalence or in antibody excess have multiple protruding Fc regions and, therefore, bind strongly to Fc receptors in macrophages, which ingest and degrade them. Small complexes, formed in antigen excess, have only one Fc region per complex. Therefore, they bind poorly to Fc receptors on macrophages and are less efficiently destroyed. Instead, they may be deposited in small blood vessels in the skin, kidneys, joints, and brain, where they may activate the complement system, causing inflammation and the destruction of tissue.
Although it appears that antigen–antibody complexes have a rigid “lock-and-key” appearance, antibodies are dynamic entities that undergo structural fluctuations ( ; ). Conformational changes may even be required for binding. These adjustments include side chain shifts of up to 20 to 30 nm, aromatic ring rotations, conformational changes, and even small rotations between VH and VL domains ( ; ; ).
It is estimated that a human is able to make at least 10 6 to 10 9 different antibody molecules. Special genetic mechanisms have evolved to produce the very large number of immunoglobulin molecules that develop in response to antigen stimulation without the need for an excessive number of genes ( ).
Immunoglobulin molecules are produced by three separate gene pools encoding the κ, λ, and H chains, respectively. The gene pools for light and heavy chains reside on different chromosomes. In each pool, separate gene segments that encode for different parts of the variable regions of light and heavy chains can be brought together by site-specific recombination events during B cell differentiation. The light chain gene pools contain one or more constant (C) genes and sets of variable (V) and joining (J) gene segments. The H chain gene pool contains a set of C genes and sets of V, diversity (D), and J gene segments. To make an antibody molecule, a VL gene segment is recombined with a JL gene segment to produce a V gene for the light chain, and a VH gene segment is recombined with D and JH segments to produce a V gene for the heavy chain ( Fig. 47.3 ). Each of the assembled gene segments is then cotranscribed with the appropriate constant region sequence to produce a messenger RNA molecule that encodes for the complete polypeptide chain. By variously combining inherited gene segments encoding for VL and VH regions, vertebrates can make thousands of different light chains and thousands of different H chains that can associate to form millions of different antibody molecules.
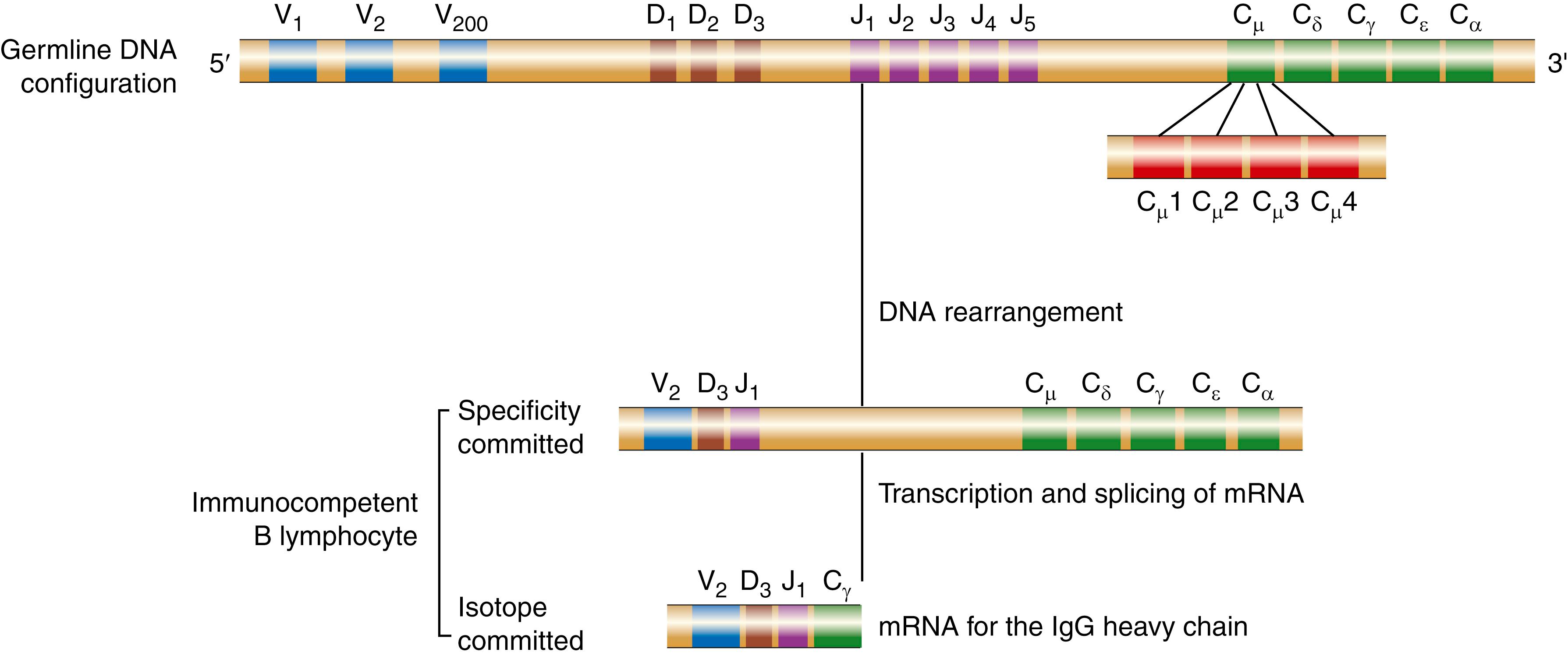
The baseline repertoire of antibody molecules can be expanded still further by somatic hypermutation, which seems to be activated by exposure to antigen. Thus, the selective role of antigen in the presence of somatic mutation appears to lead to fine-tuning of the immune response and to a virtually unlimited diversity of antibody molecules. Somatic point mutations in immunoglobulin genes occur in B cells (not germ cells) during the lifetime of the animal or of humans ( ). The somatic hypermutations are largely confined to the H chain and L chain variable region genes and the introns immediately surrounding them. It is estimated that close to one mutation will occur in the H or L chain V region of an individual cell with each cell division. This not only increases antibody diversity but may cause a change in the affinity with which the antibody binds its ligand. Those emerging B cells that can bind the antigen more avidly have an advantage over other B cells that do not bind the antigen as avidly. As the concentration of antigen falls, those B cells that have more avid receptors dominate the population of responding cells. This results in greater affinity of the antibodies being produced on rechallenge than in the initial response. Thus, this process of somatic hypermutation can result in the presence in immunized individuals of high-affinity antibodies that are much more effective on a weight basis.
All B cells initially make IgM antibodies. Some later switch to make antibodies of other classes (isotypes: IgG, IgA, etc.) that have the same antigen-binding site (idiotype) as the original IgM antibodies ( allelic exclusion ; Table 47.2 ). Such class switching in combination with allelic exclusion allows the same antigen-binding sites (same VH and light chain) to be distributed among antibodies with many different biological properties (secondary effector functions) ( ).
| Type of Variation | Distribution | Variant | Location | Examples |
|---|---|---|---|---|
| Isotypic | All variants present in serum of a normal individual | Classes | C H | IgM, IgE |
| Subclasses | C H | IgA1, IgA2 | ||
| Types | C L | κ, λ | ||
| Subtypes | C L | λOz+, λOz− | ||
| Subgroups | V L , V H | V κI , V κII , V HI , V HII | ||
| Allotypic | Allelic forms not present in all individuals | Allotypes | Mainly C H /C L Occasionally V H /V L |
Gm group (human γ chain; e.g., IgG1, G1m3, G1m17) b 4 , b 5 , b 6 , b 9 (rabbit light chain) |
| Idiotypic | Antigenic individuality specific to each immunoglobulin molecule | Idiotypes | V H /V L | Determinant identified by antibody specific to an individual immunoglobulin |
Thus, the gene organization mechanism permits the assembly of immunoglobulin molecules with a variety of specificities. Antibody diversity depends on the presence of multiple gene segments, their rearrangement into different sequences, the combination of different light and heavy chains in the assembly of immunoglobulin molecules, and somatic mutations.
The various classes of human immunoglobulins and their properties ( ; ) are summarized in Tables 47.3 and 47.4 .
| WHO Designation | IgM | IgG | IgA | IgD | IgE |
|---|---|---|---|---|---|
| Heavy chains | μ | γ | α | δ | ε |
| Heavy chain subclasses | μ 1 , μ 2 | γ 1 , γ 2 , γ 3 , γ 4 | α 1 , α 2 | — | — |
| Light chains | κ or λ | κ or λ | κ or λ | κ or λ | κ or λ |
| Molecular formula | IgM(κ) (2μ2κ) 5 | IgG(κ) 2γ2κ | IgA(κ) (2α2κ) 1-3 | IgD(κ) 2δ2κ | IgE(κ) 2ε3κ |
| IgM(λ) (2μ2λ) 5 | IgG(λ) 2γ2λ | IgA(λ) (1α2λ) 1-3 | IgD(λ) 2δ2λ | IgE(λ) 2ε2λ | |
| IgA(κ) (2α2κ) 2 S † | |||||
| IgA(λ) (2α2λ) 2 S | |||||
| Number of four-chain units per molecule | 5 | 1 | 1–3 | 1 | 1 |
| Heavy-chain molecular weight, kDa | 70 | 50–60 | 55 | 62 | 70 |
| Light-chain molecular weight, kDa | 23 | 23 | 23 | 23 | 23 |
| Sedimentation coefficient, S20W | 18.0–19.0 | 6.7–7.0 | 6.6–14.0 | 6.9–7.0 | 7.9–8.0 |
| Molecular weight, kDa | 900 | 143–160 | 159–447 | 177–185 | 187–200 |
| Electrophoretic mobility | γ1–β1 | γ2–α1 | γ2–β2 | γ1 | γ1 |
| Carbohydrate content, % | 7–14 | 2.2–3 | 7.5–9.0 | 12–13 | 11–12 |
| Heavy-chain allotypes | — | Gm | Am | — | — |
| Light-chain allotypes | Km(κ) ∗ | Km(κ) ∗ | Km(κ) ∗ | Km(κ) ∗ | Km(κ) |
| Valency for antigen binding | 5 (10) | 2 | 2.4 (? polymeric forms) | 2 | 2 |
| Number of domains on the heavy chains | 5 | 4 | 4 | 4 | 5 |
∗ Formerly designated Inv marker.
† Dimer in external secretions carries secretory component -S.
| IgM | IgG | IgA | IgD | IgE | |
|---|---|---|---|---|---|
| Physiologic | |||||
| Normal adult serum concentration, mg/mL | 1.2–4.0 | 8.0–16.0 | 0.4–2.2 | 0.03 | 17–450 ng/mL |
| International units/mL | 69–322 | 92–207 | 54–268 | — | <100 |
| Percentage total immunoglobulin | 13 | 80 | 6 | 1 | 0.002 |
| Intravascular distribution, % | 41 | 48 | 76 | 75 | 51 |
| Synthetic rate, mg/kg/day | 2.2 | 35 | 24 | 0.4 | 0.003 |
| Catabolic rate in serum, % per day (or half-life, days) | 10.6 (5–6) | 6 (18–23) | 24 (5–6.5) | 37 (2.8) | 90 |
| Biological | |||||
| Agglutinating capacity | +4 | ± | +2 | − | − |
| Complement-fixing capacity via classical pathway | +4 | + | − | − | − |
| Homologous anaphylactic hypersensitivity | − | − | − | − | +4 |
| Heterologous guinea pig anaphylaxis | − | + | − | − | − |
| Fixation to homologous mast cells and basophils | − | ± | − | − | +4 |
| Cytophilic binding to macrophages | − | + | ± | − | − |
| Placental transport to fetus | − | + | − | − | − |
| Rheumatoid factor–binding activity | − | + | − | − | − |
| Present in external secretions | ± | + | +4 | − | +2 |
| Other Characteristic Properties | |||||
| IgM—Produced early in immune response, first effective defense against bacteremia | |||||
| IgG—Combats microorganisms and their toxins in extravascular fluids | |||||
| IgA—Defends external body surfaces IgD—Present on lymphocyte surface of immunocompetent cells, important for B-cell activation and/or immunoregulation |
|||||
This glycoprotein is the major class of antibody secreted into the blood in the early stages of a primary antibody response. Normally, the secreted form of IgM is a pentamer composed of five four-chain units, a macroglobulin with a 19S sedimentation rate, and a molecular weight of 900 kDa. However, in human autoimmune disorders, such as systemic lupus erythematosus (SLE), the monomeric 7S form may be detected in appreciable amounts in serum. Selective IgM deficiency is a rare disorder associated with the absence of IgM and normal levels of other immunoglobulin classes. The cause of this disorder is unknown.
Because pentameric IgM has a total of 10 antigen-binding sites, it is more efficient than 7S monomeric IgM or IgG molecules in cross-linking antigen and in activating the complement system when bound to antigen. Thus, this high efficiency in binding and activating complement, coupled with its early appearance during the course of infection, makes IgM a particularly potent agent in combating microbial invasions.
Each IgM pentamer contains one copy of another polypeptide chain, called a J (joining) chain , which has a molecular weight of 15 kDa. This accessory polypeptide is produced by IgM-secreting cells. It is an acidic glycoprotein with a high content of cysteine residues and, thus, is disulfide linked between two adjacent IgM monomeric Fc regions at the carboxy-terminal end. Presumably, oligomerization is initiated at this site.
IgM is also the first class of antibody to be produced by developing B cells. The immediate precursors of B cells, called pre-B cells, make μ chains but not light chains, which accumulate in the cells. Pre-B cells then begin to synthesize light chains, which combine with μ chains to form four-chain monomer IgM molecules. The two μ-chain and two light-chain component is inserted into the plasma membrane, where it functions as a receptor for antigen. At this point, the cells have become B lymphocytes and can respond to antigen.
Perhaps because of its large size, secreted pentamer IgM is not found to any significant extent in tissue spaces; it is confined to the blood circulation and does not cross the placenta. IgM is a minor component of secretory immunoglobulins at mucosal surfaces and in breast milk.
IgM is phylogenetically the most primitive of the immunoglobulins, and most variants of the genes from the μ chain appear to have evolved into heavy chain genes for the other immunoglobulin classes. Additional physical and biological properties of IgM, as well as the other classes of immunoglobulin, are given in Tables 47.3 and 47.4 .
IgG is the best-studied isotype at both structural and functional levels ( ). Antibodies of this class constitute the major immunoglobulin in the blood. They are copiously produced during the secondary immune response. The Fc region of IgG molecules binds to specific receptors on phagocytic cells, such as macrophages and polymorphonuclear leukocytes, thereby increasing the efficiency with which the phagocytic cells can ingest and destroy infecting microorganisms that have become coated with IgG antibodies in response to infection. These receptors on antibodies attached to target cells also guide natural killer (NK) lymphocytes to destroy them through the process of antibody-dependent cell-mediated cytotoxicity (ADCC). The best-known function of IgG is complement activation via the classical cascade. The Fc region of IgG can bind to and thereby activate the first component of the complement system, which unleashes a biochemical attack that kills the microorganisms. At least two molecules of IgG are required for complement activation compared with one molecule of IgM, which has five Fc regions.
IgG molecules are the only antibodies that can pass from mother to fetus. Cells of the placenta that are in contact with maternal blood have receptors that bind the Fc region of IgG molecules and mediate their passage to the fetus. The antibodies first are ingested by receptor-mediated endocytosis and then are transported across the cell and released by exocytosis into the fetal blood. Other classes of antibodies do not bind to these receptors and, therefore, cannot pass across the placenta. The ability of IgG to cross the placenta provides a major line of defense against infection for the first weeks of an infant’s life. Normally, the human fetus begins to receive significant quantities of maternal IgG transplacentally at around 12 weeks’ gestation. The quantity increases steadily until, at birth, cord serum contains a concentration of IgG comparable with that of maternal serum. Barring any immunologic disorders, adult levels of IgG are reached by the seventh year of life and remain relatively constant thereafter.
IgG antibodies have a high diffusion coefficient, which enables them to diffuse into the extravascular body spaces more readily than other Ig classes. IgG, being the predominant immunoglobulin in these spaces, carries the major burden of neutralizing bacterial toxins and of binding microorganisms to enhance their phagocytosis. Furthermore, only IgG antibodies coating target cells, such as tumor cells, can sensitize them for extracellular killing by ADCC; the NK cells responsible for ADCC also possess Fc receptors for IgG.
There are four subclasses of human IgG. These four subclasses reflect the existence of four antigenetically distinct H chains (γ1–γ4), which are similar but not identical in amino acid sequence and general properties. For example, IgG1 is the dominant subclass in adult humans. IgG3 is the most effective binder of complement, followed by IgG1 and IgG2. IgG4 in most cases fails completely to bind complement by the classical pathway. All subclasses except IgG2 have been demonstrated to cross the placenta. A summary of the physical and biological properties of IgG is given in Tables 47.3 and 47.4 and for the subclasses in Table 47.5 .
| IgG1 | IgG2 | IgG3 | IgG4 | |
|---|---|---|---|---|
| Physiologic | ||||
| Percentage distribution of total normal serum IgG | 66 ± 8 | 23 ± 8 | 7.3 ± 3.8 | 4.2 ± 2.6 |
| Synthetic rate, mg/kg/day in serum | 25 | ? | 3.4 | ? |
| Fraction catabolic rate, % per day (half-life, day) | 8 (23) | 6.9 (23) | 16.8 (7) | 6.9 (23) |
| Ratio of κ/λ | 1.4–2.4 | 1.0–1.1 | 1.1–1.3 | 5.0–7.0 |
| Allotypic markers (Gm types) | a, z, f, x | n | bo, bi, bz, g, st, etc. | ? |
| Biological | ||||
| Complement-fixing capacity via classical pathway | +2 | ± | +3 | − |
| Heterologous skin-binding capacity | + | − | + | + |
| Placental transport to fetus | + | ± | + | + |
| Macrophage receptor | + | − | + | − |
| Reaction with protein A | + | + | − | + |
| Dominant Antibody Activities | ||||
| Antitetanus toxoid | +2 | + | + | ± |
| Antidiphtheria toxoid | +2 | + | + | ± |
| Antithyroglobulin | +2 | + | + | ± |
| Anti-DNA | +2 | +2 | ± | ± |
| Anti-Rh | +2 | − | − | ± |
| Anti–factor VIII | − | − | − | + |
| Antidextran | − | + | − | − |
| Antilevan | − | + | − | − |
| Antiteichoic acid | − | + | − | − |
| Number of inter–heavy chain disulfide bonds in hinge region | 2 | 4 | 5 | 2 |
| Position of light–heavy chain disulfide bond on the heavy chain | N214 | N131 | N131 | N131 |
Antibodies of this class constitute the major class of antibody in secretions (milk, saliva, tears, and respiratory and intestinal secretions). It exists as a four-chain monomer (like IgG) or as a dimer of two such monomer units. IgA molecules in secretions are dimers that carry a single J chain, similar to the one associated with pentameric IgM, and an additional glycopolypeptide chain of 70 kDa called secretory component (SC). IgA dimers pick up SC from the surface of the epithelial cells lining the intestine, the bronchi, or the milk, salivary, or tear ducts. SC is synthesized by the epithelial cells and is initially exposed on the nonluminal (external) surface of these cells, where it serves as a receptor for binding dimer IgA. The resulting dimer IgA–receptor complexes are ingested by receptor-mediated endocytosis and are transferred across the epithelial cell cytoplasm in the form of a membrane vesicle, which fuses with the plasma membrane on the luminal side of the epithelial cell. The extramembrane portion of the IgA receptor is then enzymatically cleaved and released as part of the secretory IgA molecule ([α 2 ,L 2 ,] 2 − Jα) into the lumen. The amino-terminus of the dimer IgA receptor remaining attached to dimer IgA is the SC ( Fig. 47.4 ). Thus, the fully assembled dimeric secretory IgA molecule is the synthetic product of two distinct types of cells: plasma cells and epithelial cells. In addition to this transport role, SC may protect the dimer IgA molecules from being digested by proteolytic enzymes in secretions.
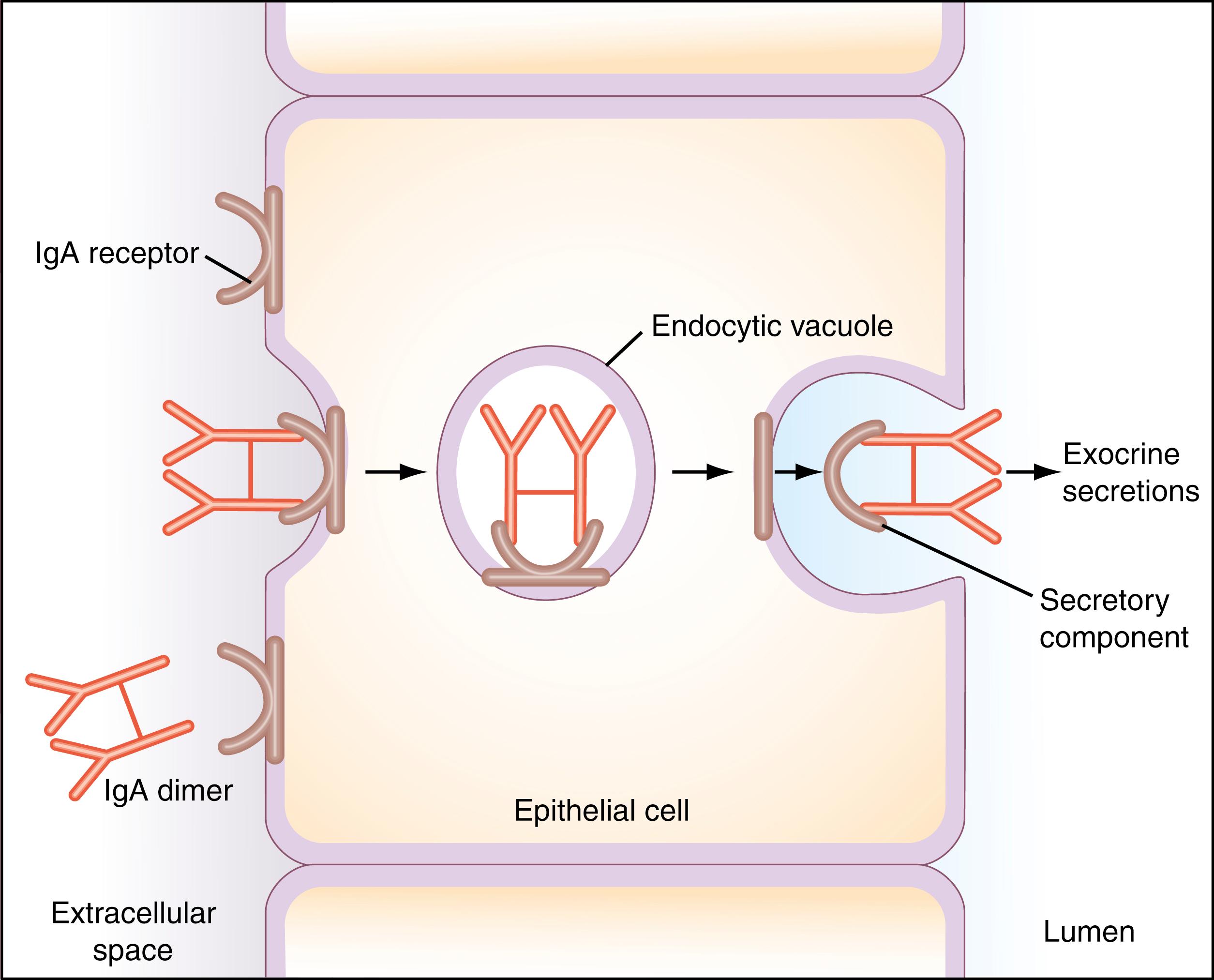
In humans, it has been possible to classify IgA antibodies into two subclasses—IgA1 and IgA2—based on differences in antigenic structure and variation in the arrangement of interchain disulfide bridges. Whereas IgA2 is a minor component of serum IgA, this subclass is the dominant form in secretions. Furthermore, secretory IgA reaches adult levels sooner than serum IgA. The IgA system in the intestinal tract of humans, for example, may be fully developed by 2 years of age, whereas serum IgA levels do not normally reach adult concentrations until 12 years of age.
Because of its presence near external membranes, secretory IgA constitutes a first line of defense against microorganisms in the external environment. It has been postulated that IgA inhibits the adherence of microorganisms to the surface of mucosal cells, thereby preventing their entry into body tissues. One property of secretory IgA that is important in this respect is its multivalence, which is associated with high avidity of binding to antigens, which may be especially relevant in the neutralization of viruses. Antiviral activity by IgA antibodies has been demonstrated in individuals given either of the polio vaccines. Secretory IgA may also combine with certain antigens in food, preventing their absorption into the bloodstream and, thus, reducing the incidence of allergic reactions. For example, IgA immunodeficiencies can lead to increased levels and incidence of humoral antibodies directed against antigens derived from food and intestinal organisms.
IgA possesses the following effective properties: it fixes complement through the alternative pathway; through a specific Fc receptor on macrophages, it can serve as an opsonin for phagocytosis; and it can induce eosinophil degranulation through a specific receptor, which has implicated IgA in antiparasitic responses. The physical and biological properties of IgA are listed in Tables 47.3 and 47.4 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here