Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter provides a practical approach to the laboratory evaluation of hemostatic and thrombotic disorders. Any assessment of hemostatic or thrombotic disorders must start with a thorough history and physical examination. These can provide clues to guide subsequent laboratory testing, diagnosis, and management.
Physiologic hemostasis is a complex interplay of cellular or plasma elements: the adhesion of platelets to damaged endothelium, the aggregation of platelets to form a temporary plug, the successive activation of coagulation factors to form a stabilizing fibrin clot, clot retraction to repair endothelial damage, and finally clot breakdown through fibrinolysis ( Fig. 127.1 ). Commonly available laboratory tests focus on the individual components of hemostasis by testing coagulation proteins, platelets, and fibrinolytic proteins; this chapter is organized into similar components to provide a structured laboratory approach to the patient with a hemostatic or thrombotic problem. Yet the clinician must be mindful that in the body, the components of hemostasis work together to form a product that is more than the sum of its parts.
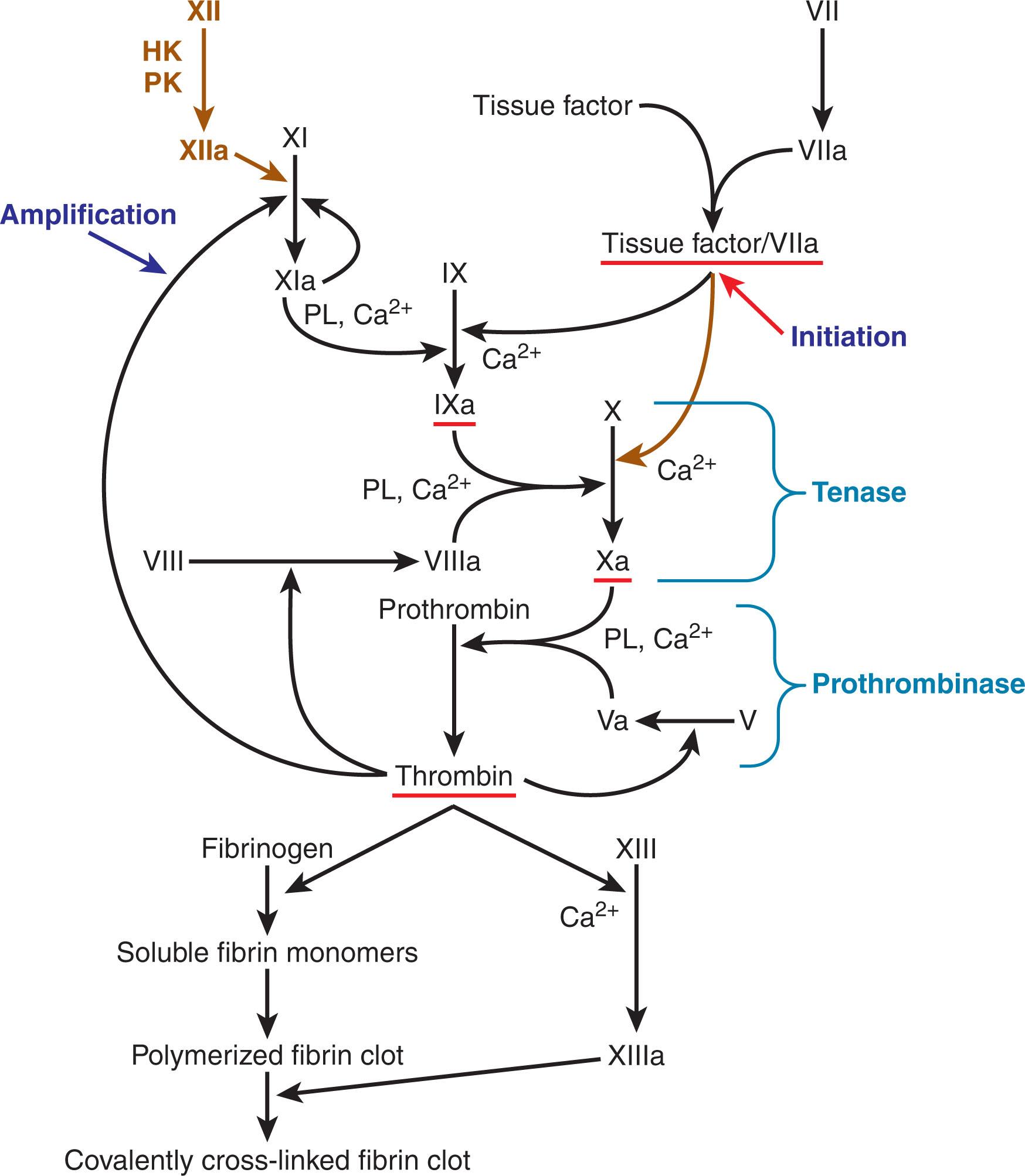
For over half a century, the process of fibrin clot formation has been conceptualized as a “coagulation cascade.” This is based on the waterfall hypothesis of Davie, Ratnoff, and MacFarlane, who almost simultaneously reported a sequence of proteolytic reactions starting with factor XII (Hageman factor) activation by surface contact and ending with thrombin’s proteolysis of fibrinogen to form fibrin. However, upon its introduction, this hypothesis of successive necessary proteolytic reactions already appeared to be too simplistic. Nearly a decade earlier, Ratnoff had identified that deficiencies of factor XII were not associated with bleeding; others soon established that deficiency of factor XII’s cofactors (prekallikrein and high-molecular-weight kininogen) also did not result in a bleeding phenotype. In 1977, Osterud and Rappaport recognized that the factor VIIa/tissue factor complex can activate factor IX to factor IXa. Broze later recognized that this complex cannot directly activate factor X, because of the presence of tissue factor pathway inhibitor (TFPI); factor IX activation is a prerequisite in vivo (see Chapter 124 ). Though deficiency of factor XII and its antecedents is not associated with bleeding, factor XI deficiency is (see Chapter 134 ). In 1991, Gailani and Broze explained this by proposing that factor XI can be activated independently of factor XII, as formed thrombin cycles back to activate factor XI and thus amplifies its own formation. Factor XII, long considered to have no role in coagulation in vivo, has been found to play a role in thrombus formation and angiogenesis. Our understanding of hemostasis has advanced still further in recent years, as blood coagulation research is increasingly performed under flow conditions with cellular elements in vitro and in animal models.
The physiologic “coagulation cascade” now appears to be an intricate system with built-in shortcuts and feedback loops. It is triggered by factor VIIa in complex with tissue factor, and results in the activation of zymogens that become serine proteases. This system functions in concert with platelet activation and fibrinolysis ( Fig. 127.2 ). However, clinical laboratory testing of coagulation proteins is not based on this current understanding—it follows the original Ratnoff-Davie-Macfarlane surface-activated coagulation cascade hypothesis. These tests attempt to mimic in vivo processes by carrying them out in vitro, and thus do not capture the true complexity of physiologic hemostasis. They are still useful in diagnosing coagulation protein deficiencies and important bleeding disorders. However, they can be misleading, as they can also detect abnormalities of questionable clinical significance. The clinician must therefore understand the distinction between the complexity of physiologic hemostasis and the simplistic picture presented by laboratory tests.
As we proceed in reviewing tests for coagulation proteins, we can place them into three technical categories:
Immunologic tests , which include enzyme-linked immunosorbent assays (ELISA), immunoelectrophoresis, and immunoturbidometric (latex immuno-agglutination) tests. These tests are quantitative and detect specific proteins with polyclonal or monoclonal antibodies. Their sensitivity and specificity depend on the antibody used (polyclonal versus monoclonal) and the presence of interfering substances (e.g., rheumatoid factor, human anti-mouse antibody (HAMA), and other autoantibodies).
Chromogenic or amidolytic assays , which measure the activity of the serine proteases of the coagulation system as they react with synthetic peptides. The reaction (and thus the activity of serine protease) can be measured as the synthetic peptide releases a colored dye. Chromogenic assays are affected by the specificity of the peptide substrate. A disadvantage of these assays is that their narrow measure of an enzyme’s activity may not correlate with its biologic activity in vivo or its activity in clot-based assays.
Clot-based or coagulation assays are functional and compare the clotting potential of a patient’s plasma with control plasma with a known clotting potential. These tests are more difficult and time-consuming to perform than the others, and are susceptible to interference from other factors, such as anticoagulant effect and hemolysis-icteric-lipemic (HIL) interferences. However, they most closely approximate in vivo hemostasis.
The three assays most often used to screen for coagulation protein defects are: (1) the activated partial thromboplastin time (APTT), induced in vitro by surface-activation of prekallikrein, high-molecular-weight kininogen, and factor XII; (2) the prothrombin time (PT), induced in vitro by the addition of excess tissue factor; and (3) the thrombin clotting time (TCT), a test of fibrinogen integrity and thrombin inhibition. The PT, APTT, and TCT are clot-based assays that measure the rate of clot formation and are based on the waterfall hypothesis of coagulation. They trigger a sequence of proteolytic reactions in the intrinsic, extrinsic, and common pathways ( Fig. 127.3 ). The reactions culminate in the proteolysis of fibrinogen to form a fibrin clot, which causes soluble proteins to precipitate in the patient’s plasma sample. These soluble proteins can be detected by either increased electric impedance or decreased optical clarity, depending on the instrumentation used to measure the result.
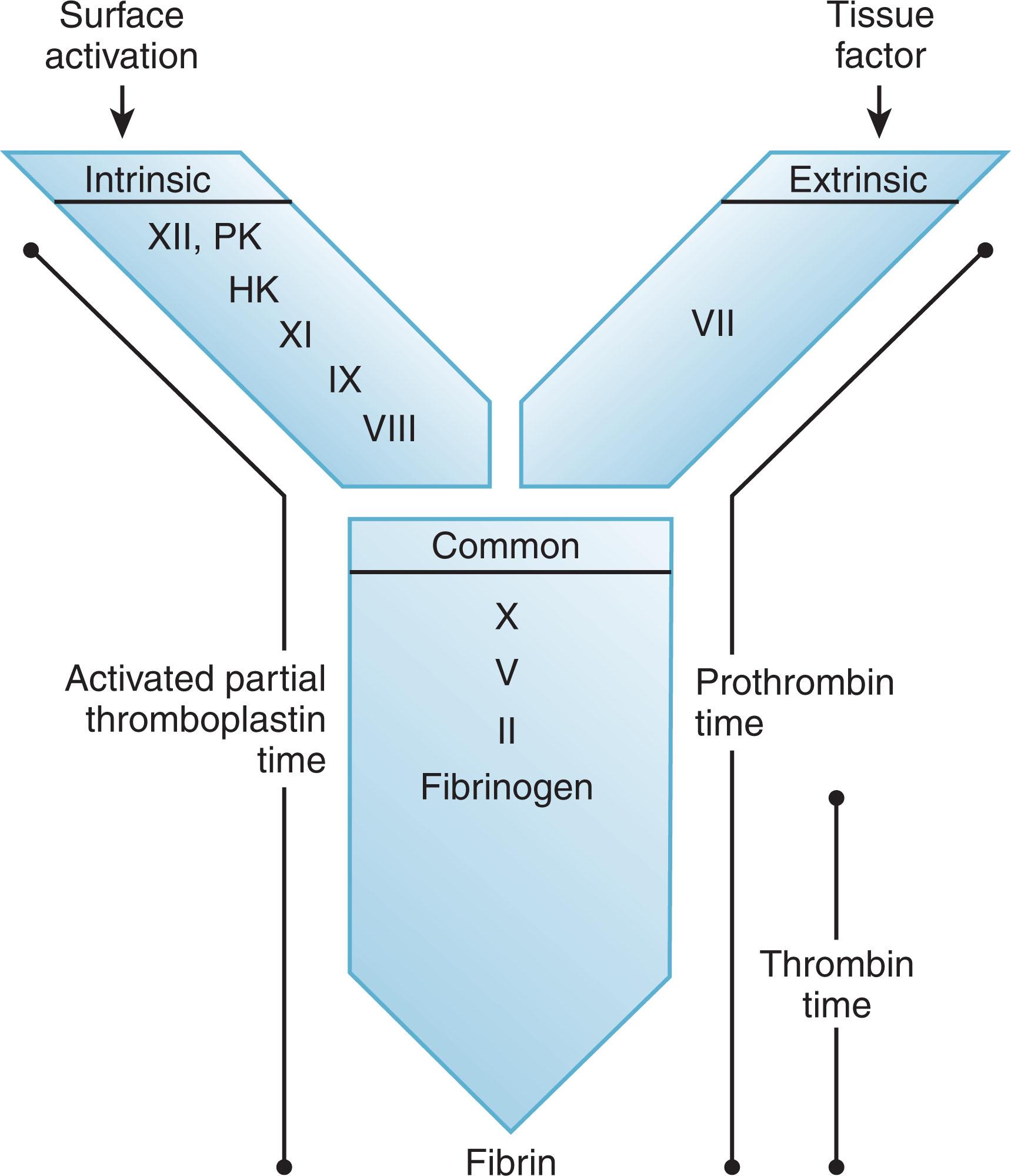
Any defect in one of the coagulation proteins along the pathway to clot formation will give an abnormal result (i.e., a delayed time to clot formation) (see Fig. 127.3 ). For example, factor XI deficiency will lead to a prolonged APTT. Furthermore, because clot formation depends on a series of reactions, any substance (e.g., inhibitory antibodies, anticoagulants) that interferes with the assay downstream from a specific coagulation protein will lead to an abnormal result. For example, profound fibrinogen deficiency will lead to a prolonged APTT and PT, even when the levels and function of all the upstream coagulation proteins are normal.
In the APTT assay, activation of the blood coagulation system occurs when factor XII and its cofactors encounter a negatively charged surface such as kaolin, celite, silica, or ellagic acid (hence the intrinsic pathway's alias as the “contact pathway”). This causes the protein to change shape, allowing its autoactivation and subsequent initiation of the cascade of proteolytic reactions seen in the coagulation system. The APTT measures many proteins that are critical to physiologic hemostasis, including factor VIII and factor IX. However, it also measures proteins like factor XII, prekallikrein, and high-molecular-weight kininogen that are not necessary for physiologic hemostasis. To perform the APTT assay, equal parts of a negatively charged surface and phospholipid mixture are incubated with patient plasma for a predetermined time. Calcium chloride is then added to recalcify the citrated plasma, and the time to clot formation is measured. The APTT assay measures all the proteins of the intrinsic system (prekallikrein, high-molecular-weight kininogen, factor XII, factor XI, factor IX, and factor VIII) and the proteins of the common pathway (factors X, V, prothrombin [factor II], and fibrinogen). These proteins have different threshold levels to which they must fall before the APTT shows an abnormality. For example, most commercial APTT reagents detect a decrease in factor VIII when the protein level decreases to 35% to 45% of normal (i.e., 0.35 to 0.45 U/mL). Alternatively, factor XII and high-molecular-weight kininogen levels must fall to 10% to 15% of normal before the APTT becomes significantly abnormal, however small decreases in these factors may cause slight prolongation. The sensitivity of the screening tests for detection of specific abnormalities varies with the factor being tested, the commercial reagent used in the assay, and the equipment platform for measurement. Each clinical laboratory should determine the level of decrease for each coagulation factor that produces an abnormal APTT with their current equipment and reagents.
In the PT assay, addition of excess tissue factor creates a nonphysiologic change in the normal stoichiometric relationship of coagulation factors, allowing factor VIIa to overcome the inhibitory effect of TFPI; the factor VIIa/tissue factor complex then activates factor X to factor Xa (this bypasses the usual physiologic requirement for this process to occur through factor IX activation). To perform the PT assay, one part patient plasma and two parts tissue thromboplastin (human or rabbit tissue-derived or recombinant human tissue factor) are added to phospholipid and calcium. The time required to clot formation is measured. The PT assay measures the extrinsic pathway of coagulation, which consists of factor VII and the proteins of the common pathway (factors X, V, II, and fibrinogen). Like the APTT, the PT becomes abnormal at different threshold levels for different factors, depending on the commercial reagent and the equipment platform. For example, factor VII levels must generally fall below 35% to 40% before the PT becomes abnormal.
The PT can also be used to monitor warfarin therapy if the test reporting is modified so it can be interpreted universally. Because of the plethora of commercially available PT reagents and coagulation instruments, it is impossible to know the normal range for the PT from any given laboratory. The international normalized ratio (INR) was thus developed to standardize the reporting of the PT and to create a universal benchmark for monitoring warfarin therapy. INR=(patient PT (sec)/mean normal laboratory PT (sec)) ISI , where the international sensitivity index (ISI) for a given thromboplastin reagent is a measure of its responsiveness to reductions in the levels of the vitamin K-dependent coagulation factors, factors II, VII, IX, and X. The ISI (provided by each reagent's manufacturer, but ideally locally validated) is based on the degree of variation of the thromboplastin reagent from the World Health Organization (WHO) reference thromboplastin. This reference has an arbitrarily assigned value of 1.0. INR derivation can be performed by developing a calibration line for any given thromboplastin, using orthogonal regression or linear regression, and incorporating the PT determined with the WHO standard. Determination of the ISI requires samples from a minimum of 20 healthy donors and 60 patients on stable warfarin therapy with INR values ranging from 1.5 to 5.0. The higher the ISI, the less sensitive the thromboplastin. In practice, changes in the INR are considered interchangeable with changes in the PT. However, the INR is correctly used only to characterize the degree of PT prolongation in samples from patients taking vitamin K antagonists such as warfarin.
In the TCT assay, exogenous thrombin is added to examine the integrity of its major substrate, fibrinogen. To perform this assay, purified thrombin is added to plasma, and the time to clot formation is measured. The TCT is thus a direct measure of the conversion of fibrinogen to fibrin (see Fig. 127.3 ). When performing this assay, it is essential to use the minimal amount of α-thrombin (3000 U/mg specific activity) that will reproducibly “clot” fibrinogen, usually 2 to 6 U/mL, aiming for a clotting time (CT) of approximately 20 seconds with pooled normal plasma to achieve maximal sensitivity and render the test clinically useful. This assay is distinguishable from the clottable fibrinogen assay (Clauss assay) by the amount of thrombin used. The clottable fibrinogen assay uses far more thrombin (100 U/mL) and determines the amount of functional fibrinogen in patient plasma compared with known levels of functional fibrinogen in calibration plasma. A prolonged TCT, as indicated by values outside the 95% confidence interval for the time to clot of a large population of normal donor samples, suggests reduced fibrinogen levels (usually <100 mg/dL), abnormal fibrinogen function, or the presence of an inhibitor of the exogenous thrombin (e.g., heparin or a direct thrombin inhibitor). The TCT can also be prolonged if there is interference with fibrin polymerization, which can be caused by elevated levels of fibrinogen degradation products or the presence of a paraprotein. Some drugs, such as valproic acid, also prolong the TCT.
When the APTT, PT, or TCT suggest a coagulation protein defect, the plasma concentration of the coagulation factors should be quantified. Factor assays determine the nature and degree of severity of coagulation protein defects and can also be used to monitor factor replacement therapy. Coagulation protein defects can take three forms: a true protein deficiency; an abnormal protein that cannot participate in its physiologic function(s); or an inhibitor that targets the active site of the protein or enhances its clearance. Inherited protein deficiencies and abnormalities can be caused by deletions, insertions, and missense/nonsense mutations in individual genes. Inhibitors are generally immunoglobulins, although abnormally produced endogenous heparin, fibronectin, or cryoglobulins can also serve as acquired inhibitors to coagulation proteins.
If a coagulation protein defect is suspected, clinical laboratory testing can be done with immunologic, chromogenic, or clot-based assays. Clot-based assays for coagulation proteins are functional: they will be abnormal with both true deficiencies and dysfunctional proteins. These take the form of one-stage assays that use the APTT (for factor XII, prekallikrein, high-molecular-weight kininogen, factors XI, IX, and VIII) or the PT (for factors VII, X, V, and II) as the platform. Specific factor–deficient plasma is the key to clot-based factor assays as it provides the required specificity for each assay. These assays are based on the principle that when plasma (either from a reference standard or from a patient) containing the factor is added to plasma completely deficient in that factor, it can “correct” (i.e., shorten) the prolonged CT. For example, a factor VIII assay requires APTT reagent and calcium chloride, WHO reference calibration plasma, factor VIII–deficient plasma (which has < 1 U/dL of factor VIII and normal levels of all other factors), and patient plasma. Several different dilutions of the reference plasma are set up, creating a range of known concentrations of factor VIII. Factor VIII–deficient plasma is added to each dilution and APTTs are performed according to laboratory protocol. A calibration curve is set up by plotting the known concentration of factor VIII ( x -axis) against the time to clot in seconds ( y -axis). This process is repeated, substituting patient plasma for reference plasma. If the patient plasma has decreased levels of factor VIII, it cannot compensate for the absence of factor VIII in the factor VIII–deficient plasma and the time to clot formation is prolonged compared with a similar dilution of reference plasma. By comparing the time to clot of the patient's plasma to the calibration curve, the level of factor VIII activity in the patient’s plasma can be quantified.
Immunologic assays can be used to establish the quantity (as opposed to the quality) of coagulation proteins. When used together with clot-based tests, these assays can detect a protein with reduced function, but normal production. For example, an abnormal fibrinogen (dysfibrinogenemia) can be detected by measuring clottable fibrinogen and fibrinogen antigen on the same sample. If fibrinogen clottability (function) is less than 70% of the amount of fibrinogen antigen (production), the protein produced is likely functionally abnormal, however the cut-off for the ratio of activity to antigen is not well validated.
There has been a significant growth in the development of replacement strategies for hemophilia A and B patients (see Chapter 133 ). These include extended half-life recombinant factor VIII and factor IX and in the case of FVIII, by-passing agents. The development and increasing use of the new replacement products introduce several challenges for both the clinician and the coagulation laboratory. The APTT-based (one-stage) factor assays are traditionally used for the diagnosis and monitoring of replacement in this patient population. Due to the heterogeneity of the extended half-life products, there is wide and significant variability in the activity levels between one-stage and two-stage or chromogenic factor assays. In response to this, the United Kingdom Haemophilia Centre Doctors’ Organisation published a guideline for the laboratory measurement of factor replacement therapies. This guideline summarizes current, as well as soon to be released products and the recommended assay and reagents for laboratories to use. Collaboration between the treating clinicians and the laboratory is paramount to ensure that the appropriate assays for monitoring the replacement products are available.
An inhibitor is generally suspected when a prolonged clot-based assay does not correct after mixing patient plasma in equal quantities with normal plasma (i.e., in an immediate 1:1 mix), or if an apparent factor deficiency is not consistent with the patient’s clinical history. Screening for inhibitors is accomplished by using a 1:1 mix in a clot-based assay. If coagulation factor–deficient plasma is mixed with normal plasma, clotting will not be impaired, as the normal plasma will compensate for the deficient factor in the plasma and “correct” its prolonged CT. However, if inhibitor-containing plasma is mixed with normal plasma, clotting will be impaired, because the specific inhibitor will decrease the activity of the coagulation factor in the normal plasma as well. Specific inhibitors of coagulation proteins are generally time-dependent, that is, clotting may not be impaired immediately after the 1:1 mix is performed. However, if the mixture is incubated for 2 hours at 37°C, the CT will be prolonged.
Inhibitors of specific coagulation proteins increase the risk for bleeding. The most common acquired inhibitor is an antibody against factor VIII (see Chapter 133, Chapter 134 ). These can occur as an alloantibody in persons with hemophilia, or as an autoantibody in those with previously normal hemostasis. Patients with an autoantibody-type acquired factor VIII inhibitor present with a prolonged APTT and hemophilia-like bleeding. Both males and females can develop this type of inhibitor. It is characteristically seen in older adult patients, patients with B-cell malignancies, patients with connective tissue disorders such as systemic lupus erythematosus, and in the postpartum period (see Chapter 134 ). Management decisions in these patients are influenced by the severity of the bleeding and the titer of the inhibitor. Quantitative measurement of factor VIII inhibitors is performed using the Bethesda method, often using the Nijmegen modifications for improved sensitivity and specificity. The Bethesda method is based on the observation that if a coagulation factor is incubated with plasma containing its specific inhibitor, the factor will be progressively neutralized. If the amount of factor added and the duration of incubation are standardized, the inhibitor potency can be measured based on how much of the factor is inhibited. One Bethesda unit is defined as the amount of factor VIII inhibitor that will neutralize 50% of 1 unit of factor VIII in normal plasma after 2 hours of incubation at 37°C. Normal pooled plasma is considered to contain 1 unit of factor VIII. The Bethesda assay is performed by incubating dilutions of the patient plasma with normal pooled plasma for 2 hours at 37°C. At the end of the incubation period, the factor VIII and the percentage residual factor activity is calculated and plotted against a standardized lin/log graph of Bethesda units versus residual factor VIII activity. The factor VIII percentage nearest to 50% (between 30% and 60%) is chosen for calculating the potency of the inhibitor to minimize overestimating (<50%) or underestimating (>50%) the inhibitor titre. At 50% residual factor activity, the test plasma contains, by definition, 1 Bethesda inhibitor unit per mL. The Nijmegen modification allows for more accurate determination of inhibitor titers at low levels of factor VIII inhibition. Increases in pH and decreases in protein concentrations can lead to increased inactivation of factor VIII, potentially leading to false-positive results with the standard Bethesda assay. The Nijmegen modification buffers normal plasma with 0.1 M imidazole and uses immunodepleted factor VIII–deficient plasma in the control mixture.
Inhibitors directed against other coagulation factors are rare, and their associated hemorrhagic manifestations are variable, ranging from minor bleeding to life-threatening events (see Chapter 134 ). Autoantibodies against von Willebrand factor (vWF) can develop in the context of a paraproteinemia, whereas autoantibodies against prothrombin are a rare complication of systemic lupus erythematosus. Inhibitors to other factors can be measured with modifications of the Bethesda method.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here