Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Numerous publications demonstrate how ligament fiber function depends on the initial length and attachment site and the specific joint position of both flexion-extension and tibial rotation.
These concepts form the basis for the placement of knee ligament grafts, provide insight into single- and double-bundle cruciate reconstructions, and form the basis for the tensioning rules regarding knee flexion and load applied for ligament grafts at surgery.
The tension developed in ligament fibers depends on its tibiofemoral attachment site, initial length, initial joint position, and subsequent joint displacement.
Fibers function only when the distance between the fiber attachment sites reaches the length of the fiber to initiate loading.
Collagen fibers are not highly elastic and demonstrate failure at relatively low levels of elongation or strain.
In the early writings of Fick in 1911, the description of ligaments having fibers grouped into bands was presented, along with the concepts that not all fibers of a ligament would be tense at one time. Fick also noted that knee flexion angle played an important role in determining which fibers were under tension and those that were slack. Since then, numerous publications have appeared that demonstrate how ligament fiber tension and function depends on the initial length and attachment site and on the specific joint position of both flexion-extension and tibial rotation. These concepts are important because they form the basis for the placement of knee ligament grafts and provide insight into the concepts of single- and double-bundle anterior cruciate ligament (ACL) and posterior cruciate ligament (PCL) reconstruction. In addition, the ligament fiber length-tension properties form the basis for the tensioning rules regarding knee flexion and load applied for ligament grafts at surgery. Major disagreement still exists among investigators on these points, as is discussed in this chapter.
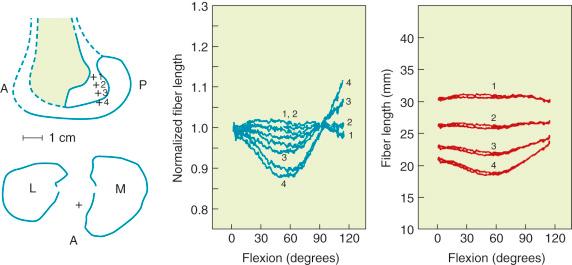
It is first important to understand the length-tension behavior of individual ligament fibers. The viscoelastic properties of ligament fibers allow them to initially undergo elongation under load in resisting joint displacements. The tension developed in the ligament fibers depends on its tibiofemoral attachment site, initial length, initial joint position, and subsequent joint displacement. In Figure 4-1 , a force-versus-length curve is shown for human ACL fibers. The “L0” point describes the initial loading of the ligament fiber; when the ligament bone attachment distance of the fiber is less than L0, the fiber is slack. The fiber functions only when the distance between the fiber attachment sites reaches the length of the fiber to initiate loading. The fiber functions only within a narrow elongation or strain, which has been estimated at strains of 5% or less. Failure of the individual fiber begins in the upper range estimated at the 8% level of strain.
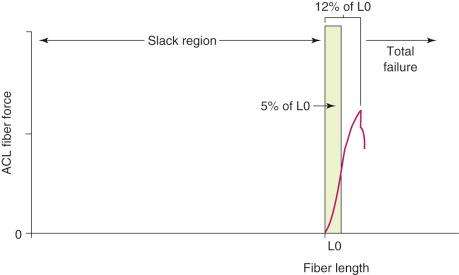
Collagen fibers are not highly elastic and demonstrate failure at relatively low levels of elongation or strain. Because ligament fibers function only within a small range of lengths and the fiber lengths vary with knee rotations and translations, these fiber length patterns can be measured under in vitro conditions. Beynnon and coworkers have measured fiber length patterns in vivo (described further in Chapter 10 ).
The 3-dimensional microgeometry of ligament fibers is only partially understood on a macroscopic and ultrastructural level.
The arrangement of ligament fibers in a parallel manner such as the MCL, FCL, or a tendon provides a structure with a high maximum load to failure because the majority of the fibers are loaded in a symmetrical manner and share the loading profile. The structure will fail at a relatively small displacement.
The capsular structures are designed to provide a low stiffness, allowing elongation or compliance, which is built into their microgeometry. Failure of capsular structures can be difficult to detect as a microtearing process occurs throughout the entire capsule. Before ultimate failure of the capsule, the microfailure process produces a residual elongation or slackening.
The length-tension behavior and failure pattern of the ACL and PCL are also dissimilar, with different fiber regions brought into the loading based on the position of the knee.
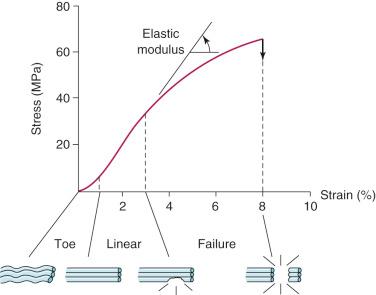
The 3-dimensional microgeometry of ligament fibers is only partially understood on a macroscopic and ultrastructural level. From a macroscopic level, Figures 4-2 to 4-5 show the concepts of how ligament fibers determine functional properties and the mechanisms of ligament failure. Figure 4-2 shows a stress-strain curve for a typical collagen fiber. The initial toe region involves straightening of the collagen fibers, followed by a linear region. At the end of the linear region, failure of individual collagen fibers occurs, which progresses to final failure of the whole structure. Note that the total fiber strain to failure is only 8% in this illustration. Figure 4-3 shows the effect on the structural properties of a ligament on increasing the cross-sectional area. There is an increase in stiffness and ultimate force to failure. Figure 4-4 shows the effect of different lengths of a collagen structure under load, both having the same cross-sectional area. In the illustration, there is a 50% decrease in stiffness in the ligament that is twice as long. This effect has been noted in ACL semitendinosus-gracilis tendon (STG) reconstructions where aperture fixation of the graft directly at the tunnel site may improve mechanical properties of the graft in addition to decreasing graft-bone tunnel motion. Figure 4-5 shows the effect of fiber microgeometry on structural properties. The finger trap illustration shows a major decrease in stiffness and ultimate strength because the collagen fibers are not loaded in a symmetric manner.
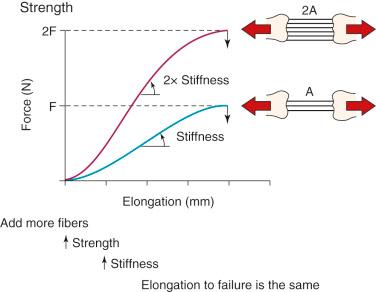
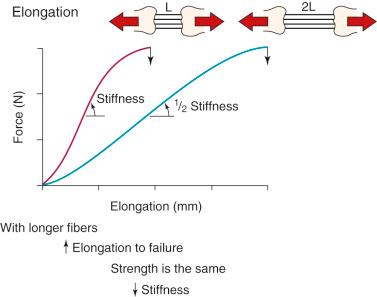
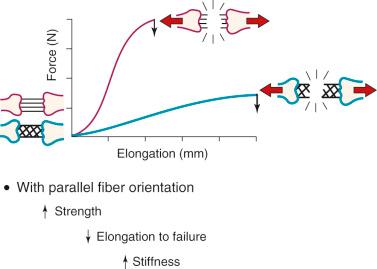
The arrangement of ligament fibers in a parallel manner such as the medial collateral ligament (MCL), fibular collateral ligament (FCL), or a tendon provides a structure with a high maximum load to failure because the majority of the fibers are loaded in a symmetric manner and share the loading profile. The structure will fail at a relatively small displacement, as is true for the MCL and FCL. Alternatively, the capsular structures are designed to provide a low stiffness, allowing elongation or compliance, which is built into the microgeometry, similar to that shown in the illustration of the finger trap in Figure 4-5 . Certain portions of the capsule may have an arrangement in which some fibers exist in a more parallel array, such as the posterior oblique ligament of the posteromedial capsule. Failure of capsular structures can be difficult to detect because a microtearing process occurs throughout the entire capsule. The capsule may appear slack and elongated without an obvious failure or the capsule may be avulsed off its femoral or tibial attachment and the failure site identified and repaired at surgery. Before ultimate failure of the capsule, the microfailure process produces a residual elongation or slackening that, in chronic knee injuries, may require plication procedures to restore tension and function. An example is plication of posteromedial or posterolateral capsular structures so that they will function in full extension and resist knee hyperextension. The length-tension behavior and failure pattern of the ACL and PCL are also dissimilar, with different fiber regions brought into the loading based on the position of the knee.
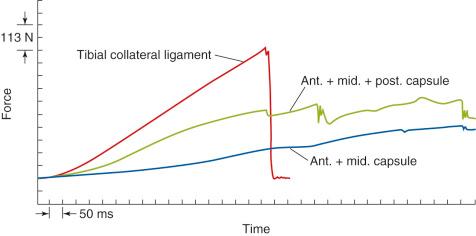
An example of the force elongation properties of the MCL and medial capsular structures is shown in Figure 4-6 . This example is from a rhesus primate knee, in which the medial structures were loaded in uniaxial tension. The high failure load of the MCL compared with the capsular structures is shown. The capsule undergoes a failure process that is somewhat continuous because different portions of the less stiff capsule are brought into the loading sequence and fail, without an abrupt failure.
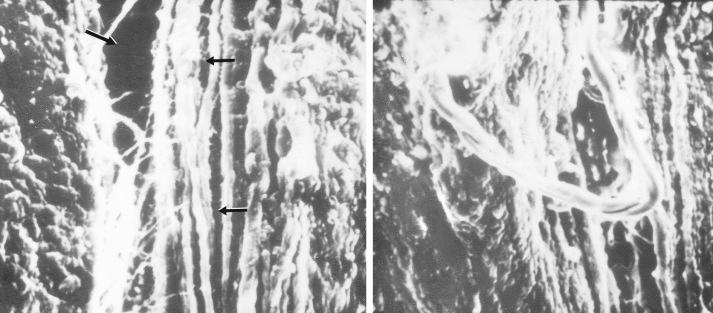
An example of a scanning electron microscopic analysis of failure of the ACL ligament is shown in Figure 4-7 . In this series of experiments, the ACL of a rhesus primate knee was loaded to one half of its expected failure load. Scanning electron microscopic examination showed that some portions of the fiber bundles had separated and had lost some of their normal wavy attachment. This is not an artifact, because normal ACL fibers will show a wavy appearance. In certain portions of the ACL, there is complete failure of individual fibers that were brought into the loading sequence owing to their fiber length at the knee position selected for the loading test. In Figure 4-8 , failure of a major fiber bundle is shown with failure of the individual fibers at different places within the fiber. Note that failure of a small vessel has also occurred at the site of fiber failure, which is unusual given the expected elongation that vessels undergo before ultimate failure.

Many factors determine ligament length patterns during knee motion; however, one of the most sensitive factors is the fiber's femoral attachment (even more so than the tibial attachment).
The length-tension pattern of cruciate ligament fibers is complex and is in marked contrast to more simplified descriptions that the ACL anteromedial fibers lengthen with knee flexion and the posterolateral fibers lengthen with knee extension.
A ligament functions with fibers of different lengths and their loading, which depends on attachment site characteristics.
There is an approximate region in the ACL and PCL at which one region lengthens with knee flexion, another region shortens, and fibers between these two regions undergo less elongation. However, there are major differences among investigations regarding where these regions are located.
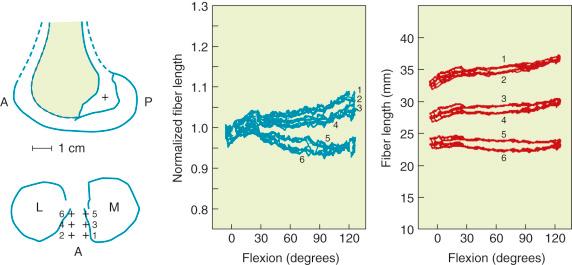
Our data concluded that the line for the ACL is primarily oriented in a proximal to distal direction and that fibers anterior to the line become longer in flexion and that posterior fibers become shorter.
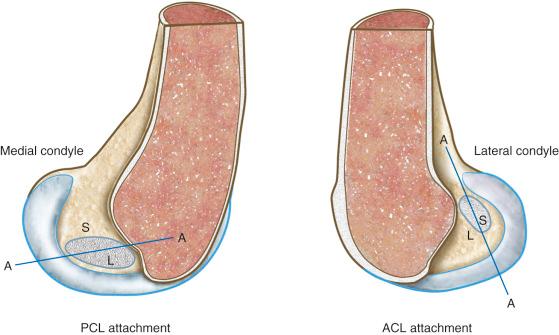
The region for the line for the PCL is oriented in an anterior to posterior direction, with distal fibers becoming longer with knee flexion and proximal fibers lengthening with knee extension.
The effects of changing the tibial attachment of an ACL graft in cadaveric specimens were investigated. Moving the fiber attachment on the tibia in a medial to lateral direction had only a small effect on fiber length and normalized length patterns. In contrast, moving the fiber in an anterior to posterior direction had a strong effect on fiber length.
The placement of an ACL graft in an anterior to posterior location within its femoral footprint has significant effects in determining the tension and loading conditions of the graft. The placement of a PCL graft in a proximal to distal femoral direction has similar profound effects on its length-tension behaviors.
The ACL or PCL graft should be tensioned at the knee flexion angle under which the graft will be at its longest length. Tensioning a graft at a flexion range in which the graft is in its shortest position would be expected to produce failure of portions of the graft as it elongates at a different knee flexion position.
Many factors determine ligament length patterns during knee motion; however, one of the most sensitive factors is the fiber's femoral attachment (even more so than the tibial attachment). This concept is highly important for understanding ACL and PCL ligament fiber function and the location of ligament grafts for reconstruction.
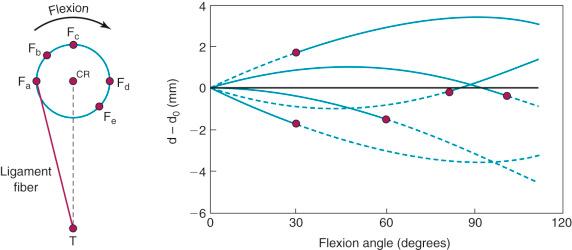
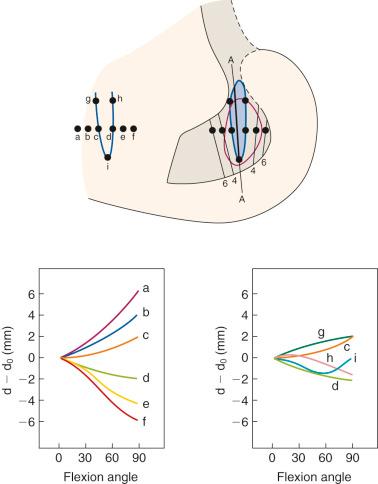
The effect of the femoral attachment described by Grood and associates is illustrated in Figure 4-9 . In this model, the tibial attachment is assumed to be stationary, and flexion of the knee occurs about a fixed center of rotation (CR). The location of the CR is at the average rotation center of the human knee joint. A fiber that attaches anterior to the CR, such as fiber F a , will lengthen with knee flexion up to 90 degrees as it rises up to the top of the circle and thereafter shortens with knee flexion. The opposite effect is shown by fiber F d , which shortens with knee flexion and, at 90 degrees of flexion, would start to lengthen. The length patterns of the fibers are shown in the accompanying graph. This shows the complex length pattern of cruciate ligament fibers and is in marked contrast to more simplified descriptions that the ACL anteromedial fibers lengthen with knee flexion and the posterolateral fibers lengthen with knee extension, which is a gross oversimplification. The circle model is still only an approximation because the knee does not have a fixed CR; this provides an additional complexity in the analysis of ACL and PCL fiber function.
Contour plots are used to simplify the description of ligament length changes; a typical contour map is shown in Figure 4-10 . A single contour line expresses all of the attachments that would produce the same maximum fiber strain. The region bound by the contour line with the smallest strain (2% elongation) provides the most isometric femoral attachments. Note in Figure 4-10 that the tibiofemoral separation distance increases dramatically anterior to the contour line, and correspondingly, the length decreases posterior to the line with knee flexion. It should also be noted that the concept of ligament isometricity is a misnomer because it is not expected that a ligament shows this behavior with a set minimal strain or elongation during knee flexion, as already discussed. A ligament functions with fibers of different lengths and their loading, which depends on attachment site characteristics. The disadvantage of the contour map is that the length of the individual native ligament fibers is not represented, and therefore it is unknown at what point the ligament fibers in any region are tense or slack. The contour map is most useful to show the length changes that would occur for ligament grafts placed at certain positions within the ACL and PCL attachment sites at the maximum elongation or strain of the graft. In addition, note the knee position at which the graft is tensioned to not produce excessive graft elongation, which would either overconstrain the joint or result in graft failure.
A number of studies show there is an approximate region in the ACL or PCL at which one region lengthens with knee flexion, another region shortens, and fibers between these two regions undergo less elongation. However, there is a major discrepancy in the investigations regarding where the regions are located within the cruciate ligaments. Our data and those of others have concluded that the line for the ACL is primarily oriented in a proximal to distal direction and that fibers anterior to the line become longer in flexion and posterior fibers become shorter ( Fig. 4-11 ; see also Fig. 4-10 ). The region for the line for the PCL, in contrast, is oriented in an anterior to posterior direction, with distal fibers becoming longer with knee flexion and proximal fibers lengthening with knee extension. Thus, the line that divides the shortening and lengthening regions has a completely different orientation for the ACL than the PCL. This is one of the most basic concepts of how ligament fibers function. Many current illustrations for ACL femoral attachment sites incorrectly show that the proximal fibers lengthen with knee flexion and distal fibers lengthen with knee extension. In fact, there are fibers within the ACL proximal and distal femoral attachment regions, governed by the contour plot behavior (see Fig. 4-10 ), that do not show this reciprocal tension behavior with knee flexion and extension. The data for these studies are not based on true fiber attachment separation distances, and these differences have rather profound effects on where ACL and PCL grafts are placed and tensioned. The PCL fiber length-tension behaviors under loading are shown in Chapter 15 .
It should be noted that both Sidles and colleagues and Hefzy and coworkers reported that the contour maps describing fiber elongation patterns were strongly dependent on whether an anterior or posterior displacement load was applied to the tibia and the contour maps provided were under loading conditions for the respective ACL or PCL. A more complete description of contour maps for the femoral and tibial attachments is provided for the reader, and the effect of tibial attachment sites is discussed in Chapter 7, Chapter 15 .
The effects of changing the tibial attachment of an ACL graft in cadaveric specimens is shown in Figure 4-12 . The six ACL fiber locations on the tibia all originate at the approximate center of the ACL femoral attachment. Moving the fiber attachment on the tibia in a medial to lateral direction had only a small effect on fiber length and normalized length patterns. In contrast, moving the fiber in an anterior to posterior direction had a strong effect on fiber length. An anterior shift of the tibial attachment of 7.5 mm increased the fiber length by 5 to 8 mm. An anterior tibial attachment resulted in the fiber length behavior to become tauter in flexion. In Figure 4-13 , the strong effect of moving the femoral attachment location in an anterior to posterior location on changing the length with knee flexion is shown. The location of the tibial insertion site is unchanged. The effect of moving the femoral attachment in a proximal to distal direction is shown in Figure 4-14 . The more distal attached fibers were markedly shortened and had a concave length pattern, whereas the longer proximal fibers had a slight convex pattern. In summary, these studies show the complex behavior of the cruciate ligaments in which individual fibers and fiber regions are loaded to resist coupled motions of tibial translation and rotation. For the surgeon, the placement of a graft for the ACL in an anterior to posterior location within its footprint has significant effects in determining the tension and loading conditions of the graft as demonstrated. As is discussed in Chapter 15 , placing the PCL graft in a proximal to distal direction has similar profound effects on its length-tension behaviors. The surgeon does have the ability to approximately select the range of knee flexion (low flexion, high flexion positions) under which the graft will be at its longest length and, therefore, should be tensioned at this position. Tensioning a graft at a flexion range at which the graft is in its shortest position would be expected to produce failure of portions of the graft as it elongates at a different knee flexion position. Within each graft, there will be asymmetric loading of the graft fibers. The contour plots show the different fiber elongations of fibers in the center of the graft compared with the fibers at the periphery of the graft. The concept of placing more than one graft is designed to allow adjustments to more closely simulate ligament fiber behaviors, although it is probable that the surgeon is adding collagen fibers to fill the ACL or PCL footprint and that native cruciate fiber behavior is never achieved.
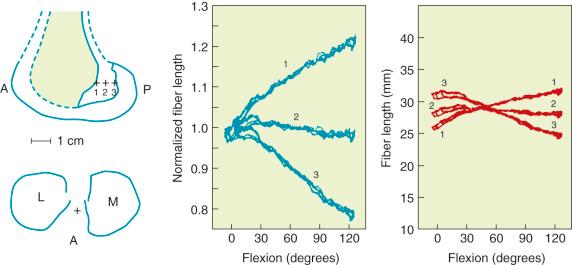
In agreement with Grood and associates' studies, Markolf and colleagues reported in a cadaveric in vitro biomechanical study that positioning the femoral ACL tunnel in an anterior to posterior direction is more critical in terms of graft forces and function than a proximal to distal direction.
Mathematical theory developed to determine all pairs of femoral and tibial attachments that remain the same distance apart (isometric) as the knee is moved through small (infinitesimal) motions.
The pairs of attachment points are represented by a curve for all possible femoral attachments and a second curve for all possible tibial attachments.
There are important scientific objections to using this model to define ligament function and possible graft attachment locations.
Shape of the curves depends on the knee flexion angle that is chosen; different curves can be computed for different flexion angles.
The curves are based on small knee motions. There are tibial attachment locations that are as isometric that are not on the Burmester curve when a wider range of knee motion is considered.
As already discussed, one of the primary scientific bases for ligament fiber function and graft placement is the measurement or computation of fiber length patterns. An alternative approach for determining ligament function proposed by Menschik is frequently referenced in the literature. This approach is based on the four-bar model of ACL and PCL ligament function shown in Figure 4-15 , first proposed by Strasser, and on the mathematical theory for knee motions developed by Burmester. This mathematical theory was developed to determine all pairs of femoral and tibial attachments that remain the same distance apart (isometric) as the knee is moved through small (infinitesimal) motions. The pairs of attachment points are represented by the two curves shown in Figure 4-16, one for all possible femoral attachments and one for all possible tibial attachments, which are described as a cubic curve because of their mathematical form. A single tibial and femoral attachment of a ligament is obtained by drawing a straight line through the instant center of knee flexion controlled by the four-bar mechanism where the cruciate links cross each other. The MCL tibial and femoral isometric attachments are the points at which a line crosses the tibial and femoral cubics.
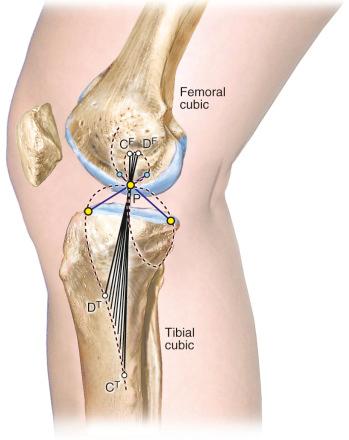
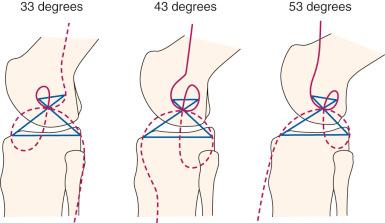
The Burmester curves have been used by many authors to explain the attachment locations of the collateral ligaments and capsular structures, and references are available proposing these curves explain ligament function. However, there are important scientific objections to using this model to define ligament function and possible graft attachment locations. The shape of the curves depends on the knee flexion angle chosen, and different curves can be computed for different flexion angles (see Fig. 4-16 ). Menschik published the curves for 43 degrees of flexion without providing the specific criteria used for computing the curves at this particular position of knee flexion. We computed the curves for other flexion angles that differed markedly in shape and had different sites for the attachment of the collateral ligaments that may not be close to their anatomic attachment (see Fig. 4-16 ). In addition, the Burmester curves are based on small knee motions, and it has been determined that there are tibial attachments locations that are as isometric that are not on the Burmester curve when a wider range of knee motion is considered.
The mechanical behavior of ligaments varies and depends on the material properties of the fibers, the geometric arrangement of collagen fibrils and fiber bundles, the proportion of different types of fibrous constituents, the effect of the surrounding ground substance, the ligament insertion site, and underlying bone.
Strain rate describes the rate of deformation used in mechanical studies (length change/initial length per unit of time).
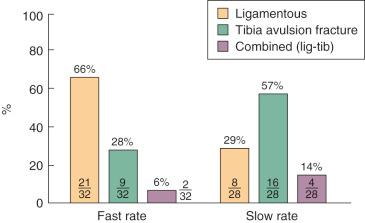
Significant difference in the predominant mode of failure of the specimens tested at the fast strain rate compared with those tested at the slow rate. At the fast rate, two thirds of the specimens demonstrated a ligamentous failure and 28% failed by tibial avulsion fracture. At the slow rate, 57% of the specimens failed by tibial avulsion fracture and 29% failed by ligamentous failure.
Under slow strain rate conditions, the bone-insertion area of the tibia was the weakest component. At the fast strain rate, representing the more physiologic loading condition, the ligament and bone components were more balanced as to strength properties and failed at a similar maximum load and energy to failure.
Mechanism of failure usually involves both the bony and ligamentous components in a progressive manner until complete failure occurs.
Ligamentous failure occurs by an initial tensile failure of collagen fibers, followed by a pulling-apart shear failure of the disrupted fibers. Rupture of collagen fiber bundles also occurs throughout the ligament.
The study showed for the first time that an ACL-ligament-bone preparation failed at a higher load and at greater elongation, and absorbed significantly more energy, at a fast rate of deformation than at a slow rate, which is important for interpreting in vitro ligament biomechanical studies.
The visual determination of continuity of a ligament at surgery provides an inadequate determination of the extent of ligament damage that has actually occurred and its functional capacity.
The fibrocartilaginous zone at the ligament insertion site is believed to be advantageous in producing a gradual change in mechanical properties, decreasing the stress-concentration effect of the ligament's insertion into the stiffer bone structure.
The ligaments of the knee have unique anatomic and mechanical characteristics that cannot be generalized from one ligament to another. The mechanical behavior of ligaments depends on the material properties of the fibers, the geometric arrangement of collagen fibrils and fiber bundles (as already described), the proportion of different types of fibrous constituents, and the effect of the surrounding ground substance. The ligament insertion site and underlying bone are additional portions of the ligament unit that must be considered when evaluating overall mechanical properties.
One approach for investigating each component of a ligament unit is to use a bone-ligament-bone specimen, such as a femur-ACL-tibia complex (FATC) preparation. This allows the mechanical properties of all of the components of the ligament unit to be studied together. The combined interaction of the components determines the properties of the entire structure in vivo. The problem still exists in loading the bone-ligament-bone unit in a manner that allows in vitro data to be extrapolated to in vivo loading conditions. This represents a problem because there are so many different in vivo loading conditions at the time of ACL failure; it is simply not possible to reproduce these conditions in the laboratory. There is less of a problem in studying the effect of one factor or variable on the mechanical properties of a ligament-bone unit because the same loading conditions are used throughout the experiment.
We conducted a study on primates to determine the effect of altered activity levels on the mechanical properties of ligaments.
The results showed that 8 weeks of immobilization produced a marked decrease of nearly one half of the maximum failure load and significant weakening of a functional ligament unit.
The decrease in strength properties was associated with resorption of haversian bone and weakening of the cortex beneath the ligament's insertion site at both the tibia and femur.
An exercise program had little effect in preventing the decline in the strength properties of the ligament unit during immobilization and only partial recovery in strength properties occurred after 20 weeks of resumed activity.
Following immobility, an extended period of time may be required before the functional capacity of a ligament unit returns to normal.
Disuse-induced changes following fracture or ligament reconstruction may extend well into the time period after which normal activity has been resumed by the patient
The strain rate describes the rate of deformation used in mechanical studies (length change/initial length per unit of time). We have conducted a series of fast and slow strain rate tests on ACL bone-ligament-bone specimens in rhesus monkeys. A slow rate of deformation was chosen to represent the strain rates used in many prior in vitro experiments. A fast rate of deformation was chosen to represent the physiologic in vivo loading conditions to which ligaments may be subjected. The ligament units were tested as right-left pairs at the two strain rates to exclude animal variability. The femur and tibia were at an angle that corresponded to 45 degrees of knee flexion to allow as nearly uniform loading of the ligament as possible. In this experiment, a distraction of the joint was produced. An alternative loading sequence would be to induce anterior translation. Both of these loading profiles have been used experimentally.
Dividing the extension rate by the initial length of the ligament produced approximate strain rates of 0.662 sec -1 at the fast rate and of 0.00662 sec -1 at the slow rate, a 100-fold difference. At the fast rate, the ligament underwent the initial 15% to 20% elongation in approximately 0.25 seconds.
An example of a load-versus-time record from a typical test at the fast strain rate is shown in Figure 4-17 . The curve demonstrates an initial concave toe region, followed by a fairly linear region up to the first significant failure. At this point, the load is termed the linear load because it denotes the approximate point at which the ligament preparation enters into the major failure region. Minor failures occur first, with a drop in load. Decreases in load after the linear load indicate successive failures until complete failure, at which time the load falls to zero. The maximum load occurs in the major failure region. The time axis is proportional to the relative displacement of the grips and represents elongation of the ligament after the compliance of the testing device and the grips are computed. Because the extension rate is known, specimen elongation at the linear load, maximum load, and zero load (complete failure) is determined. The area under the load-deformation curve indicates the energy absorbed by the specimen up to complete failure. The slope of the curve in the linear region provides the approximate stiffness of the preparation. It was not possible in this experiment to calculate the cross-sectional area of the ACL with any degree of reproducible accuracy. Force values are reported as load rather than stress (force per unit area). Alternative experiments, discussed later, employ other testing configurations in which ligament strain is measured by sensitive optic techniques.
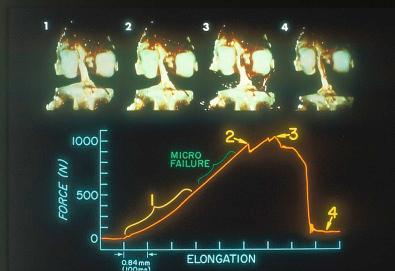
The anterior cruciate bone-ligament-bone specimens showed a complex and varied mode of failure. These included avulsion of minor to major bone fragments from insertion sites, failure at the bone-ligament interface without bone avulsion, and ligament failure that occurred initially by tensile pull-apart failure of fiber bundles and then by shear failure among the disrupted fibers. The predominant mode of failure of each specimen was classified as one of the following: (1) ligamentous failure, (2) tibial avulsion failure, (3) combined ligament failure/tibial avulsion fracture, (4) femoral avulsion fracture, or (5) combined ligament failure/femoral avulsion fracture.
A statistically significant difference was found in the predominant mode of failure of the specimens tested at the fast strain rate compared with those tested at the slow rate ( P < .05, Fig. 4-18 ). At the fast rate, two thirds of the specimens demonstrated a ligamentous failure and 28% failed by tibial avulsion fracture. At the slow rate, 57% of the specimens failed by tibial avulsion fracture and 29% failed by ligamentous failure.
The results of the strain rate properties according to the modes of failure are shown in Table 4-1 . Under slow strain rate conditions, the bone-insertion area of the tibia was the weakest component, demonstrated by the reduced maximum load and energy absorbed to failure. At the fast strain rate, representing the more physiologic loading condition, the ligament and bone components failed at a similar maximum load and energy. The overall difference in strength properties was caused in part by increased strength of the bone component at the fast rate. At the slow rate, failure by tibial avulsion fracture was interpreted as premature failure, indicating a decrease in the specimen's potential strength. The size of the animals was not a factor contributing to the predominant mode of failure. The different types of failure were distributed throughout each strain rate group regardless of the weight of the animal. In addition, juvenile animals were not used, which could have biased the results toward a higher frequency of tibial avulsion fractures.
| Strain Rate | n | Failure Mode | Maximum Load (kgf) | Strain to Maximum Load (%) | Energy (kgf-cm) | Strain to Failure (%) |
|---|---|---|---|---|---|---|
| Fast | 21 | Ligamentous | 92.8 ± 17.5 | 51.4 ± 7.5 | 39.8 ± 8.9 | 61.0 ± 8.9 |
| 9 | Tibial avulsion fx | 100.0 ± 19.3 * | 51.6 ± 7.0 * | 38.4 ± 13.2 | 55.0 ± 12.4 | |
| Slow | 8 | Ligamentous | 93.0 ± 10.6 | 47.8 ± 6.0 | 39.9 ± 9.7 † | 59.0 ± 9.3 † |
| 16 | Tibial avulsion fx | 84.1 ± 2 * | 45.6 ± 7.4 * | 30.9 ± 9.3 † | 50.9 ± 8.0 † |
* Mean value significantly different from that of the same failure mode at the other strain rate.
† Mean value significantly different from that of other failure mode at the same strain rate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here