Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term J wave syndromes denotes a clinical spectrum of diseases that differ from each other in etiology and clinical characteristics, yet they have similar electrocardiographic features and appear to share arrhythmogenic mechanisms. They include disorders caused by genetic mutations (Brugada syndrome), acquired disorders (myocardial ischemia and hypothermia), and diseases of unclear etiology (idiopathic ventricular fibrillation [VF] with early repolarization) ( Table 99.1 ). The proposed arrhythmogenic mechanism shared by these diseases is a marked abbreviation of the action potential affecting some myocardial areas more than others. The resulting increase in dispersion of ventricular repolarization underlies the electrocardiographic hallmark of these disorders: elevation of the J-point (also termed J wave ) followed by different degrees of ST segment elevation. When dispersion of ventricular repolarization reaches a certain threshold (i.e., when areas of markedly different action potential duration coexist in contiguous myocardial areas), malignant reentrant polymorphic ventricular tachyarrhythmias occur. This common arrhythmogenic mechanism explains why disorders with fundamentally different etiologies, such as an inborn malfunction of cardiac sodium channels in Brugada syndrome versus acute coronary occlusion in the case of acute myocardial infarction, cause ventricular arrhythmias of identical morphology and mode of onset. As shown in Fig. 99.1 , arrhythmias in the various J wave syndromes are polymorphic and invariably start with a ventricular extrasystole preceded by a short coupling interval.
| Congenital a | Acquired a | ||||
|---|---|---|---|---|---|
| Congenital Brugada Syndrome | Idiopathic VF With J Waves | Acquired Brugada Syndrome | Myocardial Ischemia | Hypothermia | |
| Etiology | Genetic mutations leading to malfunctioning ion channels ( SCN5A, CACNA1C, CACNB2b, GPD1L, SCN1B, KCNE3, SCN3B, SCN10A ) b | Unknown in most cases Genetic mutations in some ( KCNJ8, CACNA1C, CACNB2b, CACNA2D1, ABCC9 ) |
Medications blocking sodium channels c | Acute coronary vessel obstruction with transmural myocardial ischemia | Experimental or clinical lowering of body temperature |
| Site of J wave and ST segment elevation d | Right precordial leads | Inferior and inferolateral leads | As in congenital Brugada syndrome | Depending on the occluded coronary artery | Inferolateral leads |
| Clinical arrhythmias | VF at rest, mainly in adult males, sometimes triggered by fever and drugs c | VF at rest, mainly in young adults of both sexes, sometimes triggered by fever | VF may rarely occur during drug exposure | VF during the hyperacute phase of ST-elevation myocardial infarction | VF during clinical or experimental hypothermia |
| Effective therapy | Intravenous isoproterenol and oral quinidine | Intravenous isoproterenol and oral quinidine Reports of bepridil and cilostazol |
Discontinuation of the culprit drug Intravenous isoproterenol and sodium bicarbonate intuitively effective |
Myocardial revascularization Quinidine intuitively effective |
Quinidine, cilostazol, and milrinone are effective in experimental hypothermia |
a The distinction between congenital and acquired forms is not always straightforward. For example, some data suggest that individuals with inborn J waves are at increased risk for ischemic VF in the event of acute coronary occlusion (see text).
b Although 21 different genes have been reported to cause Brugada syndrome and are assessed routinely on genetic testing panels, a ClinGen gene disease validity classification framework performed by experts concluded that SCN5A is the only gene with definitive evidence as a cause for Brugada syndrome.
c Drugs causing acquired Brugada syndrome by blocking sodium channels may provoke arrhythmias in patients with the congenital form. See www.brugadadrugs.org .
d Type I Brugada in the inferolateral leads and non–type I J waves in the right precordial leads are also associated with arrhythmic risk (see text).
Three points are noteworthy. First, although some J wave syndromes (e.g., idiopathic VF [see Chapter 100 ] or hypothermia ) appear to be entirely caused by repolarization abnormalities, depolarization delay is important in others. Specifically, conduction delay to the right ventricular outflow tract (RVOT) and through ischemic myocardial zones contribute to the arrhythmias of Brugada syndrome (see Chapter 95 ) , and ischemic VF, respectively. In fact, recent evidence suggests that delayed conduction is also important in malignant early repolarization without Brugada syndrome (see later).
Second, although J wave syndromes are classified as congenital or acquired (see Table 99.1 ), both characteristics may coexist. For example, patients with congenital Brugada syndrome are predisposed to develop the drug-induced form of this disease when challenged with specific drugs ( http://www.brugadadrugs.org ). Patients with genetic mutations causing loss of function of the sodium channel not severe enough to manifest Brugada syndrome, and seemingly healthy patients who have J waves in their baseline electrocardiogram (ECG), appear to be at increased risk for ischemic VF during acute myocardial infarction; whereas patients with idiopathic VF may develop recurrent VF as a result of J wave augmentation during therapeutic hypothermia. , Finally, it is important to emphasize that J waves are often observed in healthy individuals who have the early repolarization ECG pattern. Although this ECG pattern has long been considered benign, case-controlled series recently demonstrated a significant association between the early repolarization pattern and idiopathic VF. It is therefore imperative to distinguish the very common and benign early repolarization ECG pattern from that of the rare and malignant J wave syndromes. In this regard, it is important to note the following: the early repolarization pattern has conventionally been defined as the combination of J waves (in the form of J-point elevation or slurred R waves) with ST segment elevation. However, each of these components may have different diagnostic and prognostic significance. As explained later, the pattern of early repolarization most strongly associated with idiopathic VF includes tall J waves, flat ST segment, , and low-amplitude T waves. This chapter summarizes the main clinical and ECG features of the J wave syndromes with an emphasis on the morphology of the J wave and the contour of the ST segment.
The prototypical arrhythmogenic J wave was described by Osborn in the setting of experimental hypothermia : dogs subjected to worsening degrees of hypothermia and metabolic acidosis developed increasingly taller J waves and eventually VF. This electrocardiographic hallmark of impending VF involves giant J waves without ST segment elevation ( Fig. 99.2A ), and the same applies to clinically significant hypothermia in humans , (see Fig. 99.2B ). Experiments involving differential cooling of cardiac wedge preparations or cooling of preparations mimicking early repolarization demonstrated that hypothermia-related J waves are the ECG reflection of increased dispersion of repolarization caused by disproportionate abbreviation of the epicardial action potential, eventually leading to phase 2 reentry and VF (see Fig. 99.1A ). , Quinidine is extremely effective for preventing hypothermia-induced VF in tissue and animal studies, which is an interesting finding considering that quinidine is the most effective drug for preventing VF in clinical J wave syndromes, including idiopathic VF (see Chapter 100 ) and Brugada syndrome (see Chapter 95 ).
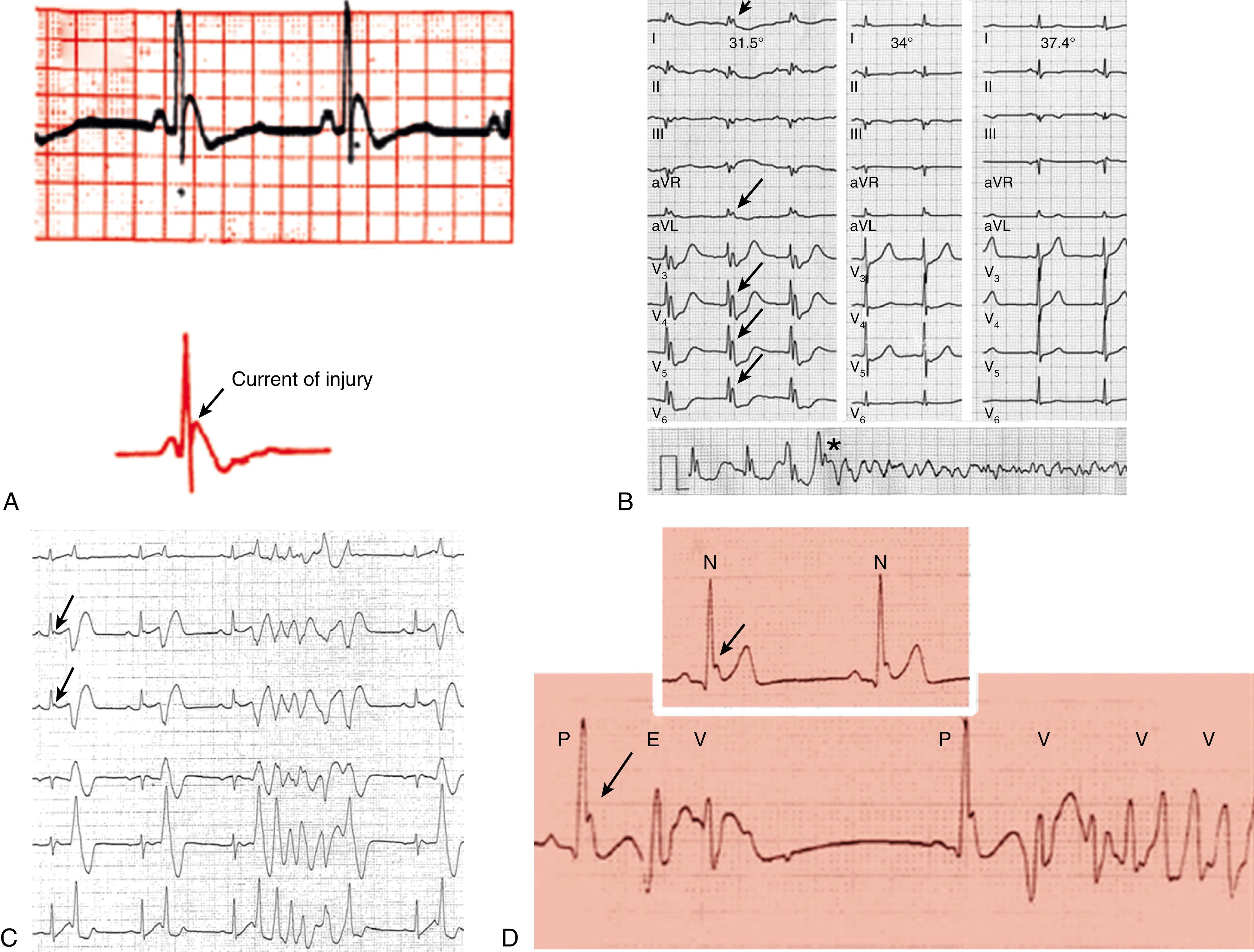
The mechanisms underlying ischemic arrhythmias are more complex, yet phase 2 reentry appears to be the cause of VF occurring shortly after the onset of acute ST segment elevation myocardial infarction. In the setting of acute coronary occlusion, the fall in the action potential dome in the ischemic zone occurs principally because of activation of adenosine triphosphate (ATP)-sensitive potassium current (I K-ATP ) secondary to a reduction in intracellular ATP levels and increase in adenosine diphosphate (ADP) levels as a result of tissue ischemia. Phase 2 reentry occurs secondary to propagation of the action potential dome from the normal zone (where it is normally present) to the ischemic region. This translates in clinical settings as the distinctive VF initiation, with short coupled extrasystoles, during ST segment elevation myocardial infarction (see Fig. 99.1B ).
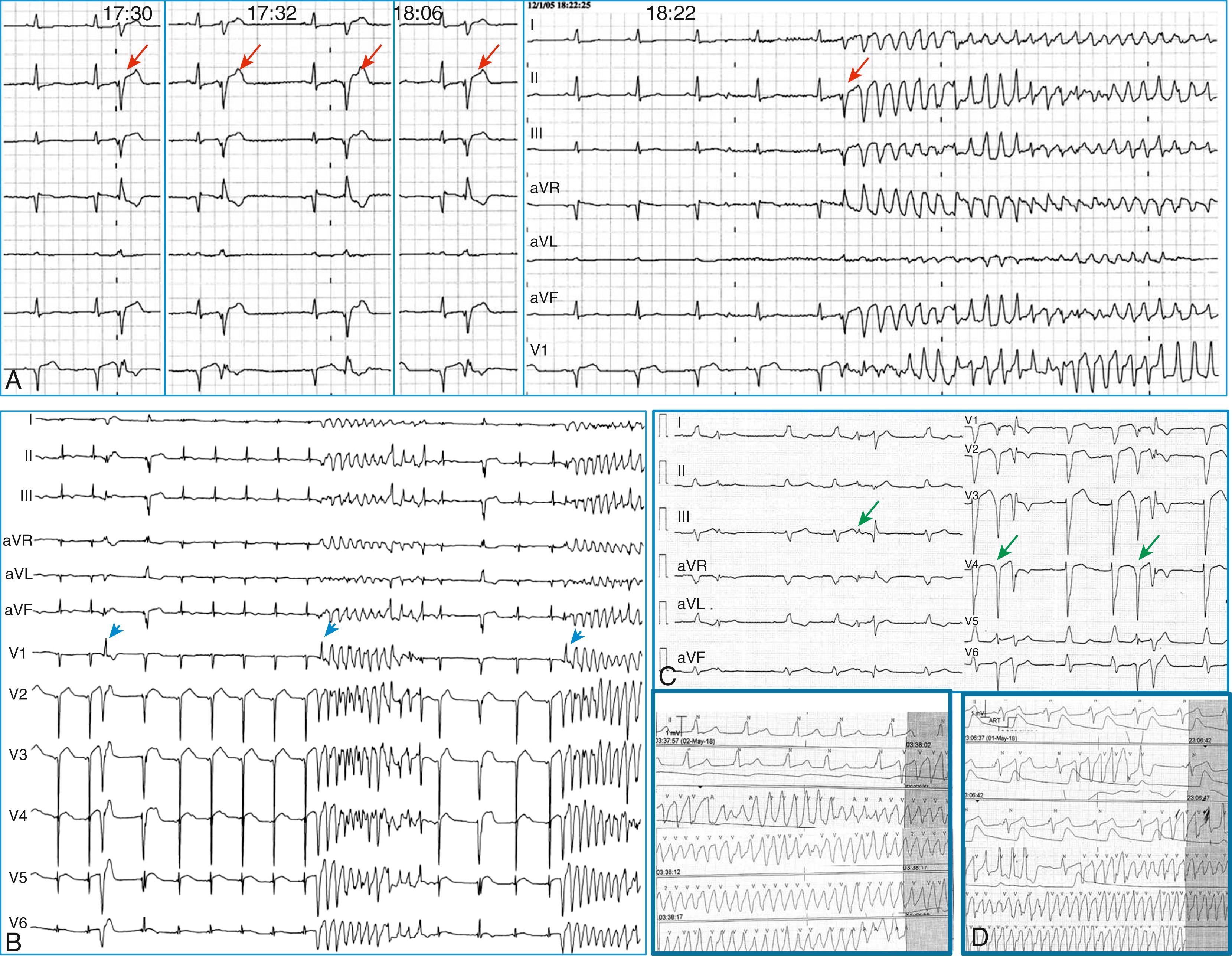
Polymorphic ventricular tachycardia (VT)/VF is triggered by short coupled ventricular extrasystoles, but in the absence of myocardial ischemia, it may occur during the recovery phase of an ischemic event, usually 3 to 5 days after a myocardial infarction or coronary bypass surgery. , Here, the arrhythmia is triggered by ectopic beats originating from Purkinje fibers within the border zone of a myocardial infarction, or less commonly from Purkinje fibers that survived the ischemic insult that are located deep within scar areas (see Chapter 101 ). As for all of the arrhythmias described here, Purkinje-related polymorphic VT is likely to cause arrhythmic storms ( Fig. 99.3 ) that are refractory to multiple antiarrhythmic drugs, yet they (almost invariably) respond to quinidine.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here