Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
While in recent years ischemic stroke has dropped to the fifth most frequent cause of death in the United States, it remains a leading cause of morbidity, mortality, and long-term disability worldwide with a devastating impact on patients, families, and communities. It is well understood that ischemic stroke represents a constellation of etiologies and mechanisms that often present with similar signs and symptoms. Advances in technology have improved understanding of stroke pathophysiology that promises to translate into more specific treatments and better outcomes.
The distinction between transient ischemic attacks (TIAs) and strokes based on the duration of ischemic symptoms has become less clinically relevant over the past couple decades. With the more widespread use of magnetic resonance imaging (MRI), it has become evident that about a third of patients with transient ischemic symptoms actually have evidence of diffusion restriction on MRI. Therefore vascular neurologists have advocated for a new, tissue-based definition of TIA that implies the absence of acute imaging abnormalities. More importantly, strokes and TIAs share pathophysiologic mechanisms, and the same preventative measures may apply to both. Therefore, the diagnostic approach to patients with transient or persistent ischemic symptoms should be the same, and treatment should be guided toward the underlying cause of the brain ischemia.
The most common ischemic stroke etiologies are large artery occlusive disease, cardioembolism, and small vessel disease.
Atherosclerosis causes stenosis or occlusion of extracranial and intracranial arteries and is directly responsible for a significant percentage of cerebral ischemic events. Atheroma formation involves the progressive deposition of circulating lipids and ultimately fibrous tissue in the subintimal layer of the large and medium arteries, occurring most frequently at branching points ( Fig. 15.1 ). Plaque formation is enhanced by blood-associated inflammatory factors as well as increased shear injury from uncontrolled blood pressure (BP). Intraplaque hemorrhage, subintimal necrosis with ulcer formation, and calcium deposition can cause enlargement of the atherosclerotic plaque with consequent worsening of the degree of arterial narrowing.
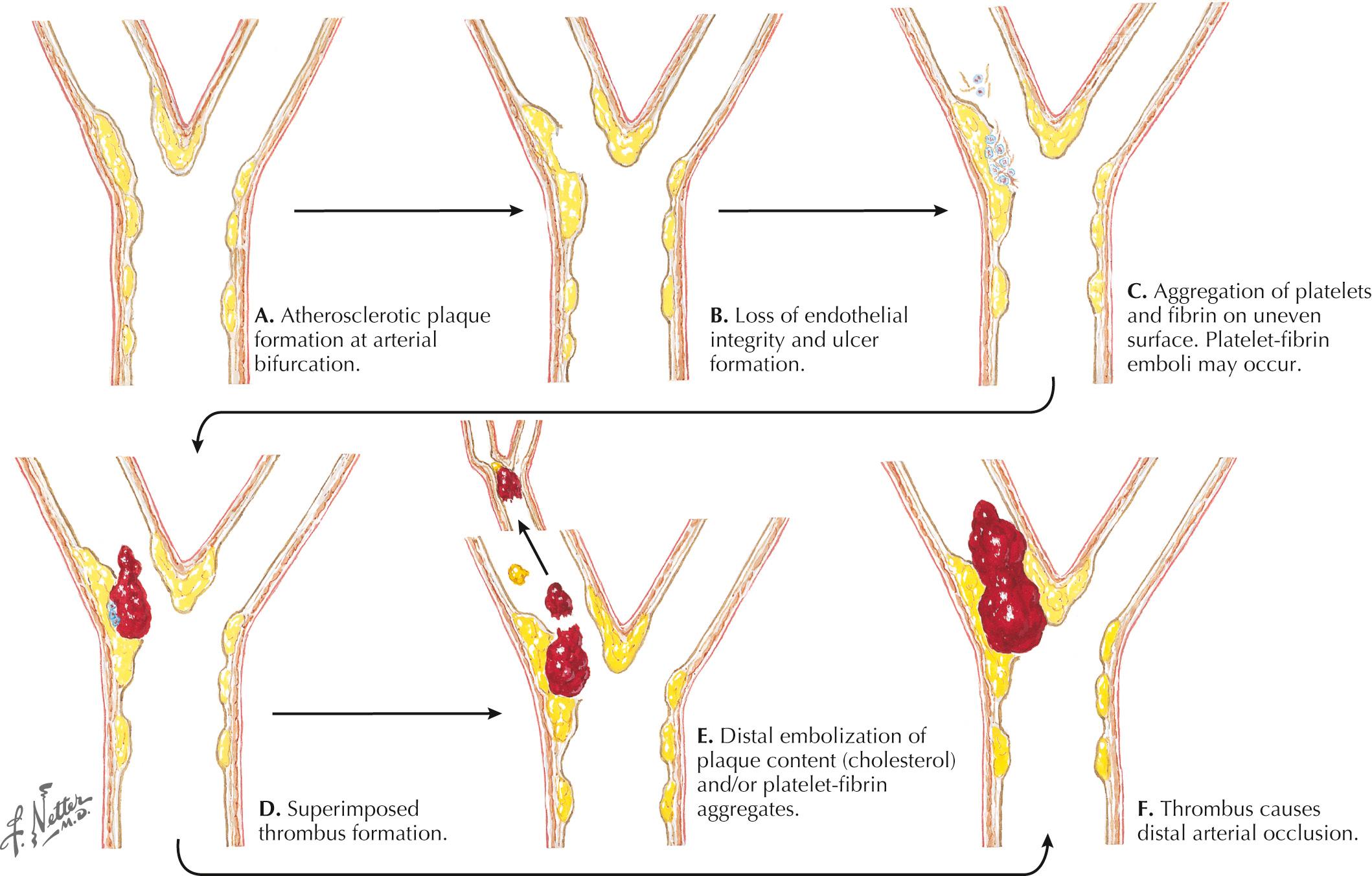
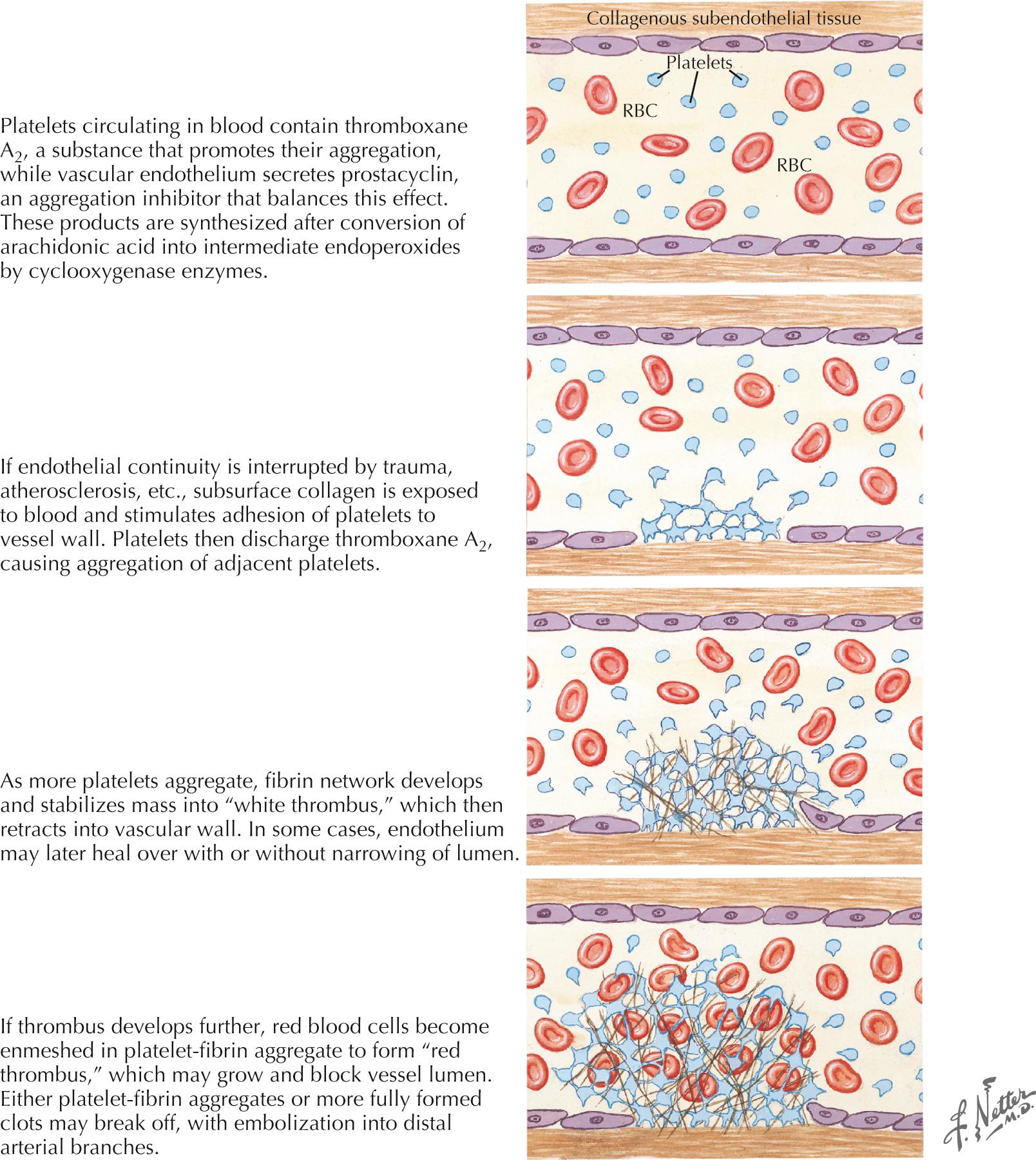
Disruption of the endothelial surface triggers thrombus formation within the arterial lumen through activation of nearby platelets by the subendothelial matrix. When platelets become activated, they release thromboxane A 2 , causing further platelet aggregation. The development of a fibrin network stabilizes the platelet aggregate, forming a “white thrombus.” In areas of slowed or turbulent flow within or around the plaque the thrombus develops further, enmeshing red blood cells (RBCs) in the platelet–fibrin aggregate to form a “red thrombus” ( Fig. 15.2 ). This remains poorly organized and friable for up to 2 weeks and presents a significant risk of propagation or embolization. Either the white or red thrombus, however, can dislodge and embolize to distal arterial branches. Large artery disease can cause ischemic strokes by either intraarterial embolism, as described previously and, less commonly, hemodynamic ischemia or hypoperfusion through a significantly narrowed vessel.
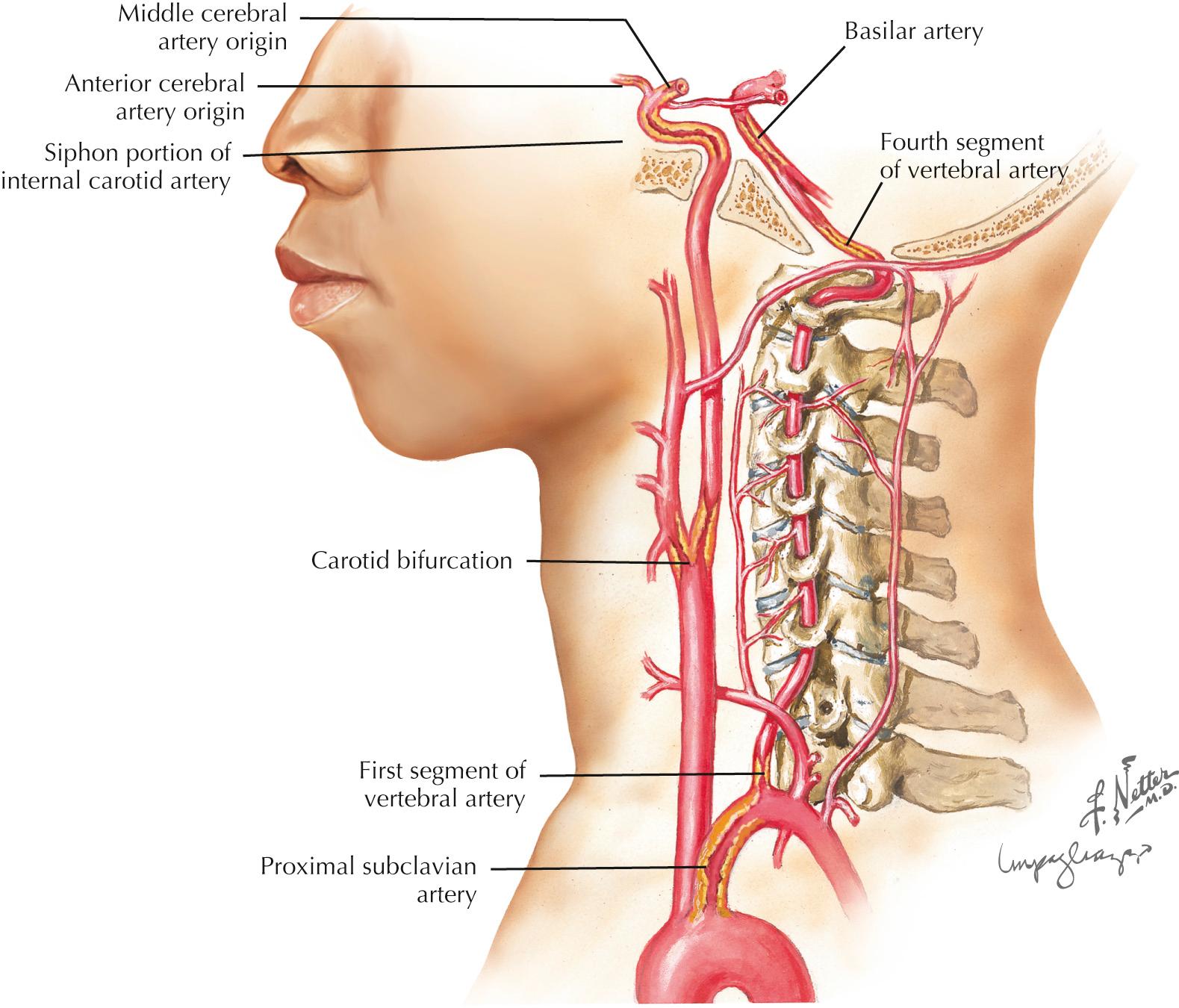
Frequent sites for carotid system or anterior circulation atherosclerosis are the origin of the internal carotid artery (ICA), the carotid siphon at the base of the brain ( Fig. 15.3 ), and the main stem of the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). The ICA at or around the bifurcation is usually affected in Caucasians, whereas in Asian, Hispanic, and African American populations, intracranial atherosclerosis may be more common than cervical carotid disease. In the vertebrobasilar system, the origins of the vertebral arteries in the neck and the distal portion of the intracranial vertebral arteries are the most commonly affected areas. The basilar artery and origins of the posterior cerebral arteries (PCAs) are other sites.
The main modifiable risk factors for large artery disease are arterial hypertension (HTN), diabetes, hypercholesterolemia, and smoking. The most important nonmodifiable risk factors are age and family history.
Several types of cardiac disease lead to cerebral embolism: atrial fibrillation (AF), ischemic heart disease, valvular disease, dilated cardiomyopathies, atrial septal abnormalities, and intracardiac tumors ( Fig. 15.4 ).
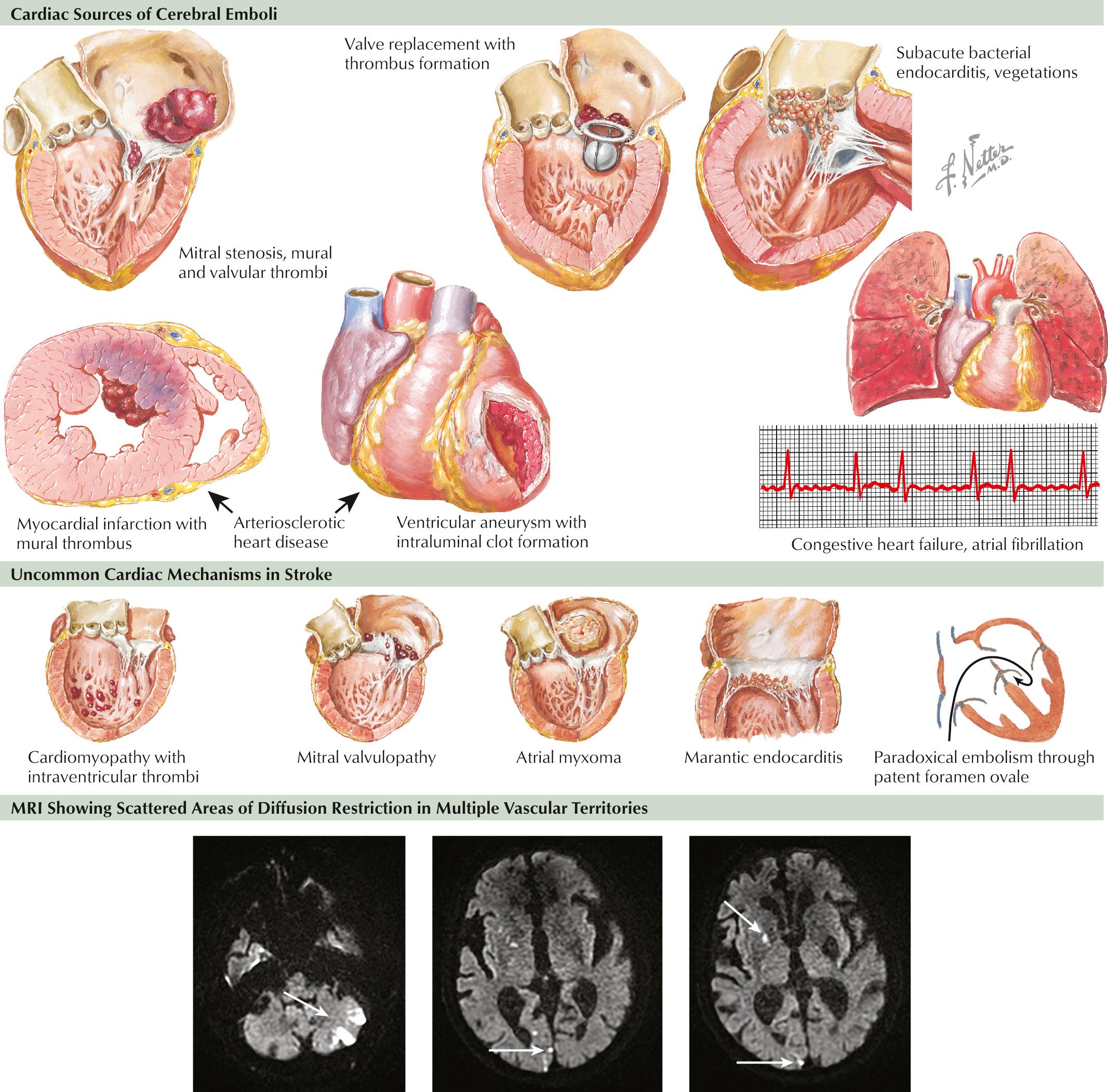
Chronic or paroxysmal AF is the rhythm most associated with cardioembolic events, with stroke often being their first manifestation. Atrial flutter is also implicated as a cardioembolic risk due to its tendency to convert back and forth with AF. Because such arrhythmias are often intermittent, careful, and at times repeated, monitoring is needed to identify their presence, as they pose a significant risk for recurrent stroke.
Within the first 4 weeks of myocardial infarction (MI), particularly with ischemia of the anterior wall, there is a higher risk of embolic stroke. More remote MIs can be a potential embolic source, particularly in patients who develop akinetic segments or left ventricular aneurysms. Mural thrombi are common in patients with dilated cardiomyopathies. Brain embolism is estimated to occur in approximately 10%–15% of these patients.
Rheumatic valvular disease, mechanical prosthetic heart valves, and infective endocarditis are well-known cardiac sources of embolism. Other relatively common abnormalities, such as mitral valve prolapse, mitral annulus calcification, and bicuspid aortic valve, have suspected embolic potential. However, these should be considered as a potential cause of stroke only after other etiologies have been excluded.
Patent foramen ovale (PFO) and atrial septal aneurysm are risk factors for stroke. A meta-analysis of case-control studies comparing patients younger than 55 years with ischemic stroke to nonstroke controls showed an odds ratio for stroke of 3 : 1 for PFO alone and of 6 : 1 for PFO with an associated atrial septal aneurysm. Potential or presumed mechanisms of stroke included venous “paradoxical embolism,” direct embolization from thrombi formed within the PFO or atrial septal aneurysm, and thrombus from atrial arrhythmias thought to be more prevalent in this population.
Intracardiac tumors are a rare but important cause of embolic stroke. Atrial myxoma and papillary fibroelastoma are the two most common and relevant neoplasms in the provocation of cardioembolism.
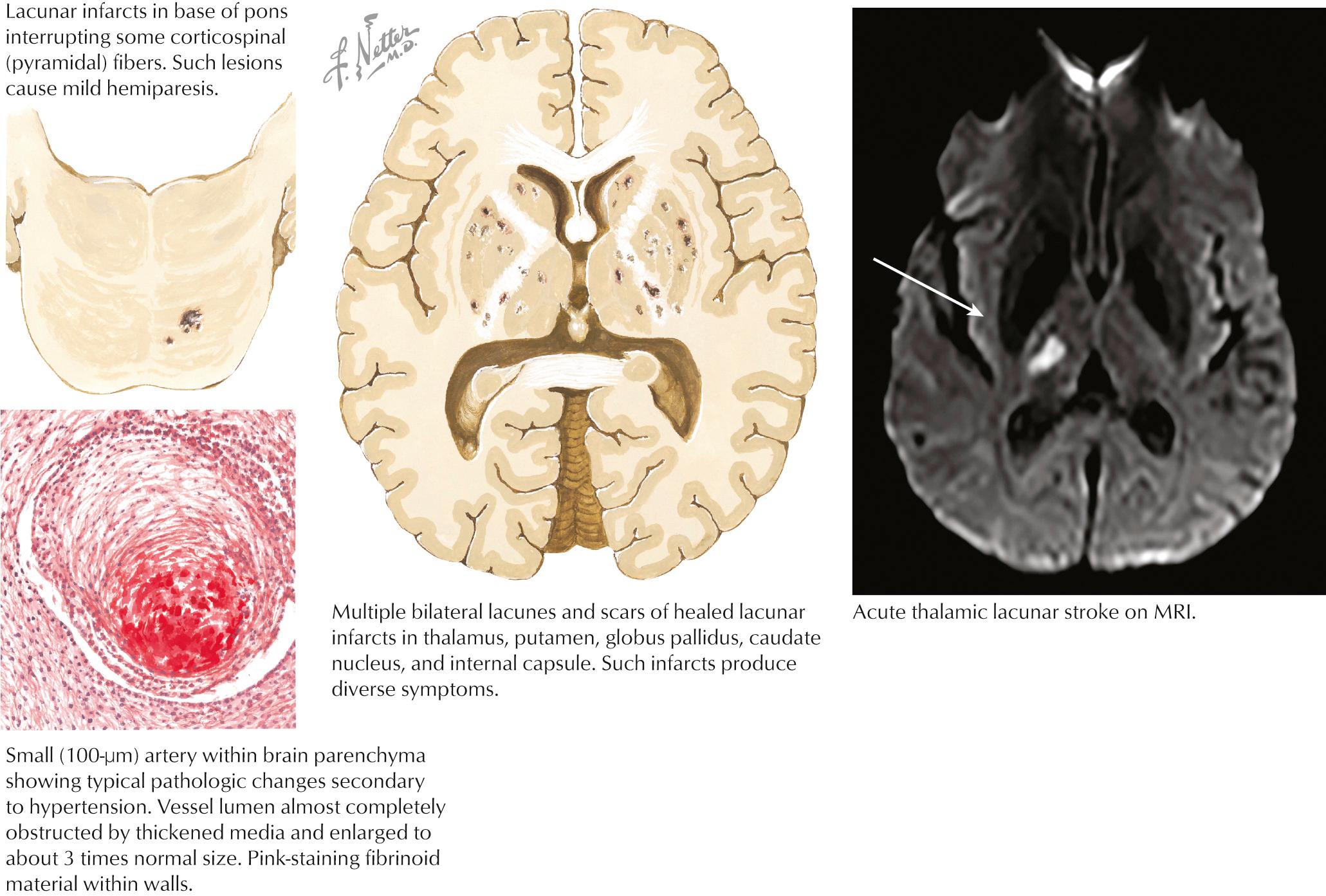
The capillary vessel and the end penetrating arteries that supply the basal ganglia, thalamus, internal capsule, and white matter tracts are not prone to atherosclerosis, as in the large-caliber cerebral circulation, but undergo a characteristic pathologic degeneration in response to endothelial damage. Fibrinoid degeneration with focal enlargement of the vessel wall, foam cell invasion of the lumen, and hemorrhagic rupture through the vessel wall characterize this process known as fibrinoid degeneration or lipohyalinosis. Occlusion of these arteries causes small (1–20 mm), discrete, and often irregular lesions called lacunes. As previously alluded to, lacunes do not involve the cortical ribbon and occur most often in the basal ganglia, thalamus, pons, internal capsule, and cerebral white matter, and may cause discrete clinical syndromes but often go clinically unnoticed. Arterial HTN and diabetes are the main risk factors ( Fig. 15.5 ).
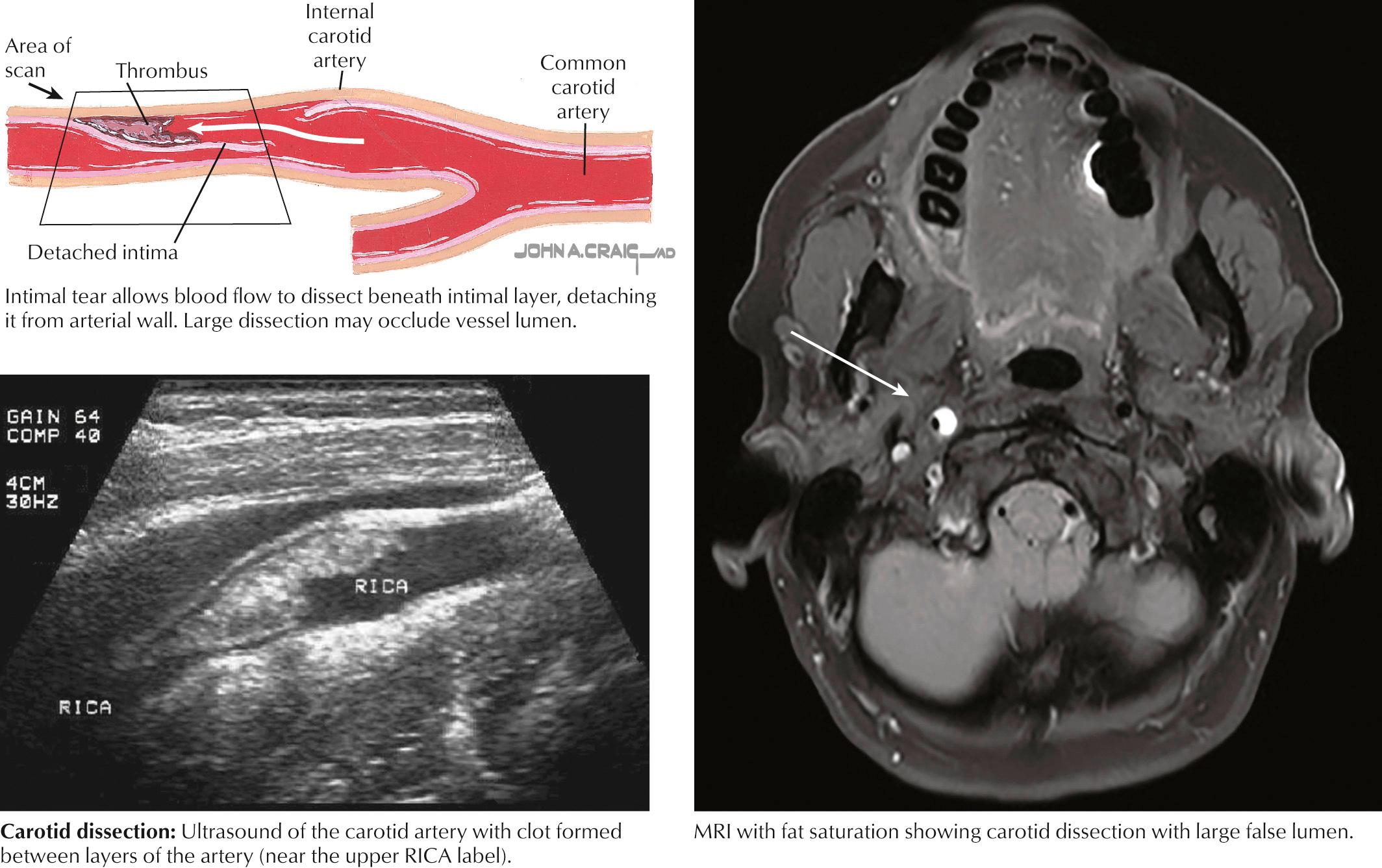
Dissection of the cervical ICA tends to occur several centimeters distal to the bifurcation or at the skull base. The cervical vertebral artery (VA) is most frequently dissected where it engages the spine, coursing through transverse foramina from C6 to C2 (V2) and then through the transverse foramen of V1 before entering foramen magnum (V3). Dissection occurring between the intima and media usually causes stenosis or occlusion of the affected artery, whereas dissection between the media and adventitia is associated with aneurysmal dilatation. Congenital abnormalities in the media or elastica of the arteries, as seen in Marfan syndrome, fibromuscular dysplasia, and type IV Ehlers-Danlos, can predispose patients to arterial dissection. Although often associated with acute trauma, arterial dissection may result from seemingly innocuous incidents, such as a fall; sports activities, particularly wrestling or diving into a wave; and paroxysms of coughing or vomiting. Dissection causes stroke by one of two mechanisms. Clot may accumulate at the intimal tear and subsequently embolize to the distal cerebral circulation. Alternatively, expanding thrombus within the media may cause some dissections to progress to severe stenosis or complete occlusion of the true lumen resulting in cerebral hypoperfusion ( Fig. 15.6 ).
Although frequently considered in the differential diagnosis of ischemic stroke, arteritis is a rare stroke etiology. Usually, central nervous system vasculitis presents as an encephalopathy with multifocal signs.
Cocaine and amphetamine are the most frequent drugs associated with ischemic strokes. Vasoconstriction and vasculitis are the proposed mechanisms. Other illicit drugs, in particular marijuana, as well as a growing list of medications (classically antidepressants) can trigger reversible cerebral vasoconstriction syndrome, which typically presents with a thunderclap headache sometimes accompanied by focal neurologic deficits.
Hematologic disorders such as polycythemia, sickle cell disease, and thrombocytosis (usually platelets >1,000,000/dL) can cause ischemic strokes by increasing blood viscosity, hypercoagulability, or both. Antithrombin III, protein S, protein C deficiencies, factor V Leiden, and prothrombin gene mutation are usually associated with venous and not arterial thrombosis but may take on importance in cases of stroke associated with PFO due to the passage of venous clots through an intraatrial defect (paradoxical embolization). Moderate to severe hyperhomocysteinemia and antiphospholipid syndrome are causes of arterial ischemic stroke, even in the absence of PFO.
A 54-year-old man presented to his optometrist after experiencing a 2-minute episode of painless transient monocular vision loss in the left eye. Two weeks earlier he had been seen in the emergency room for a 1-hour episode of transient right-hand tingling which was attributed to carpal tunnel syndrome. His past medical history was notable for 40 pack-years of smoking, poorly controlled type 2 diabetes, and untreated obstructive sleep apnea. He had two younger siblings with coronary stents. On exam, he had bilateral carotid bruits and a delayed left radial pulse. His speech was mildly dysfluent, and he made subtle naming errors for low frequency words. He was referred for an emergent carotid ultrasound, which demonstrated greater than 70% bilateral carotid stenosis with an ICA/CCA ratio of 9.0 on the left and 5.5 on the right. A contrast-enhanced MRI demonstrated small, scattered, acute, and subacute embolic ischemic strokes in the left MCA and ACA territories. He was admitted to the hospital and placed on aspirin 325 mg daily and a statin. A left carotid endarterectomy was performed with no subsequent neurologic events.
A 70-year-old white man with arterial hypertension and high cholesterol presented with 1 month of recurrent 1- to 2-minute episodes of left extremities shaking that occurred only on standing. His blood pressure was 110/80 mm Hg, and neurologic examination showed a left pronator drift, but was otherwise normal.
Head computed tomography (CT) showed small strokes in the arterial border zone between the right MCA and ACA, and right MCA and PCA distributions. Head and neck computed tomography angiography (CTA) demonstrated a right ICA occlusion. CT perfusion showed hypoperfusion in the right MCA territory, worse in the border zone areas. Collateral flow through the right ophthalmic, anterior communicating, and posterior communicating arteries was detected by transcranial Doppler and conventional angiogram. Patient was started on antiplatelet treatment as well as a statin drug, and his antihypertensive medication dose was decreased, with a subsequent increase in the systolic BP to 140–150 mm Hg. No further episodes occurred.
The previous vignettes illustrate the two mechanisms of stroke or TIA in large artery atherosclerotic disease, intraarterial embolism (the first vignette), and hypoperfusion (the second vignette). Identification of the exact mechanism has important therapeutic implications.
TIAs are common in patients with carotid artery disease and usually precede stroke onset by a few days or months. TIAs caused by intraarterial embolism from a carotid source may not be stereotypical. TIA symptoms vary, depending on which ICA branch is involved. For example, patients can have a first episode of a transient right leg weakness and weeks later have another spell characterized by expressive aphasia, right facial droop, and weakness of the right hand. This depends on the destination of the emboli. In the first example, the ACA territory is the destination, and in the later example, the MCA territory. In contrast, hemodynamic “limb-shaking” TIAs, as in the second vignette presented previously, are often stereotypical and posturally related and are usually seen in patients with high-grade ICA stenosis or occlusion. In this classic example of a hemodynamic ischemia, patients present with recurrent, irregular, and involuntary movements of the contralateral arm, leg, or both, usually triggered by postural changes and lasting a few minutes. These spells likely represent intermittent loss of cortical control and paralysis and differ from a focal seizure, in which the movements are more regular and rhythmic and usually correlate with focal repetitive cortical hyperexcitability seen on electroencephalogram.
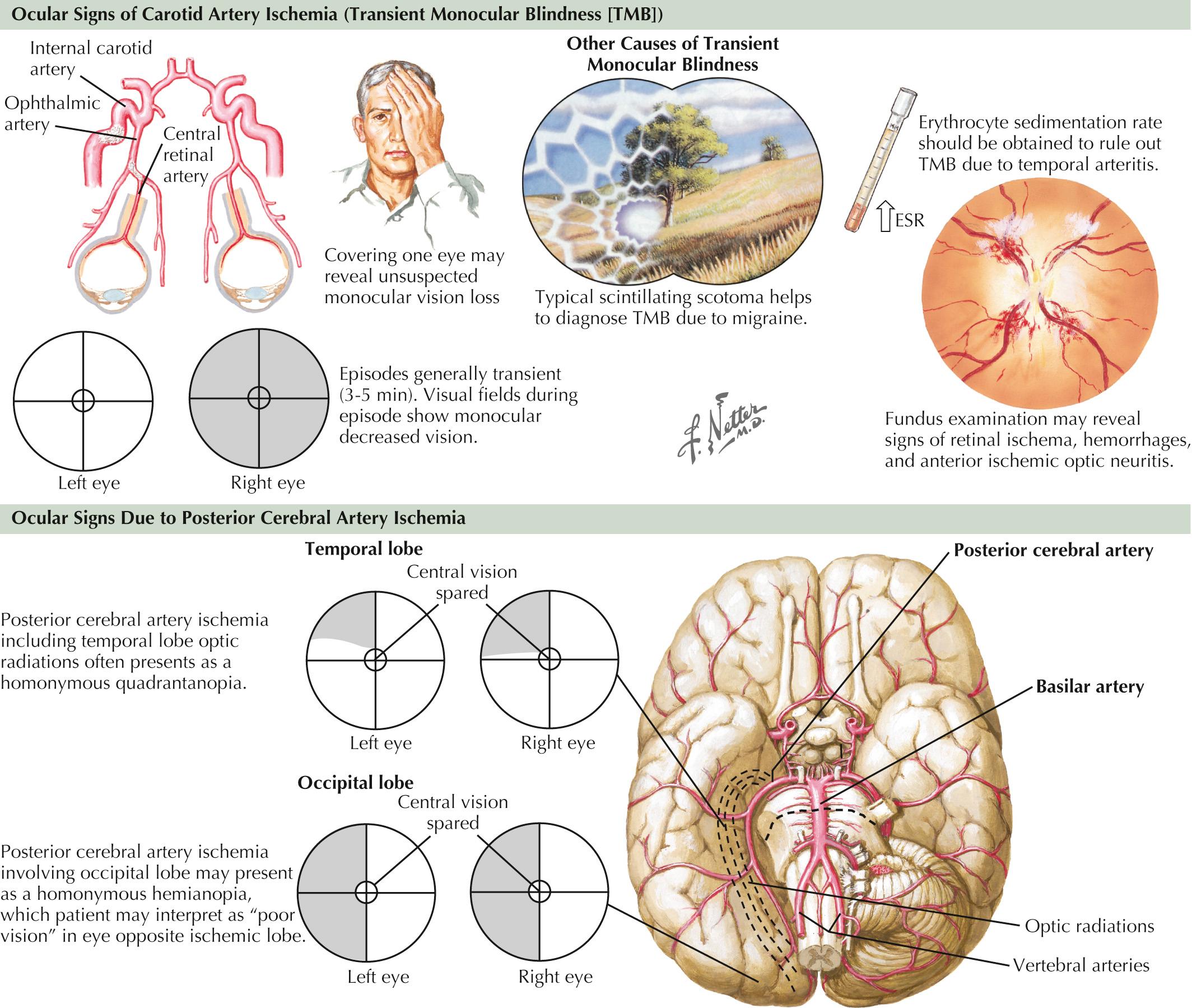
Another important clue to ICA disease is episodes of transient monocular blindness (TMB). TMB refers to the occurrence of temporary unilateral visual loss or obscuration that is classically described by careful observers as a horizontal or vertical “shade being drawn over one eye,” but most frequently as a “fog” or “blurring” in the eye, lasting 1–5 minutes. It often occurs spontaneously but at times is triggered by position changes. Positive phenomena such as sparkles, lights, or colors evolving over minutes are more typical of migrainous phenomena and help differentiate such benign visual changes from the more serious TMB, a frequent harbinger of cerebral infarct within the carotid artery vasculature. Rarely, with critical ipsilateral internal carotid stenosis, gradual dimming or loss of vision when exposed to bright light, such as glare of snow on a sunlit background, can be reported and is due to limited vascular flow in the face of increased retinal metabolic demand. Besides carotid atherosclerosis, other etiologies of TMB include cardiac embolism and intrinsic ophthalmic artery disease due to processes such as atherosclerosis or arteritis (see “ Giant Cell or Temporal Arteritis ” in Chapter 21 ), as well as decreased retinal perfusion from glaucoma or increased intraocular pressure. It is not uncommon that homonymous field deficits are reported by patients as monocular visual loss off to the affected side, and careful questioning as to whether each eye was checked independently and whether the visual difficulty involved the perception of a quadrant or one-half of the visual world is essential. For example, patients with left occipital infarctions or transient ischemia may report right-sided vision loss, but further questioning reveals that they were unable to read the right side of street signs or a license plate, and while covering the “unaffected” left eye, the seemingly abnormal right eye had retained vision within the distribution of the unaffected left homonymous field ( Fig. 15.7 ).
As in the first vignette, strokes from intraarterial embolism from ICA disease are usually cortically based. Symptoms depend on whether branches of the MCA, ACA, or both are involved. The PCA territory may rarely be affected by intraarterial emboli from ipsilateral ICA stenosis or occlusion in patients with anomalous normal vascular variants, as in a persistent fetal PCA.
Neurologic findings vary by the location of the occlusion and presence of collateral circulation ( Fig. 15.8 ). A large MCA stroke is usually seen in patients with MCA main stem occlusion without good collateral flow, whereas deep or perisylvian strokes are the most common presentation when enough collateral flow is present over the convexities.
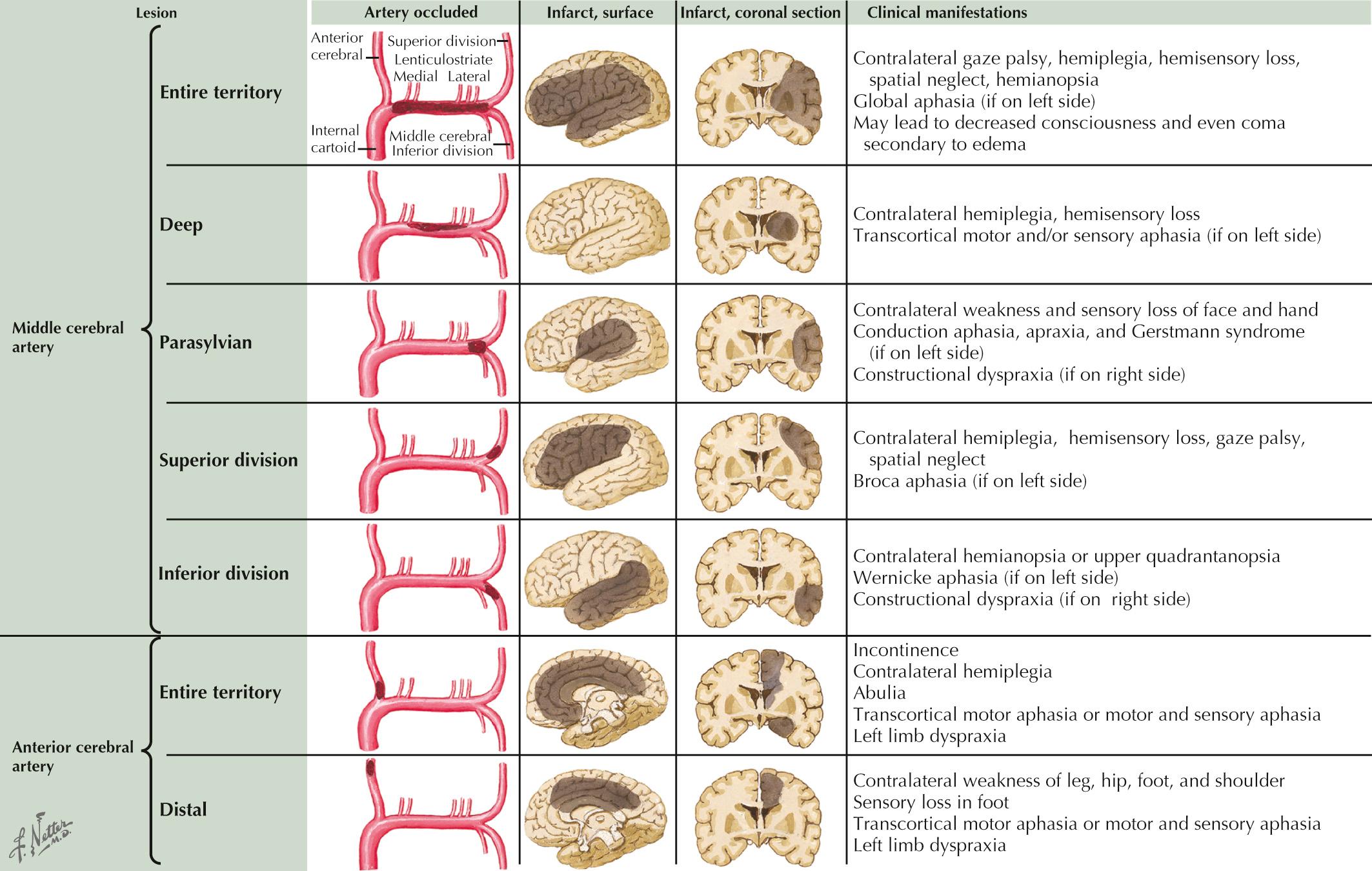
Contralateral motor weakness involving the foot more than the thigh and shoulder, with relative sparing of the hand and face, is the typical presentation of distal ACA branch occlusion. Conversely, prominent cognitive and behavioral changes associated with contralateral hemiparesis predominate in patients with proximal ACA occlusions and the involvement of the recurrent artery of Huebner (caudate and anterior limb of internal capsule infarct).
Hemodynamic strokes usually involve the border zone territory between ACA and MCA (anterior border zone), MCA and PCA (posterior border zone), or between deep and superficial perforators (subcortical border zone), and cause the typical clinical symptoms outlined in Table 15.1 .
| Stroke Location | Clinical Symptoms |
|---|---|
| Anterior border zone | Contralateral weakness (proximal > distal limbs and sparing face), transcortical motor aphasia (left-sided infarcts), and mood disturbances (right-sided infarcts) |
| Posterior border zone | Homonymous hemianopsia, lower-quadrant-anopsia, transcortical sensory aphasia (left-sided infarcts), hemineglect, and anosognosia (right-sided infarcts) |
| Subcortical border zone | Brachiofacial hemiparesis with or without sensory loss, subcortical aphasia (left-sided infarcts) |
A 70-year-old woman with history of diabetes mellitus (DM) and hypercholesterolemia presented to the ED reporting mild right-sided weakness, first noticed on awakening 2 days previously. The hemiparesis progressed to a right hemiplegia with dysarthria within 48 hours without change in the patient's level of consciousness. Head CT demonstrated a stroke involving the left centrum semiovale. Head CTA showed a distal M1 segment stenosis. The patient was started on an antiplatelet treatment and a statin. Pharmacologic treatment for her diabetes was maximized. Once stable, the patient was transferred to a rehabilitation facility with partial recovery of her motor deficits.
This vignette describes a classic course of subcortical infarct from poor perfusion of the lenticulostriate vessels secondary to a fixed lesion in the ipsilateral MCA. The patient's symptoms evolved from relatively mild hemiparesis to complete paralysis within 2 days. Unlike large MCA infarctions, there was no impairment of the patient's level of consciousness despite the progressive nature of the neurologic deficit. In contrast, large cortical MCA lesions may also evolve over 2–4 days, but due to development of cerebral edema and increased intracranial pressure, altered levels of consciousness and even coma are commonly seen.
Intrinsic occlusive disease of the MCA and ACA are more common in Asians, Hispanics, and African Americans than in Caucasians. Arterial HTN, diabetes, and smoking are the most common risk factors, with a lower incidence of high cholesterol, coronary artery disease, and peripheral vascular disease. Although TIAs can occur, they are not as common as in patients with ICA disease and usually occur over a shorter period of hours or days. When strokes occur, initial symptoms are typically noticed on awakening and often fluctuate during the day, supporting a hemodynamic mechanism.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here