Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
A number of research approaches appropriate for a neuroscientific investigation of cognition are afforded in human epilepsy surgical patients. The most common is intracranial electroencephalography (iEEG), a powerful neuroscientific tool that represents a bridge between noninvasive human functional imaging and invasive single neuron recordings in nonhuman animals. Although the approach is limited to a special population, there is an increase in the number of laboratories around the world that use this technique.
Appropriate consideration must be made for the numerous factors required to carry out successful research in this unique population, including choice of electrodes, implantation technique, electrode localization, recording setup, potential concomitant physiologic measures, and experimental approach.
Studies of cognition using iEEG have been used for all brain regions that have undergone implantation in this population, allowing insight into the complex nature of cognition in the human brain. Among the numerous studies, research on the prefrontal cortex has investigated high-level concepts such as value judgment, emotional processing, and reward expectation. Investigation of the medial temporal lobe has focused on learning, memory, and spatial navigation. New research is finding a broader role for the medial temporal lobe beyond these classical ideas, into a more general role for association of cognitive concepts and complex objects. Research using recordings from the anterior and superior temporal lobe is unpacking how we merge our audiovisual world and process sounds from simple tones to complex inputs such as music and language.
Recordings of single units and local field potentials in humans has begun to demonstrate the complexity of human cognition as enabled via distributed activity in the brain across both local and regional neural populations.
Localization of the epileptogenic zone (EZ) and eloquent cortex is crucial for planning resective epilepsy surgery. Over the years, this has necessitated the development of various techniques to localize functional cortex around the EZ, which in turn has led to groundbreaking discoveries that have profoundly influenced our understanding of the human brain, especially in regard to the neural basis and organization of higher cognition. Intracranial electrodes implanted to localize epileptic foci and eloquent cortex provide an unprecedented opportunity to study the electrophysiologic correlates (i.e., intracranial electroencephalography [iEEG]) of human cognition with a combination of spatial and temporal fidelity unmatched by other techniques. Advances in iEEG signal analysis have led to powerful new analytic approaches that have expanded the applications of iEEG recordings for cognitive research. , As a result, there has been a dramatic increase in the number of iEEG publications in recent years, which demonstrates the increasing importance of intracranial recordings for both basic and clinical neuroscience research. In this chapter we provide an overview of how intracranial recordings and related research methods can be used to study human cognition in epilepsy surgery patients, and we discuss some examples of their application.
Subjects are epilepsy patients with drug-resistant seizures who undergo intracranial electrode implantation for localization of the EZ. , Indications for iEEG monitoring are determined by a multidisciplinary treatment team based on an extensive presurgical epilepsy work-up that incudes seizure history and semiology, family history, and neuropsychologic evaluation; neuroimaging data—MRI, CT, positron emission tomography (PET), single-photon emission computed tomography (SPECT); neurophysiology data including interictal scalp EEG, video-EEG and magnetoencephalography (MEG); and functional localization data including a Wada test and functional MRI (fMRI). Research protocols must be approved by the institutional review board where the research will be taking place and must conform to the ethical guidelines of the institution’s governing bodies. In all cases, the plan for electrode placement and monitoring is determined primarily by clinical criteria. Accordingly, changes to normal risks associated with intracranial recording for seizure localization may occur as a result of research participation. The potential changes to risk and research plan must be explained to research participants in detail such that informed consent is received. It is crucial that this informed consent process be reconfirmed repeatedly immediately before starting every experimental session for the duration of the intracranial recording period, as some patients may become fatigued and wish not to participate during some portions of their hospitalization owing to having seizures or undergoing seizure-provoking procedures such as sleep deprivation. In our experience, most epilepsy patients find research participation enjoyable when they feel well and continue to participate during different intervals throughout the implantation period. iEEG research can be performed in adult subjects as well as pediatric subjects. However, the informed consent process for pediatric subjects requires special arrangement that includes explaining the research project with age-appropriate language and obtaining consent from parents or legal guardians.
The cognitive status of each subject is evaluated extensively by a neuropsychologist prior to electrode implantation, as part of the routine clinical diagnostic work-up. To maximize generalizability of results to understanding human cognition, it is desirable that the subject’s neuropsychological status fall within the normal limits of an age-matched control population. It is particularly important to ensure that subjects do not have impairment in the cognitive functions to be researched. Thus it is crucial that the cognitive functions of interest (e.g., memory, naming, executive function, or recognition) be objectively quantified prior to electrode implantation. A critical part of the determination of cognitive function comes from the results of the Wada test, which involves selective impairment of one cerebral hemisphere after intracarotid injection of anesthetic drug (e.g., sodium amytal, propofol) or from functional imaging studies to derive measures of hemispheric laterality. Such tests are used principally for the determination of candidacy for epilepsy surgery; however, the results are invaluable for assisting evaluation of cognitive function, such as hemispheric lateralization of language, general language functional performance, and memory function.
Several different types of clinical and combined clinical-research electrodes are available and can be used for intracranial seizure localization. The signals from a given electrode contact can be shared for both clinical monitoring and research purposes. It is important that the research recording not disrupt the clinical EEG monitoring, and care should be taken to communicate with clinical staff about any potential impacts that may occur or exist. There are two broad-categories of intracranial electrodes: (1) subdural (cortical surface) electrodes in the form of either grid or strip, and (2) depth electrodes ( Fig. 93.1 ). The extent of coverage is decided solely by clinical necessity based on the epilepsy work-up. Electrodes should cover a wide enough area to sufficiently include the suspected EZ or hypothesized epilepsy network. The specific implantation strategies vary widely. In the United States, surface grid and strip electrodes have traditionally been used, with a small number of depth electrodes (subdural EEG [sdEEG]). In some European countries (e.g., France and Italy), multiple depth electrodes are stereotactically implanted in strategic locations (stereo-EEG [sEEG]); this technique has been used for many decades to sample hypothesized epilepsy networks. Recently the use of sEEG has rapidly increased in North America. It is generally accepted that sEEG and sdEEG are comparable in detection of the seizure focus; however, sEEG is less invasive with shorter surgical time and fewer complications. There is also increasing evidence suggesting that surgical procedures based on sEEG techniques have a higher rate of seizure freedom. Each method has its own strengths: sEEG is better suited for cases that require bilateral electrode placement and cases in which the seizure focus is suspected of being deep from the lateral and dorsal cortical surface; on the other hand, sdEEG may be better for certain neocortical seizure foci that require functional mapping of eloquent cortex. Each institution has adopted a range of safe and effective approaches, which is beyond the scope of this chapter. It is, however, important to stress that the choice of the invasive monitoring modality should be dictated solely by clinical needs and not influenced by the research mission.
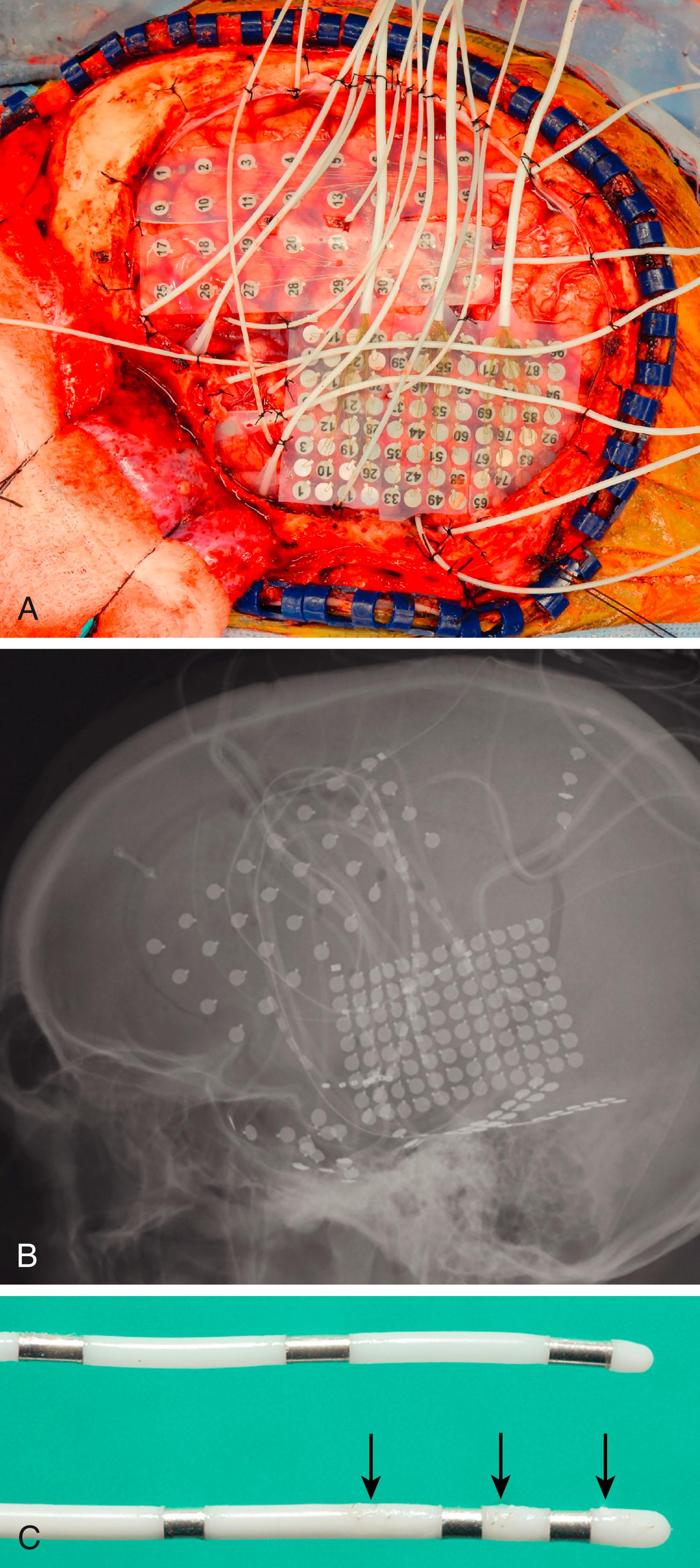
In addition to standard clinical electrodes, customized research electrodes are available that can collect research data in addition to clinical iEEG data. Some manufacturers customize electrodes to suit specific research needs (e.g., Ad Tech Corporation; PMT Corporation). Most clinical grid or strip electrodes are constructed with a center-to-center intercontact distance of 1 cm. High-density electrodes with less than 5 mm of interelectrode distance provide better spatial resolution; however, careful consideration needs to be made so as to not alter the clinical risk profile that can occur with a large wire bundle that exits the head at the same or nearby locations, which may result in skin breakdown and increased risk of infection. There are multiple different designs for depth electrodes in overall length, interval distance between electrode contacts, and diameter of electrodes. Depth electrodes can be customized to have several high-impedance microwire contacts in addition to clinical low-impedance contacts (see Fig. 93.1 ). , , , These high-impedance wires enable single- or multiple-unit recordings (i.e., recording of neuronal spiking activity of individual or groups of neurons) from the human brain and enable researchers to achieve distinct research objectives. Customized electrodes may have more electrode contacts and lead cables attached to them than standard clinical electrodes; however, single-tailed electrode cables can reduce the number of cables by combining multiple lead cables into a single bundle, thereby reducing the number of penetrations through the scalp.
Electrode implantation for research patients is essentially the same as that of a standard intracranial electrode implantation. As in purely clinical electrode implantations, preoperative planning is essential to optimize electrode coverage while minimizing mass effect caused by the electrodes. In the case of sdEEG, displacement of cortex by grids or cables may occur because of the stiffness of the base plate of the electrodes and cables. Compression of the cortical surface can be minimized by making careful cuts on the base plate of the grid electrodes and meticulously looping cables to avoid undue torsion on the grid or strip electrodes. It is also important to pay careful attention to prevent leakage of cerebrospinal fluid (CSF) by placing a tight purse-string suture at each cable exit site on the scalp. It is not unusual to see CSF leakage around cable exit sites several days after the implantation procedure. This delayed leakage is probably due to subsidence of postoperative swelling of the scalp or breakdown of tissue around cable sutures that makes previously tight seals around cables loose enough to allow leakage of CSF. As soon as a CSF leak is noticed, the source of the leak should be identified and terminated by placing additional sutures at leak sites to reduce the risk for infection. In a series of approximately 200 patients who underwent implantation with chronic intracranial electrodes at the University of Iowa over a 15-year period, there was no significant difference in the infection rates of patients who were research participants and those who were not.
Accumulation of blood in the subdural space either beneath or above the grid electrodes may occur. Although the exact mechanism of such blood accumulation is unknown, it is presumed that direct contact between the base plate of the grid electrodes and the dura mater may disturb normal hemostatic and resorptive processes. At our institution we perform the following procedures in an effort to prevent accumulation of blood in the subdural space:
Cutting multiple slits in the base plate to prevent entrapment of blood underneath the grid (making multiple cuts on the base plate of the grid electrodes also reduces the stiffness of the grid, thus allowing the grid to conform to the shape of the brain surface and avoiding compression of the cortex, which minimizes the space between the dura mater and the electrodes, where blood potentially accumulates)
Placing Surgicel (oxidized cellulose) over the grid electrodes to prevent direct contact of the grid with the inner surface of the dura mater
Performing meticulous hemostasis of the incising edge of the dura mater by means of bipolar electrocautery
sEEG is becoming an increasingly more prevalent mode of invasive monitoring relative to sdEEG, likely because sEEG obviates the need for craniotomy, facilitates bilateral exploration, and is associated with lower morbidity. , Techniques for sEEG implantation can be frame based using either a Talairach or a Leksell frame, and an increasing proportion of sEEG implantations are performed using a frameless robotic-assisted technique. Commonly, electrode targeting is based on specific anatamo-electro-clinical hypotheses that can direct subsequent resective epilepsy surgery or neuromodulation (i.e., responsive neurostimulation [RNS] or deep brain stimulation [DBS]). sEEG recordings provide simultaneous assessment of gyral surface, sulcal, and deep gray matter structures, with gray matter recording density that is similar to that of sdEEG.
Accurate localization of electrodes on the brain surface is crucial to correctly interpreting research data. Various techniques have been developed for this purpose. Preimplantation and postimplantation CT, MRI, and photographs taken during both implantation and removal surgery are the three main tools used to localize the position of electrodes. Intracranially implanted electrodes create substantial artifact and distortion of images on CT and MRI, so extra caution is required when interpreting postimplantation imaging studies. Locations of subdural contacts on exposed cortical surface are best documented by comparing photographs taken at the time of both implantation and removal operations with postimplantation MR and CT images. By matching the details of gyral and pial vessel anatomy and applying manually guided nonlinear coregistration (e.g., thin-plate spline warping) to compensate for postoperative tissue deformation, it is possible to localize surface contact locations with millimeter accuracy. , It is important to document by photograph at both implantation and removal operations because electrodes might change position relative to the underlying brain after implantation for any of several reasons, which include closure of the dura; head or limb motion that puts tension on electrode cables, as might occur during a seizure; or changes in conformation of the brain such as brain sagging during implantation or postoperative brain edema. The position of electrodes is mapped onto a three-dimensional rendering of the brain surface created from each subject’s preoperative thin-slice MRI studies by referencing gyral and sulcal patterns in imaging, photographs, and surface renderings ( Fig. 93.2 ).
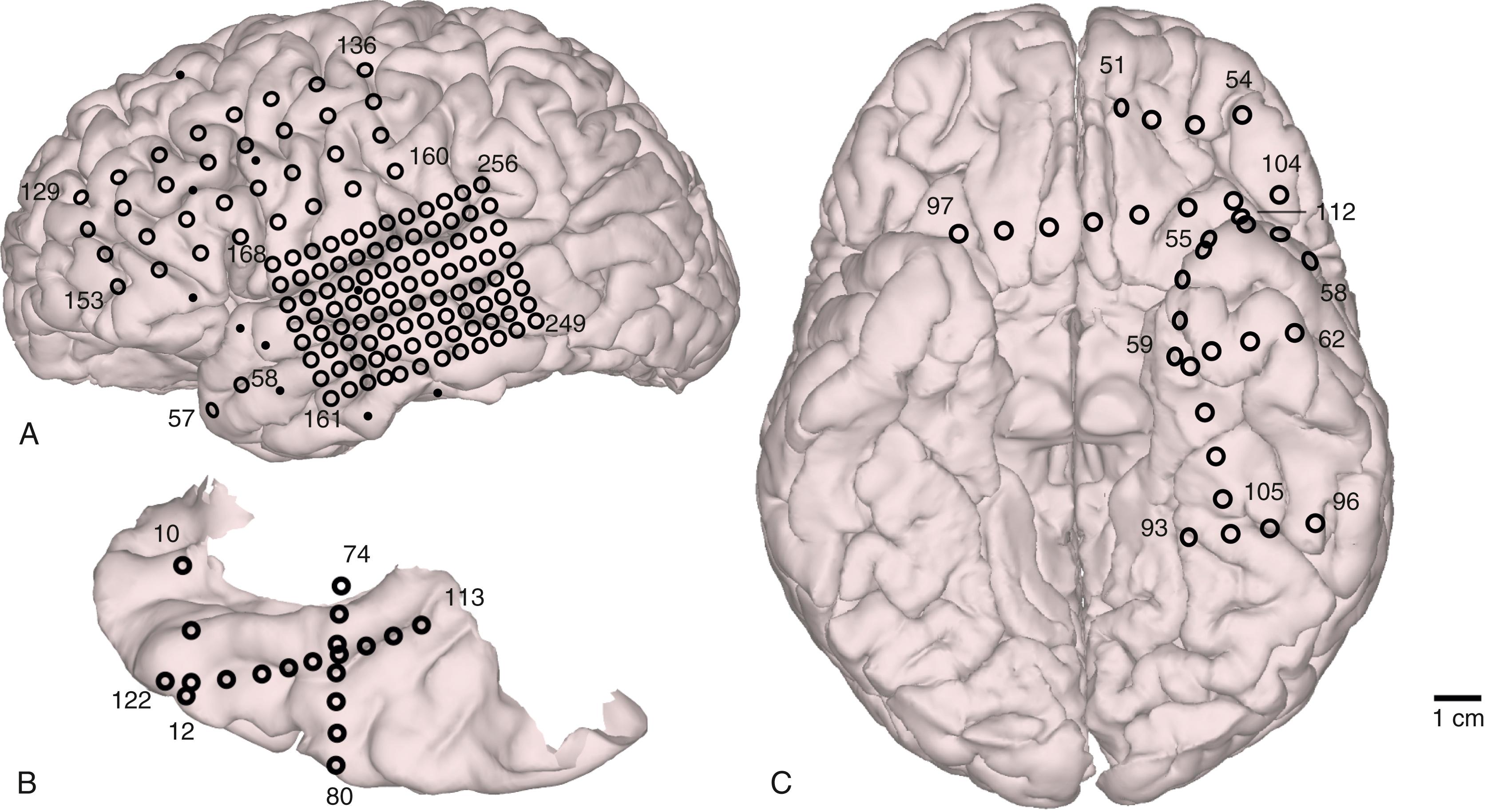
Localizing electrodes on the ventral surface of the brain is a difficult challenge because these electrodes cannot be viewed directly during surgery and consequently cannot be documented with intraoperative photography. Susceptibility artifacts created by the air-bone-tissue interface may also obscure nearby electrodes in MRI. Metal contacts are more reliably demonstrated with thin-slice CT, and their relationship to the bony structures can be observed by adjusting the level and width of the display window; the metallic artifact, however, tends to obscure adjacent brain parenchyma. It is therefore useful to compare aligned CT and MRI images, identifying electrodes in the former and observing its relationship to brain anatomy in the latter. The position of electrodes can then be mapped onto the surface rendering of the preoperative brain image with appropriate warping.
Electrode contacts on a depth electrode can be localized in relation to surrounding brain structures by means of postimplantation MRI. Only the larger, low-impedance macro contacts can be clearly delineated with postimplantation MRI, but with knowledge of the spacing of microwires positioned between these contacts it is possible to depict accurately where these recording sites are within the brain, and these locations can be depicted on the preimplantation MRI study ( Fig. 93.3 ). , , Development of techniques to automatically localize intracranial electrodes is underway.
The following sections outline some of the main approaches being used to investigate cognition in epilepsy patients.
It is technically feasible to obtain massive amounts of iEEG recording data from hundreds of electrode contacts implanted in each surgical patient. Modern signal processing methods also enable investigators to use a wide range of analytic methods to discern what physiologic events are relevant to the cognitive functions being investigated. The practical challenge is to carefully plan and execute experimental protocols so that the results are interpretable and the limitations of the methods used are appropriately recognized. The key practical data collection issues are reviewed and discussed subsequently.
Modern clinical EEG recording equipment converts EEG potentials to digital signals and has the capability of recording more than 250 channels with high sampling rates. Depending on the specific research question being addressed, research recordings may require a wider frequency bandwidth than is typical of clinical recordings. Although it is possible to use clinically recorded EEG signal for cognition research, it is better to have dedicated research recording equipment separate from the clinical EEG recording system so that research recordings can be performed more flexibly without disturbing the clinical EEG recording. In addition, the higher sampling rates (>1000 Hz) used for research recordings enable investigators to study high-frequency brain activity that is not captured with standard clinical sampling rates. It is ideal to use battery-driven head stages and optical isolation of the EEG signal from the research amplifier-recording system to minimize the chance of injuring subjects by accidental leakage of current. Most institutional review boards and hospital biomedical engineers require this level of electrical isolation for the patient. It is imperative to follow local or hospital safety regulations regarding use of research equipment on human subjects. Almost all modern neurophysiologic recording systems have a digital recording design. Recorded data can be stored on digital recording media such as hard disk drives or optical recording media. Stored data can be analyzed offline with various commercially available or custom-made software. Because data are shared among many researchers, it is important to separate a subject’s identifiable information, such as name, date of birth, or medical record number from the recorded data by replacing such information with unique research identifiers pursuant to regulations for the protection of personal health information.
At the University of Iowa we use a commercially available signal breakout box (Neuralynx) or a custom-built signal breakout box to feed the iEEG signal picked up from the subject simultaneously to both a clinical iEEG recording device and a research recording device. This makes it possible to conduct research recordings without disrupting clinical EEG monitoring. Researchers can use dedicated research recording equipment, and any required research equipment can be disconnected for subjects’ convenience when research activity is not being performed.
Contamination by ambient electronic noise can become a problem, more so for the research recordings than for the clinical EEG recordings. Among various sources of noise, power line noise is typically the most pervasive and requires the greatest attention to eliminate. It contaminates EEG signal at fundamental power line frequency the most, which is 60 Hz in the United States, and is often prominent at odd harmonics (i.e., 180, 300, 420 Hz, and so on), but can also be present at even harmonics. Although notch filtering may effectively reduce power line noise, such filtering distorts the EEG waveform and may affect the results of frequency analyses. Therefore every possible effort must be taken to reduce noise contamination at its source. At the University of Iowa, research participants are housed in a specially constructed, electromagnetically shielded room in the National Institutes of Health–funded Clinical Research Unit. A significant amount of medical and nonmedical equipment is necessary for both the medical treatment and the convenience of the subjects, who spend several days to up to 2 weeks in the room. It is useful to unplug as many power cords as possible from alternating current power outlets when research recording is being performed. If any equipment can be run on battery power, it should be turned to battery mode, including the recording equipment. Note, however, that DC to AC conversion can undo the benefit of battery power for equipment that requires an AC power source; therefore it is best to check noise levels for all relevant equipment with and without battery power. Special attention should be paid to any powered device that is in close proximity or direct contact with the patient, such as hospital beds. Some recording amplifiers now have the ability to be plugged into the main power source; however, when possible, the best noise isolation will be achieved on DC battery power. Shielding EEG connection cables can reduce the extent of noise contamination. If some equipment must be powered by alternating current, careful attention should be paid to keep the power cords away from the EEG recording equipment and connecting cables. Hospital-grade power cords must be used for all equipment, if possible, not only to reduce the noise level, but also to reduce the chance of injuring the patient by leakage of current.
Noise can be removed digitally offline after the EEG signal is recorded. Band-pass filtering is the simplest way to remove the power line noise; however, it will also distort EEG signal in the vicinity of the main noise frequency. In principle, perfectly periodic line noise contamination with constant amplitude might be removed with near infinitesimal distortion of the physiologic signal by using the smallest possible filter bandwidth. In practice, however, the energy of the contaminating signal is dispersed over a range of frequencies for three reasons: (1) fluctuations over time in noise amplitude and, to a lesser extent, (2) small fluctuations of center frequency, and, finally, (3) spectral leakage artifacts arising from windowing of the signal in time, all of which necessitate a lower bound on the filter bandwidth.
Bandpass filtering with a fixed bandwidth can be improved on through adaptive filtering, which adjusts filter characteristics over time according to noise frequency and magnitude, often through some form of thresholding within windows of shorter duration than the complete recording. One must take care to minimize spectral leakage artifacts that arise from the application of frequency windowing to brief time windows, as is implicit in the use of finite impulse response (FIR) filters, especially when the window duration cannot reliably be fixed to a multiple of the noise period. The risk of spectral leakage may also be minimized through techniques of complex demodulation that begin by segmenting the signal in the Fourier domain rather than the time domain. Such techniques are especially well suited to handling variable line noise, but they are limited to offline denoising of continuously acquired recordings, as they require access to the Fourier transform of the complete recording.
Electrical stimulation of the brain has been used for the investigation of brain function for close to a century and is still considered the “gold standard” for functional mapping of cortex to minimize the risk for postoperative functional deficits. , Electrical stimulation of the cortex is believed to either facilitate or disrupt local brain function in the vicinity of a stimulation site, depending on the stimulation parameters. Exactly how the frequency of electrical stimulation modulates neural activity within the stimulated structure is poorly understood and is the subject of many ongoing experimental investigations. With that caveat, it is generally believed that high-frequency stimulation well above 50 Hz exerts an inhibitory effect on the firing of neurons in the brain region exposed to suprathreshold currents. Stimulation in the 50-Hz range may activate sensory cortices and cause a subject to experience a sensory perception, but stimulation of language critical sites can disrupt function without generating a percept. This technique is useful for the investigation of local cortical and subcortical function not only in the clinical setting but also as a research tool. Averaged evoked potentials elicited by electrical stimulation can be used for the investigation of connectivity between remote brain sites. By manipulating stimulus parameters, stimulation site, and recording site, it is possible to delineate functional connections in various brain regions.
One of the risks of electrical stimulation is the possibility of inducing a seizure. It is important that a qualified physician continuously monitor the iEEG recording during the electrical stimulation procedure to detect the occurrence of afterdischarges or local seizure activity induced by electrical stimulation. Once an afterdischarge is detected, electrical stimulation must be stopped temporarily until afterdischarges disappear. On restarting the stimulation procedure, the stimulus intensity should be decreased. Consideration should also be given to administering a loading dose of phenytoin or fosphenytoin prior to mapping the patient, in order to decrease the risk of causing seizures during the procedure. It is important to note that the afterdischarge threshold varies across different cortical locations. From our experience, the afterdischarge threshold in the primary sensory and primary motor cortices is lower than that of other brain regions. The threshold level may also be low in areas neighboring a seizure focus, which is a reason why it is advantageous to await localization of the seizure focus, when patients have restarted antiseizure medications, prior to performing brain stimulation research. Most afterdischarges stop spontaneously within a few minutes; however, they may occasionally progress to a generalized seizure.
One major limitation of electrical stimulation mapping is the requirement for activity to propagate to the location of another recording electrode. Although a number of brain regions are covered for a chronic epilepsy monitoring investigation, the majority of the brain is uncovered. One approach to get around this issue, implemented at the University of Iowa and University College London, is to place patients within the MRI scanner and record functional activity while stimulating an implanted electrode. This allows a recording of the propagation of stimulation-induced activity across the entire brain. A notable limitation, however, is that artifact in the MRI image still occurs at the location of an implanted electrode.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here