Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fungal infections including invasive Candida infections and molds (aspergillosis and mucormycosis) are devastating infections most commonly complicating extreme preterm infants due to their underdeveloped immune system combined with the need for intensive care and any neonate with complex gastrointestinal disease.
Dermatologic findings of candidiasis and molds are critical to their prompt diagnosis and correct empiric antifungal selection.
Congenital cutaneous candidiasis is an invasive infection that requires prompt recognition and evaluation as well as systemic treatment for 14 days. Dermatologic findings of congenital cutaneous candidiasis commonly involve skin desquamation and maculopapular, hypopigmented, and/or erythematous rashes.
Cutaneous aspergillosis presents as erythematous indurated papules or white plaques that progress to ulceration and white-gray-yellow coloration or exudates. They can evolve into target lesions with a white-yellow ring with an erythematous interior and exterior ring. Voriconazole or echinocandins are first-line therapy for aspergillosis infections.
Mucormycosis presents as an erythematous to purple papule with a central ulceration or induration that evolves into a black eschar, usually in a skin area that has been covered, injured, or in a pressure area such as the back. Amphotericin is the first-line therapy for mucormycosis.
Major risk factors for invasive Candida infections include extreme prematurity during the time period the infant requires intravenous access, gastrointestinal immaturity, dysmotility, injury, or disease and exposure to broad spectrum antibiotics, acid suppression medications, and postnatal steroids.
The highest-risk patients for invasive Candida infections are infants less than 1000 g at birth or 28 weeks’ gestation until they no longer require intravenous access, due to their high mortality and risk of neurodevelopmental impairments from infection. Preventative measures including targeted antifungal prophylaxis have lowered the incidence in this group significantly, approaching zero.
Candida pathogenesis involves exposure, adherence, and colonization, followed by infection and commonly organ involvement. All infected infants need screening for end organ dissemination.
Cultures are critical for diagnosis of invasive Candida infections and should include blood, urine, and cerebrospinal fluid at the time of presentation. Additionally, peritoneal cultures should be obtained in any infant bowel perforation or necrotizing enterocolitis requiring laparotomy or drainage.
Survival and infection-related outcomes are improved with central venous catheter removal for candidemia, prompt antifungal dosing for all infected patients, and empiric therapy in high-risk patients.
Amphotericin B is the optimal choice for systemic treatment of invasive Candida infection due to Candida susceptibility patterns and cost. Fluconazole should not be the first choice until speciation and susceptibilities are known, because several Candida species are resistant to fluconazole. Additionally, if fluconazole is used for prophylaxis, a different antifungal should be selected to minimize emergence of resistance.
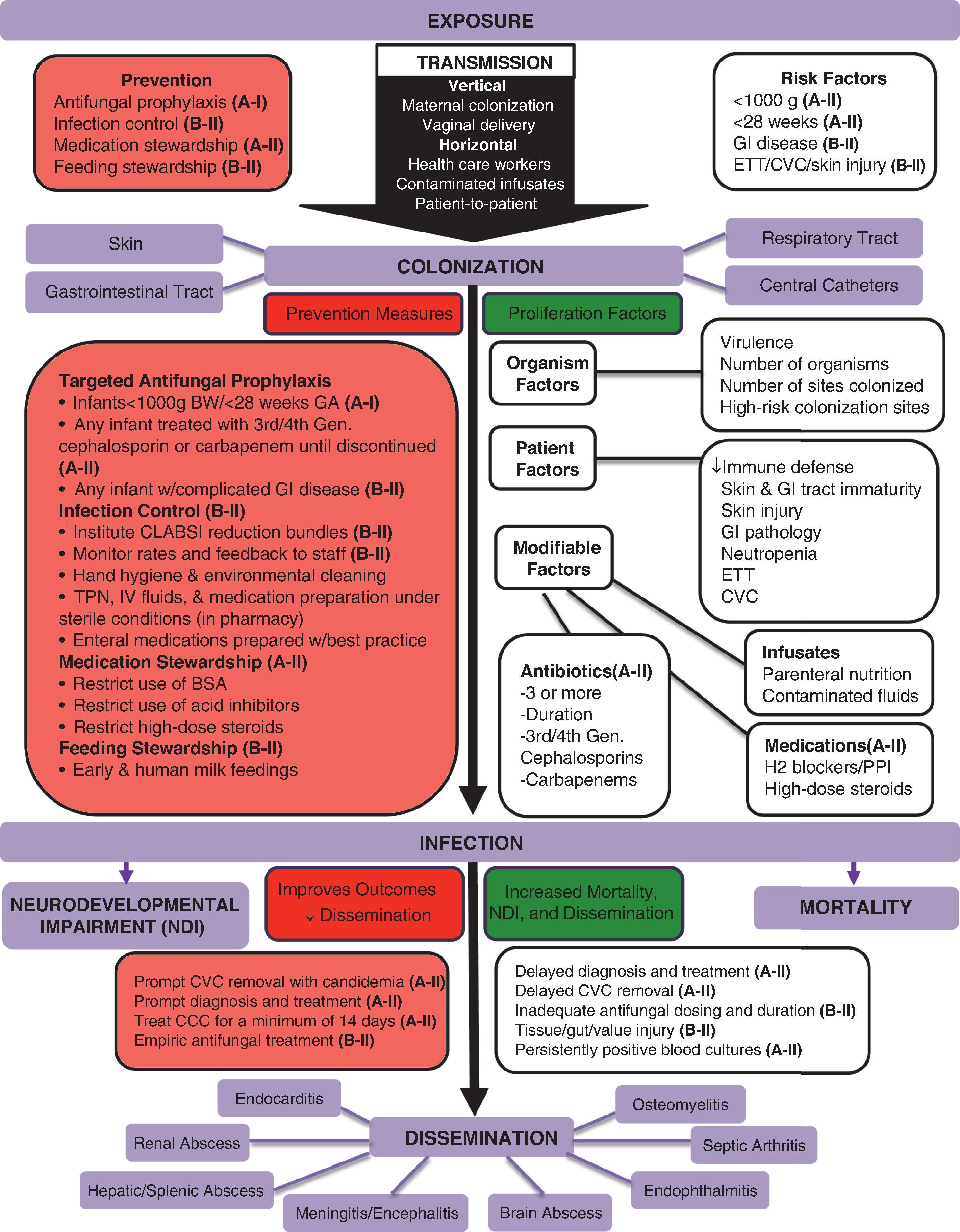
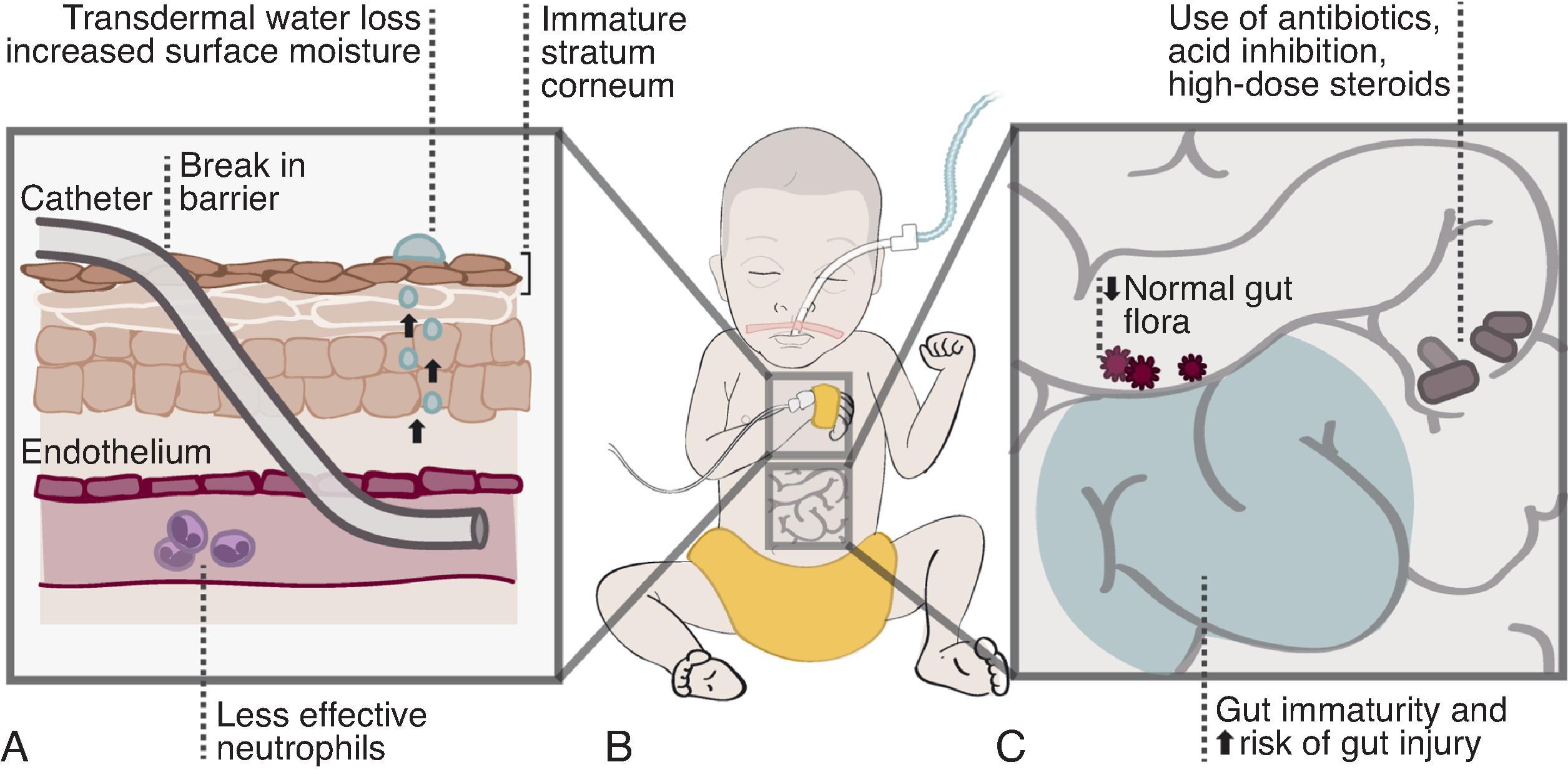
Fungal pathogenesis involves exposure, adherence, colonization, and ultimately infection. Measures can be applied for each of these four aspects to prevent infections and improve outcomes in those infected. Invasive Candida infections (ICIs) in the neonatal intensive care unit (NICU) most commonly include bloodstream and urinary tract infections, meningitis, peritonitis, and congenital cutaneous candidiasis, whereas mold infections more often present as cutaneous lesions or complicate necrotizing enterocolitis (NEC). NICU patients are at increased risk for invasive fungal infections due to their developing immune system and need for intensive care including catheters and tubes that breach important protective barriers ( Figs. 35.1 and 35.2 ). Furthermore, molds take advantage of skin that has been covered, in dependent areas, and at the site of any skin injury or complicated NEC.
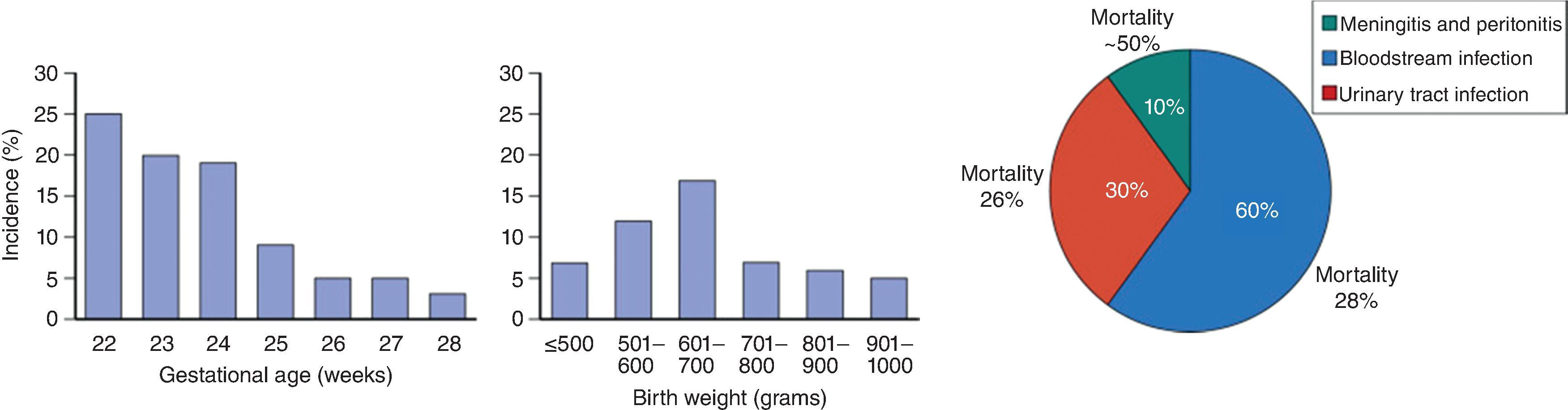
Extremely preterm infants represent the highest-risk patients in the NICU, and incidence is inversely proportional with gestational age ( Fig. 35.3 ). Many studies demonstrate rates of ICI >10% in infants less than 25 weeks’ gestation and decreasing to around 5% in infants 27 weeks’ gestation. The highest risk patients are infants <1000 g or <28 weeks’ gestation, in whom the incidence is significant and infection is associated with high mortality and neurodevelopmental impairment. Mold infections are less common but are increasing as we care for more infants at the lowest gestational ages.
Targeted antifungal prophylaxis in high-risk patients is the most effective prevention measure for ICI and has been critically studied in randomized controlled trials. For the entire NICU, a prevention bundle should include (1) targeted antifungal prophylaxis in high-risk patients <1000 g or <28 weeks’ gestation during their high-risk period while they have intravenous access (A-I evidence, Strong Recommendation ); (2) central line associated bloodstream infection (CLABSI) preventative practices (A-II, Strong Recommendation ); (3) antibiotic, medication, and feeding stewardship (A-II, Strong Recommendation ); and (4) infection control.
Infectious morbidity and mortality can be improved by (1) starting the appropriate antifungal agent and dose (A-II, Strong Recommendation ); (2) prompt central venous catheter removal when candidemia is present (A-II, Strong Recommendation ); (3) prompt recognition of dermatologic findings of invasive cutaneous fungal infections, evaluation, and systemic treatment for 14 days (A-II, Strong Recommendation ); (4) end organ dissemination (EOD) screening and treatment (A-II, Strong Recommendation ); (5) empiric antifungal therapy in high-risk patients when there is a high suspicion for fungal infection (A-II, Strong Recommendation ); and (6) antenatal treatment of vaginal candidiasis (B-II, Weak Recommendation ).
ICI is defined as the presence of Candida species in a body fluid or tissue sample and includes bloodstream infections (BSIs), urinary tract infections (UTIs), peritonitis, meningitis, congenital (or diffuse) cutaneous candidiasis, and any infection of otherwise sterile tissue such as bones and joints. , These invasive infections are diagnosed based on a positive culture of blood, urine, cerebrospinal fluid (CSF), peritoneal fluid, or tissue. For congenital cutaneous candidiasis, diagnosis requires a diffuse rash with identification of Candida or yeast from the skin, placenta, or umbilical cord. These ICIs can disseminate directly or hematogenously. They can lead to end organ abscesses due to multiple adhesive factors of Candida and damage the heart, kidneys, brain, liver, spleen, bone, and joints (see Fig. 35.1 ).
The majority of ICIs in the NICU are due to Candida albicans (∼50%), followed by C. parapsilosis (30%–40%) and to a lesser degree C. glabrata. Infections due to Candida tropicalis , C. lusitaniae , C. krusei , C. guilliermondii , and other species occur less frequently. C. albicans is the most pathogenic of the Candida species, with mortality rates two to three times higher compared with nonalbicans candidemia.
Extremely preterm infants represent the highest-risk patients in the NICU, and incidence is inversely proportional with gestational age. , There is variation in rates of ICI in NICUs as well as the incidence reported in the literature due to the factors listed in Table 35.1 .
| Reporting bias |
|
| Resuscitation gestational age cutoff |
|
| Surgical and complex gastrointestinal diseases |
|
| Targeted antifungal prophylaxis |
|
In the absence of antifungal prophylaxis, the incidence of ICIs in extremely low birth weight (ELBW; <1000 g) infants, not including congenital cutaneous candidiasis, is around 10% (see Fig. 35.3 ). The incidence decreases from >20% at 23 weeks’ gestation to 3% at 28 weeks’ gestation. The incidence of 5% in infants of 27 weeks’ gestation marks a potential cutoff for targeted antifungal prophylaxis in the first weeks after birth while other risk factors are present (central venous catheter, endotracheal tube, antibiotics, and/or parenteral nutrition). , Although bloodstream infections account for the majority of ICIs in ELBWs, Candida UTIs would increase the incidence an additional 3% to 4%, and meningitis and peritonitis (complicating any bowel perforation) an additional 1% to 2%. , The average candidemia rates are much lower in larger infants (1.32%, 0.36%, and 0.29% for birth weights of 1001–1500, 1501–2500, and >2500 g, respectively).
Proliferation is favorable under certain conditions such as when antibiotics are used, as they eradicate competitive flora; when H2 blockers and proton pump inhibitors are used, because they decrease stomach acidity, which is an important defense against Candida ; or when postnatal steroid exposure impairs granulocyte function. Longer antibiotic duration or exposure to multiple antibiotics, particularly third- and fourth-generation cephalosporins or carbapenems, are associated with increasing risk for ICI. , , Dexamethasone and high-dose hydrocortisone (>1 mg/kg/day) are associated with increased incidence of ICI, but physiologic dosing of hydrocortisone (≤1 mg/kg/day) does not appear to increase risk.
Central venous catheters, endotracheal tubes, and certain feeding practices increase the risk of ICI. Risk of ICI is decreased by receiving an early as well as an all human milk diet, because it is shown to lower rates of NEC and its associated ICI rate of 10%. While increased amounts of fresh expressed human milk are associated with fewer bacterial infections, studies have not demonstrated a decrease in ICI.
Gastrointestinal pathology is associated with an increased risk for candidemia and/or Candida peritonitis in patients with NEC, bowel perforation, gastroschisis, Hirschsprung disease, omphalocele, intestinal atresia, or tracheoesophageal fistula. , ,
Candida pathogenesis involves exposure, followed by colonization, infection, and dissemination.
As discussed above, prematurity is the greatest risk factor for ICI. This is due to these infants’ underdeveloped immune system and immature and often breached (by central catheters and endotracheal tubes) defense barriers including the skin and the gastrointestinal and respiratory tracts (see Fig. 35.2 ). Candida species are potential opportunistic pathogens for preterm infants because they are naturally present on the skin, oral, and gastrointestinal mucosa, primarily as saprophytes. Candida species can also lead to infections if the host is exposed to a large number of organisms (at birth or with poor infection control) and after colonization when risk factors allow Candida to proliferate easily or barriers are compromised (e.g., skin breakdown, NEC, or the presence of an endotracheal tube or intravenous catheter).
Colonization rates are inversely correlated with gestational age and birth weight, similar to infection. In the first weeks of life, >50% of ELBW and 25% to 50% of very low birth weight (VLBW; <1500 g) infants may become colonized, as compared with 5% to 10% of full-term infants. Skin and gastrointestinal colonization occurs initially, followed by the respiratory tract. Approximately 25% of colonized VLBW infants will progress to infection; risk is influenced by the number and location of colonized sites. Colonization of multiple sites or colonization at a single high-risk site (endotracheal tube, urine, catheter tips, drains, and surgical devices) is associated with higher ICI risk than colonization at a single low-risk site. ,
At the time of presentation, Candida often has already disseminated to other tissues, organs, or body fluids and formed microabscesses. This is due to adherence properties of Candida , its slow growth prior to clinical signs and symptoms, and the patient’s immunocompromised state. Additionally, central vascular catheters can cause local trauma to valvular, endocardial, or endothelial tissue, followed by a thrombus to which yeast can adhere. More rapid diagnosis, treatment, and prevention of ICIs have decreased EOD, but it still is important to screen for EOD. Among infants with ICI, the incidence of concomitant endocarditis is around 5%, kidney abscesses 5%, central nervous system (CNS) abscesses 4%, and endophthalmitis 3%. EOD is higher in ELBW infants and any infant with candidemia lasting >5 days.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here