Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The aims of this book are to present and explain some basic anatomical facts about how the brain is put together, to discuss aspects of how it works, and to present clinical features to aid in application and retention. This introductory chapter describes in a general way the subdivisions of the nervous system then focuses on the cellular elements found within it and the anatomical specializations that adapt these cellular elements to their respective functions.
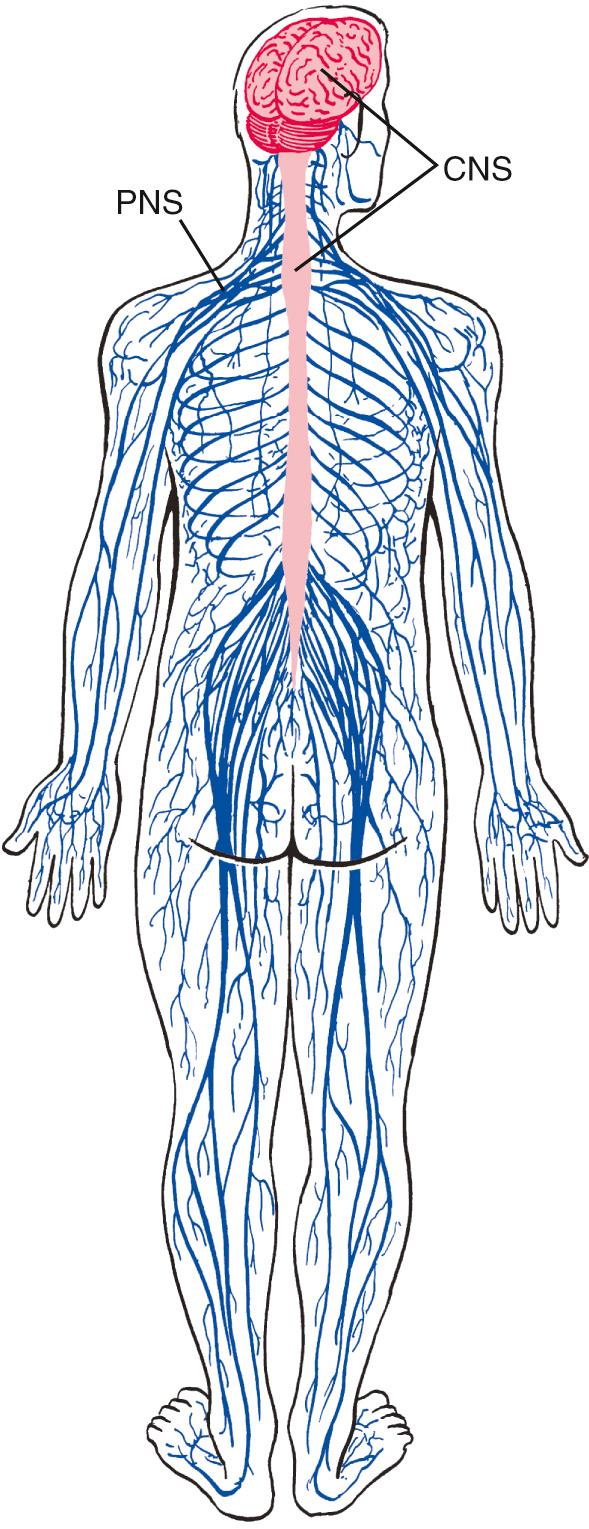
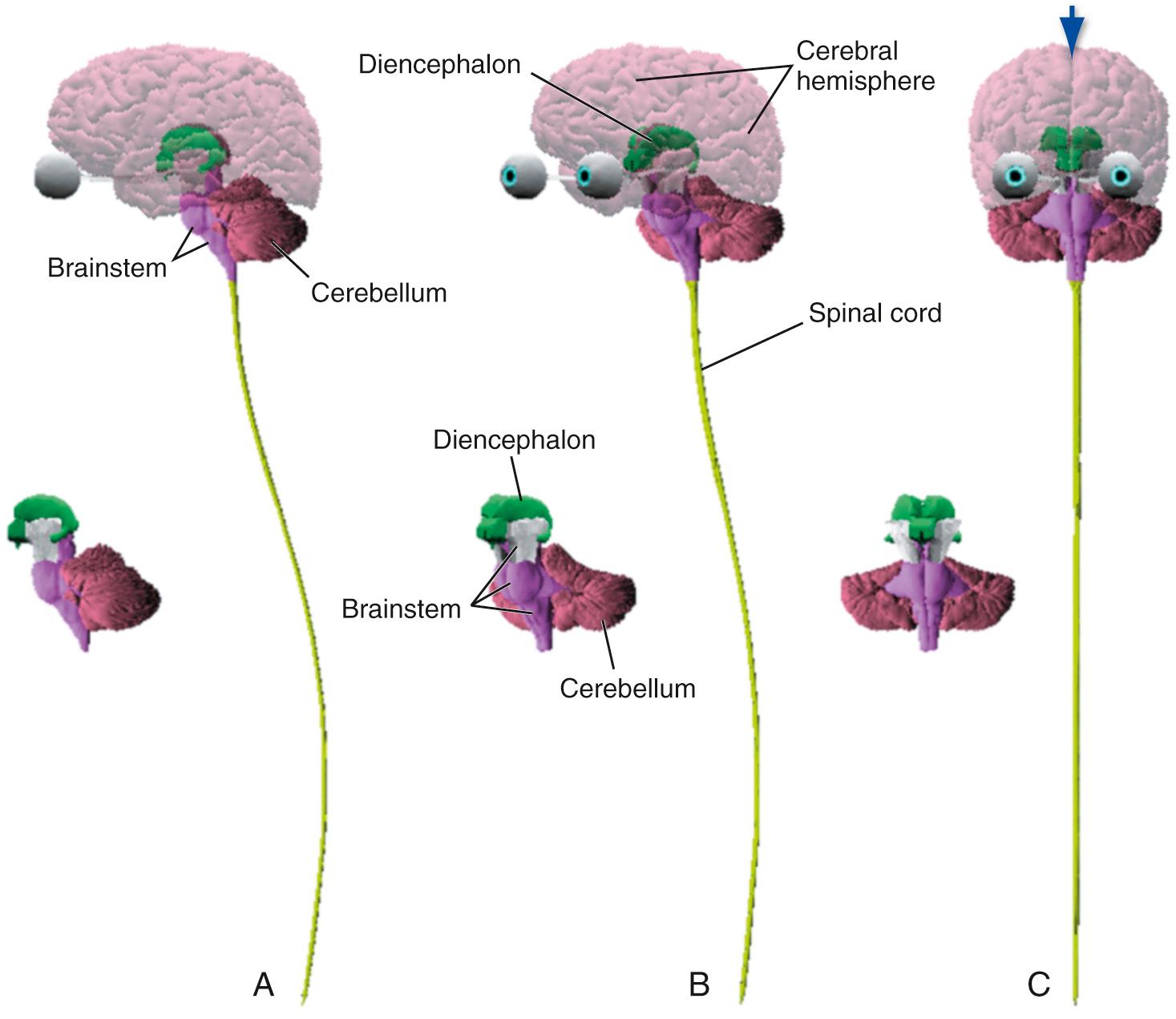
The nervous system is broadly subdivided into the peripheral nervous system (PNS) and the central nervous system (CNS) ( Fig. 1.1 ). The PNS is the collection of spinal and cranial nerves whose branches infiltrate almost all parts of the body, conveying messages to and from the CNS. The CNS, ensconced in the skull and vertebral column, is composed of the brain and the spinal cord ( Fig. 1.2 ). The brain has multiple subdivisions and is composed of the forebrain, the cerebellum, and the brainstem. The forebrain, in turn, is composed of the two massive cerebral hemispheres (separated from one another by the longitudinal fissure —a long cleft between the two hemispheres) and the diencephalon a
a Much seemingly arcane neuroanatomical terminology has a Latin or Greek derivation that actually makes sense. In this case, encephalon is Greek for “in the head” (i.e., “brain”). Diencephalon means “in between brain,” signifying that this part of the CNS is interposed between the cerebral hemispheres and the brainstem.
; in an intact human brain most of the diencephalon is hidden from view by the massive cerebral hemispheres. The brainstem is the part of the CNS, exclusive of the cerebellum, that lies between the forebrain and the spinal cord.
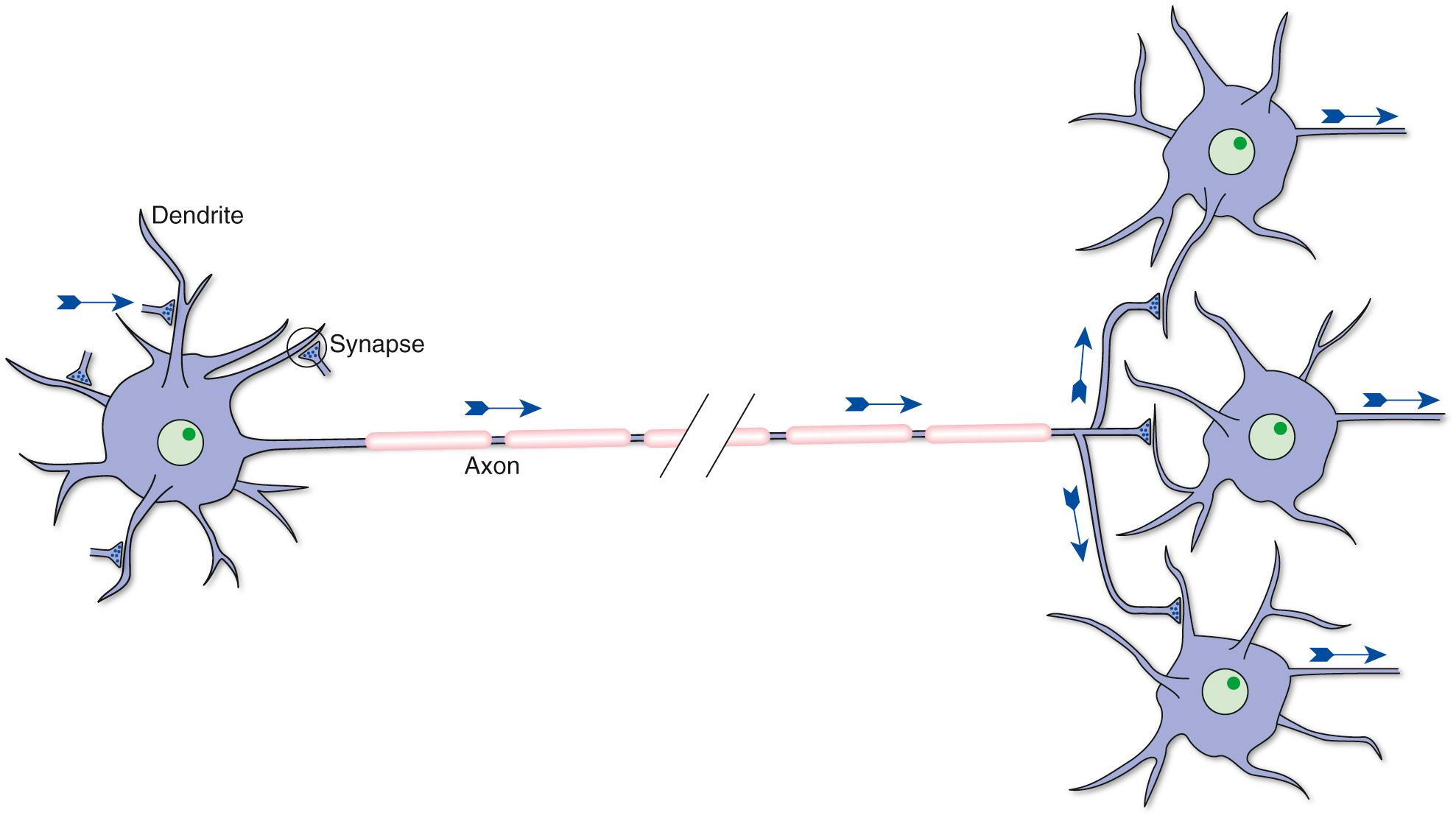
Despite the large size and widespread distribution of the nervous system, it contains only two principal categories of cells— nerve cells, or neurons, which are the information-processing and signaling elements, and glial cells, which play a variety of supporting roles. Neurons and glial cells are present in enormous numbers. There are around 100 billion neurons in the human nervous system and a similar number of glial cells.
Neurons convey information. They do so by a combination of electrical and chemical signaling mechanisms: electrical signals are used to convey information rapidly from one part of a neuron to another, whereas chemical messengers are typically used to carry information between neurons. Hence there are anatomically specialized zones for collecting, integrating, conducting, and transmitting information ( Fig. 1.3 ; Table 1.1 ). All neurons have a cell body (or soma b
b Sometimes also referred to as the perikaryon. Karyon is Greek for “nucleus,” and, strictly speaking, the perikaryon is the cytoplasm surrounding the nucleus of a neuron.
) that supports the metabolic and synthetic needs of the rest of the neuron. Most neurons have a series of branching, tapering processes called dendrites (Greek for “like a tree”) that receive information from other neurons at junctions called synapses c
c Synapse is derived from two Greek words meaning “to fasten together.”
and one long, cylindrical process called an axon that conducts information away from the cell body. The axon gives rise to a series of terminal branches that form synapses on other neurons. Hence neurons are anatomically and functionally polarized, with electrical signals traveling most often in only one direction under ordinary physiological circumstances. (The molecular underpinnings of this anatomical and functional polarization are discussed in Chapter 7, Chapter 8, Chapter 9 .)
| Part | Description | Major Organelles | Primary Functions |
|---|---|---|---|
| Dendrites | Tapered extensions of cell body | Cytoskeleton, mitochondria | Collect information from other neurons |
| Soma (cell body) | May have one, two, or many processes; typically one axon, many dendrites | Nucleus, Golgi apparatus, Nissl substance, cytoskeleton, mitochondria | Synthesize macromolecules, integrate electrical signals a |
| Axon | Single, cylindrical; may be many centimeters long; may be myelinated or unmyelinated | Cytoskeleton, mitochondria, transport vesicles | Conduct information to other neurons |
| Axon terminals (synaptic endings) | Vesicle-filled apposition to part of another neuron; most are axodendritic or axosomatic, but other configurations occur | Synaptic vesicles, mitochondria | Transmit information to other neurons |
a As discussed in Chapter 7 , the final integration of electrical signals (i.e., conversion of synaptic potentials to trains of action potentials) typically occurs at the beginning of the axon.
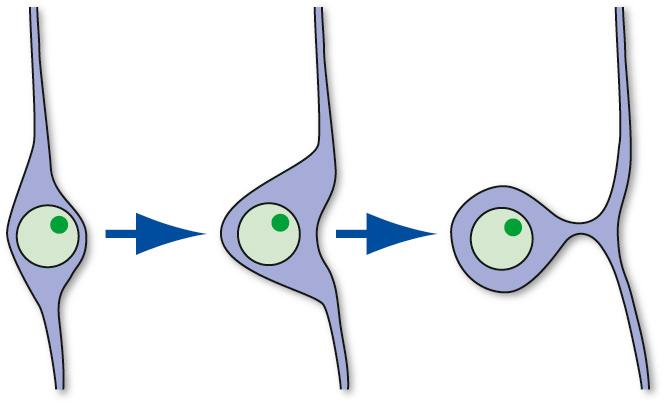
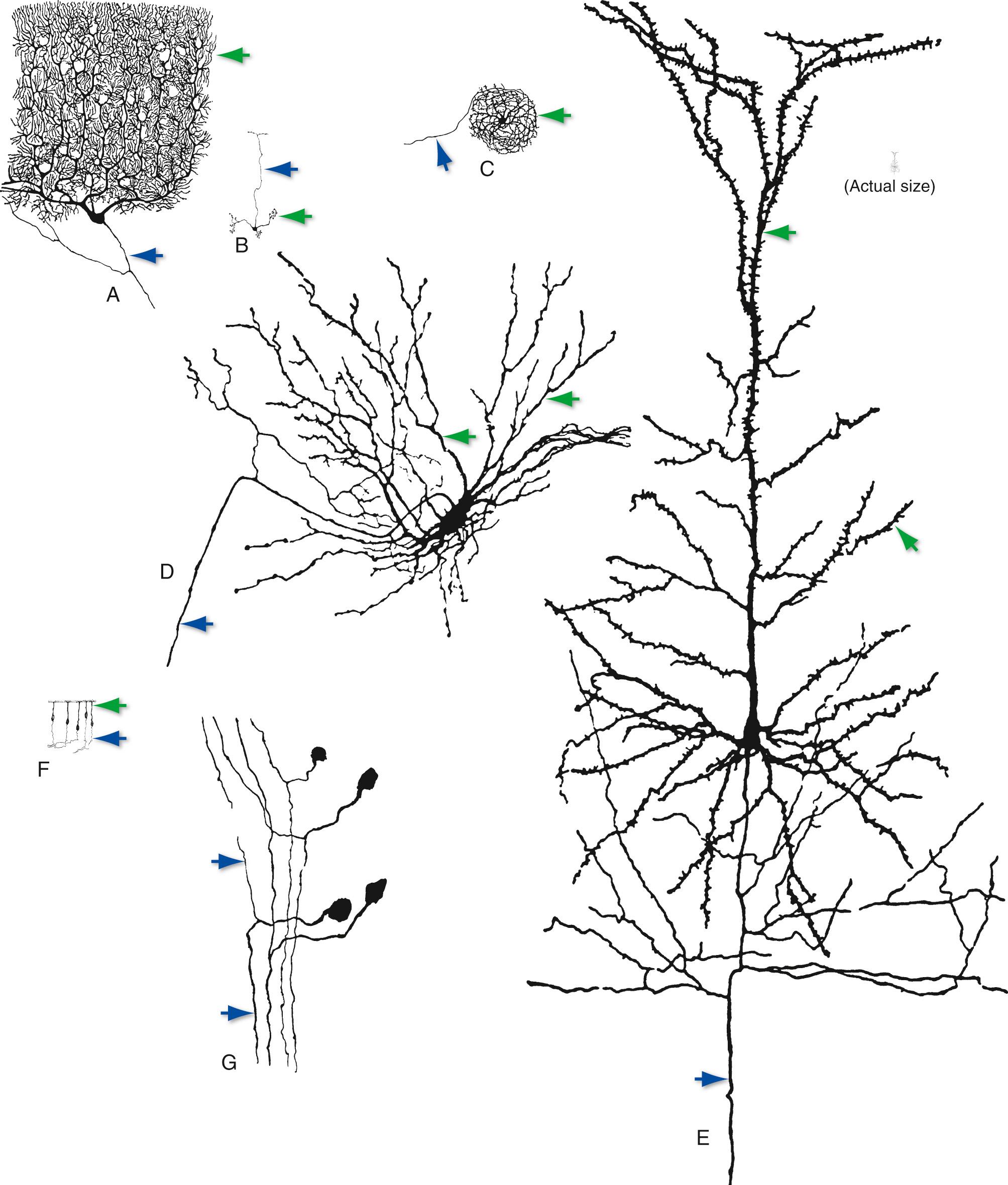
Despite the basic similarity of neurons to one another, there is wide variability in the details of their shapes and sizes ( Fig. 1.4 ). Certain aspects of somatic, dendritic, and axonal morphology give rise to a descriptive terminology for neurons. The majority of vertebrate neurons are multipolar, meaning that there are multiple dendritic projections from the cell body and almost always an axon as well (see Figs. 1.4A to E ); in many cases the pattern of the dendritic processes is characteristic of that type of neuron. Some neurons are bipolar (see Fig. 1.4F ) or unipolar d
d Although true unipolar neurons are common in invertebrate nervous systems, vertebrate neurons with a unipolar appearance are actually pseudounipolar. They start out as bipolar neurons, but during development the cell body expands asymmetrically (below), leaving behind a stalk from which both processes emerge.
(see Fig. 1.4G ), having two processes or only one, respectively. There is a broad spectrum not only of neuronal shapes but also of neuronal sizes. Cell bodies range from about 5 to 100 µm in diameter. Many axons are short, only a millimeter or so in length; but some, like those that extend from the cerebral cortex to the sacral spinal cord, measure a meter or more. For many years, the primary technique available for studying the shapes and sizes of neurons was Golgi staining, a method that infiltrates all the processes of a small percentage of neurons with heavy metals, causing them to stand out from an unstained or counterstained background (see Figs. 1.4 and 1.16A ). More recently, however, a variety of methods relying on microinjection, immunocytochemical, or other techniques have become available ( Box 1.1 ). These now make it possible to correlate the structure of an individual neuron with aspects of its function.
One drawback of Golgi staining is that it stains a subset of neurons indiscriminately (see Fig. 1.16A ), revealing relatively little about the function of an individual cell.
The last few decades have seen the development of increasingly sophisticated techniques for demonstrating the morphology of functionally identified neurons.
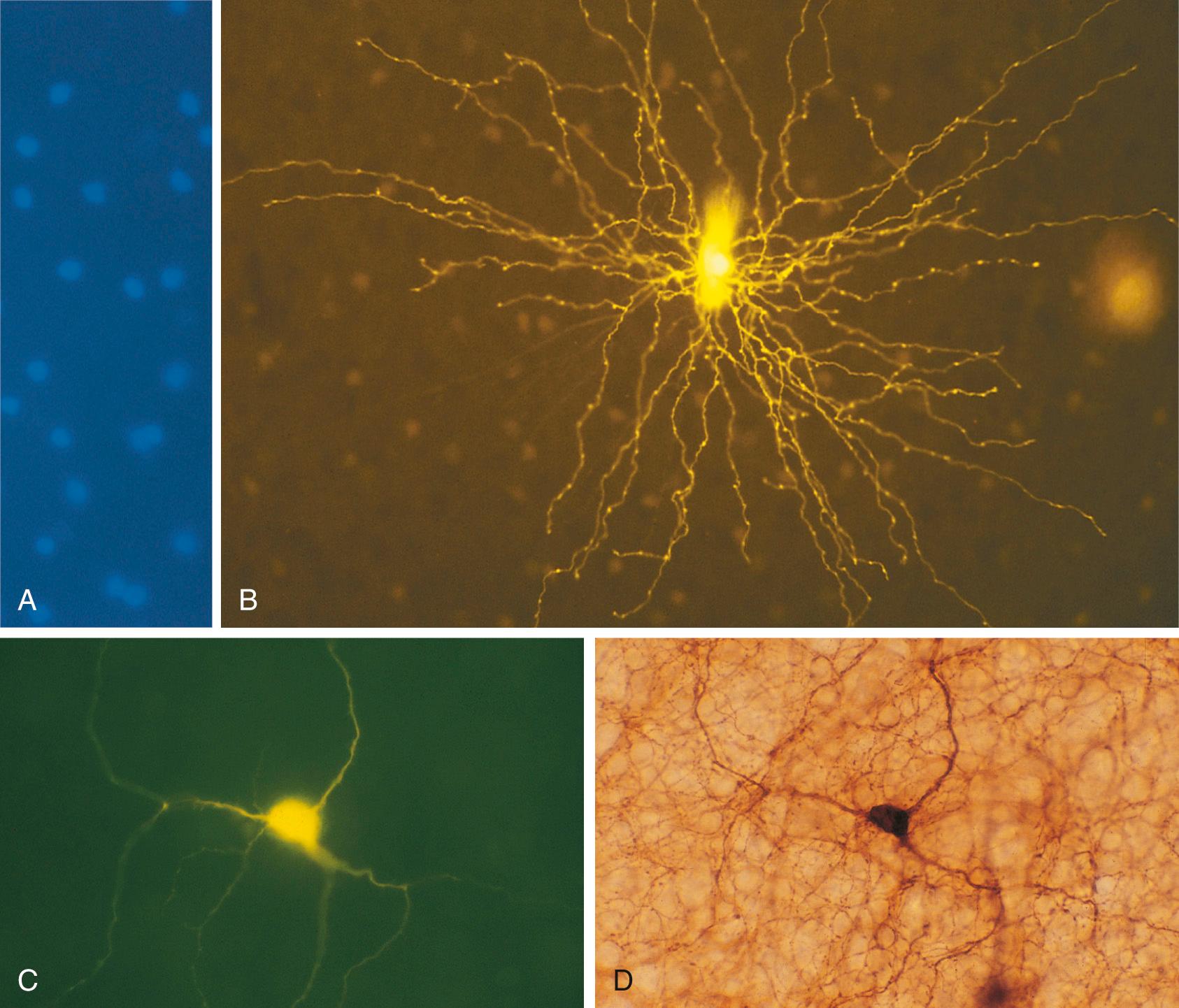
A mainstay in the study of the electrophysiological properties of individual neurons has been the use of micropipette electrodes that either impale single neurons or attach to their surfaces (see Chapter 7 ). The same electrodes can be used as tiny hypodermic needles to inject a dye or marker substance, allowing study of the anatomy of the same neuron ( Fig. 1.5 ). Such injections can reveal morphological detail comparable to that shown by Golgi staining ( Figs. 1.5 and 1.6 ).
Different classes of neurons also have chemically different interiors, and it is possible to make labeled antibodies that demonstrate some of these differences (see Fig. 1.6D ). Correlations of neuronal morphology and location with neurotransmitter content have been particularly instructive. For example, neurons that use norepinephrine as the chemical transmitter at their synapses contain this substance throughout their axons and cell bodies. Appropriate fixation and processing cause these neurons to be fluorescent. Alternatively, it is possible to make a labeled antibody to an enzyme involved in the formation of a neurotransmitter or a labeled antibody to a receptor for a given transmitter. Such methods have made it possible to map out “chemically coded” neural pathways (see Chapter 11 ). Methods for studying neurotransmitter content and electrophysiological properties can be combined to produce particularly elegant structure-function correlations (see Fig. 1.6 ).
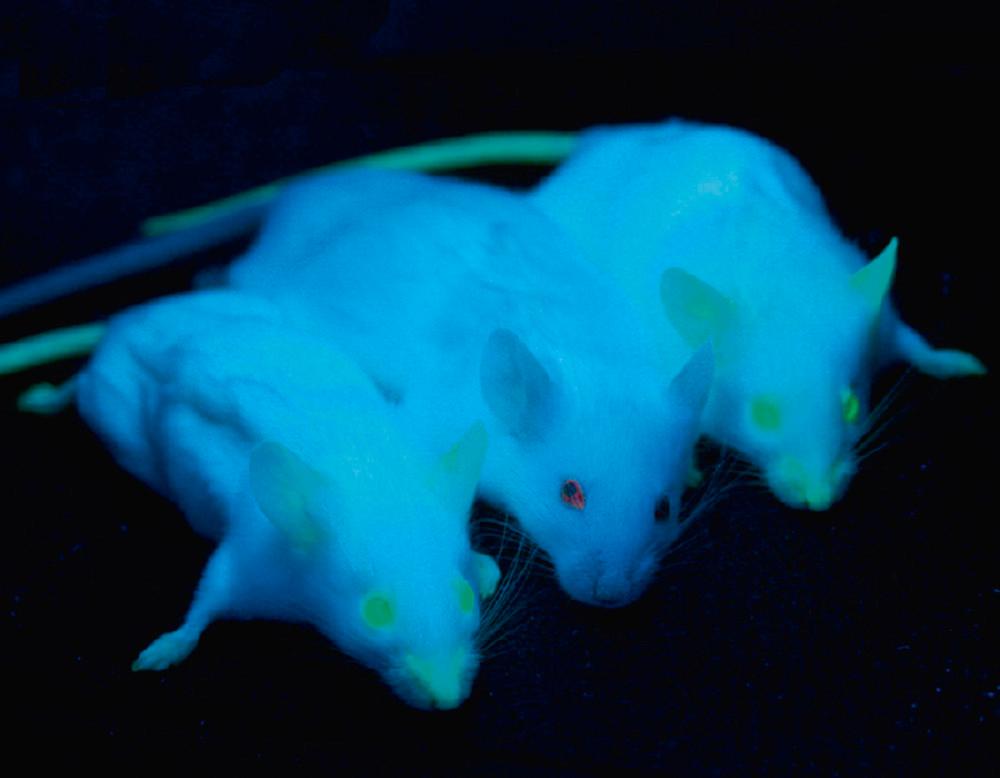
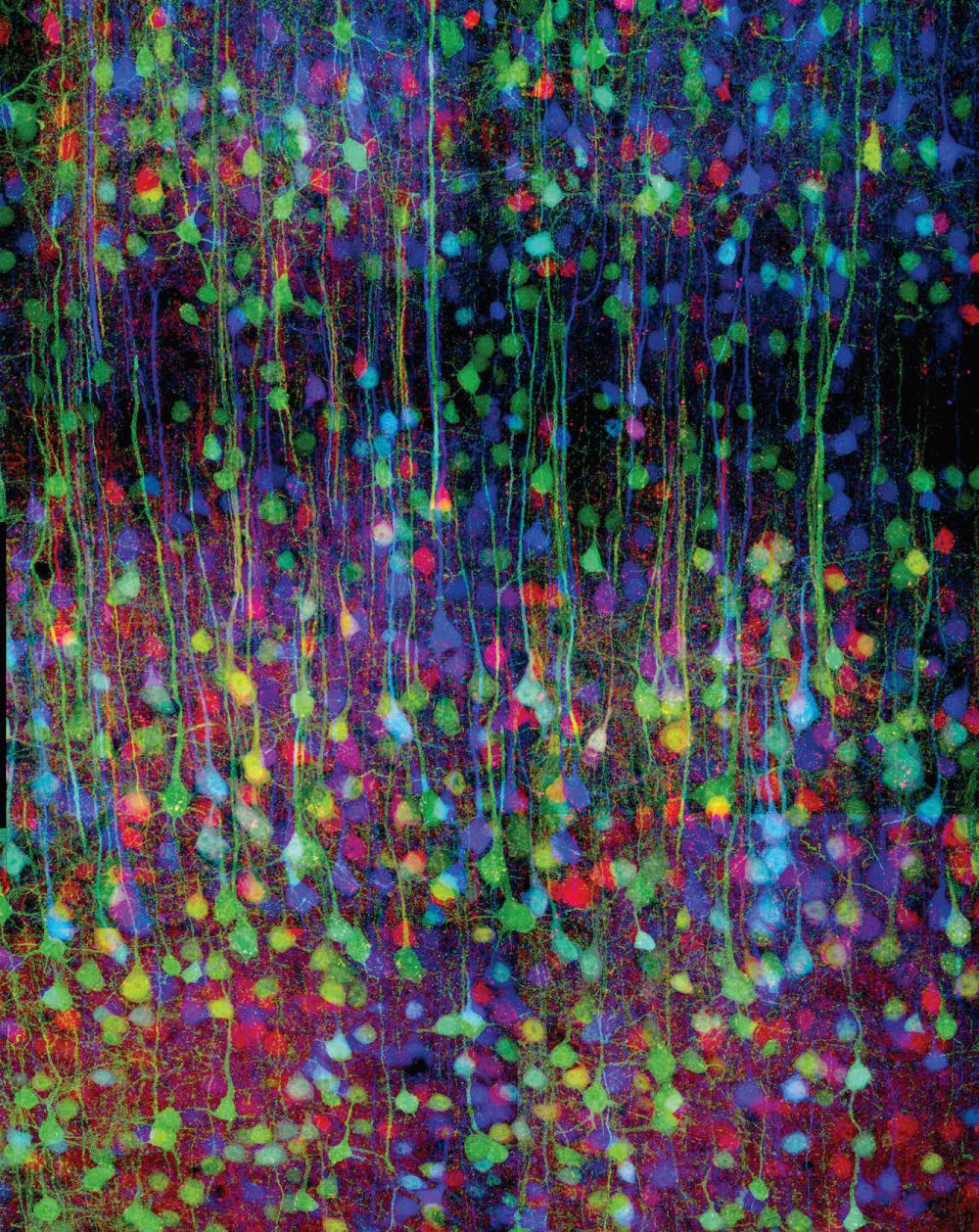
Genetic techniques have assumed a progressively more prominent role in mapping the structure and connections of neuronal populations. An early method involved engineering neurons and other cells to express green fluorescent protein ( Fig. 1.7 ), a small protein originally isolated from the jellyfish Aequorea victoria . Mutated versions of this protein fluoresce in colors other than green, and animals can be genetically manipulated in such a way that different neurons express distinctive mixtures of multiple fluorescent proteins ( Fig. 1.8 ). The effect is almost one of having multiple distinguishable populations of Golgi-stained neurons so that the interconnections of networks of neurons can be traced.

Neurons can also be classified according to their connections. Sensory neurons either are directly sensitive to various stimuli (e.g., pain or temperature changes) or receive direct connections from nonneuronal receptor cells (e.g., rods and cones for vision or hair cells for hearing). Motor neurons end directly on muscles, glands, or other neurons in PNS ganglia. Most sensory and motor neurons live partly in the PNS and partly in the CNS (see Fig. 1.10 ), whereas almost all other neurons reside entirely in the CNS and interconnect other neurons. Some are local interneurons and have all their processes (e.g., dendrites, cell body, and axon) confined to a single small area of the CNS. Others are projection neurons, with long axons connecting different areas of the CNS, as in a neuron in the cerebral cortex whose axon reaches the spinal cord. In a strict sense, the human nervous system is composed almost entirely of interneurons and projection neurons: there are at most 20 million sensory fibers in all of the spinal and cranial nerves combined and no more than a few million motor neurons. Even taking into account the autonomic neurons that innervate muscles and glands (see Chapter 10 ), more than 99% of our neurons are interneurons or projection neurons. However, the words sensory and motor are often used in a much broader sense to refer to cells and axons that carry information related to sensory stimuli and to the generation of responses, respectively.
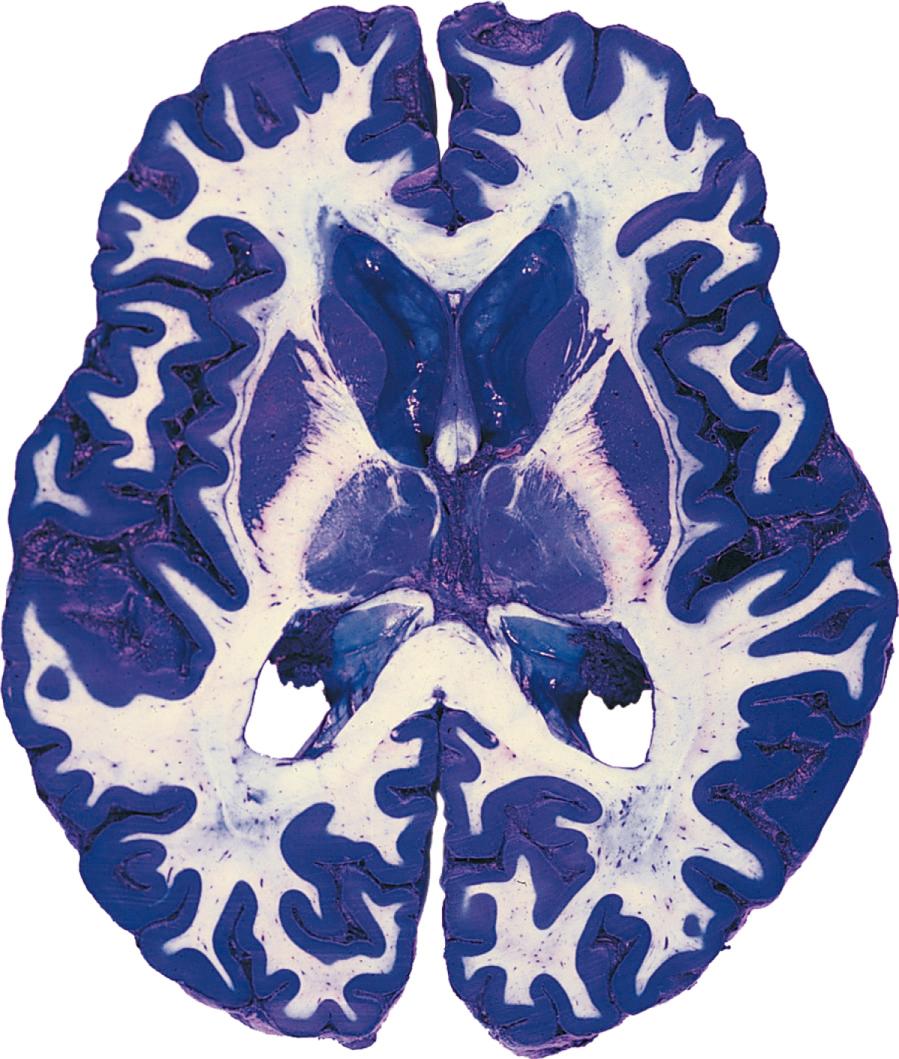
For the most part, the CNS is easily divisible into gray matter and white matter ( Fig. 1.9 ). Gray matter refers to areas where there is a preponderance of cell bodies and dendrites. (In life, however, gray matter is actually a pinkish-gray color because of its abundant blood supply.) White matter refers to areas where there is a preponderance of axons; many axons have a myelin sheath (described later in this chapter) that is mostly lipid and therefore has a fatty, white appearance.
Specific areas of gray matter are often called nuclei, e
e Thus the term nucleus has two meanings in the CNS—it can mean either the nucleus of an individual cell or a collection of neuronal cell bodies (e.g., nucleus accumbens).
particularly if the contained cell bodies are functionally related to one another. An area where gray matter forms a layered surface that covers some part of the CNS is referred to as a cortex. The cerebral and cerebellar cortices are the two most prominent examples. Occasionally, descriptive names are used for particular areas of gray matter (e.g., the putamen, a nucleus in each cerebral hemisphere named for its shape and location), but these are relatively infrequent .
In contrast, subdivisions of white matter (i.e., collections of axons) go by a bewildering variety of names, f
f These are mostly descriptive terms that also make sense, carried forward from the days when the appearances of these structures were better known than their functions. Fasciculus and funiculus mean “little bundle” and “string,” respectively. Lemniscus means “ribbon” and is used for tracts that are flattened out in cross section. Peduncle means “little foot,” and is used for a site where axons funnel down into a compact bundle.
such as fasciculus, funiculus, lemniscus, peduncle, and, most commonly, tract. Many tracts have two-part names that provide some free information about the nature of the tract: the first part of the name refers to the location of the neuronal cell bodies from which these axons originate, and the second part refers to the site where they terminate. Thus a spinocerebellar tract is a collection of axons with cell bodies in the spinal cord and synaptic endings in the cerebellum.
The spinal cord provides a reasonably clear example of the separation of neural tissue into gray matter and white matter ( Fig. 1.10 ). Sensory axons, whose pseudounipolar cell bodies are located in the dorsal root ganglia of spinal nerves, enter the spinal cord and divide into a large number of branches, some of which terminate on neuronal processes in the spinal gray matter and some of which terminate on cell bodies in the medulla of the brainstem. Motor axons emerge from multipolar cell bodies located in the spinal gray matter, leave the spinal cord, and travel with spinal nerves and innervate skeletal muscle. The white matter contains long descending tracts (from the brainstem and forebrain), long ascending tracts (to the brainstem, cerebellum, and forebrain), and local axons interconnecting different spinal levels. The gray matter, on the other hand, contains motor neuron cell bodies, endings of incoming sensory axons, second order sensory cell bodies (whose axons enter long ascending tracts of white matter to relay sensory information to the brainstem and forebrain), cell bodies of the preganglionic neurons, and endings of long descending tracts and local interneurons. This division into white and gray matter is rarely absolute; for example, axons in long descending tracts obviously must pass through some gray matter before reaching their targets (see Chapter 10 ).
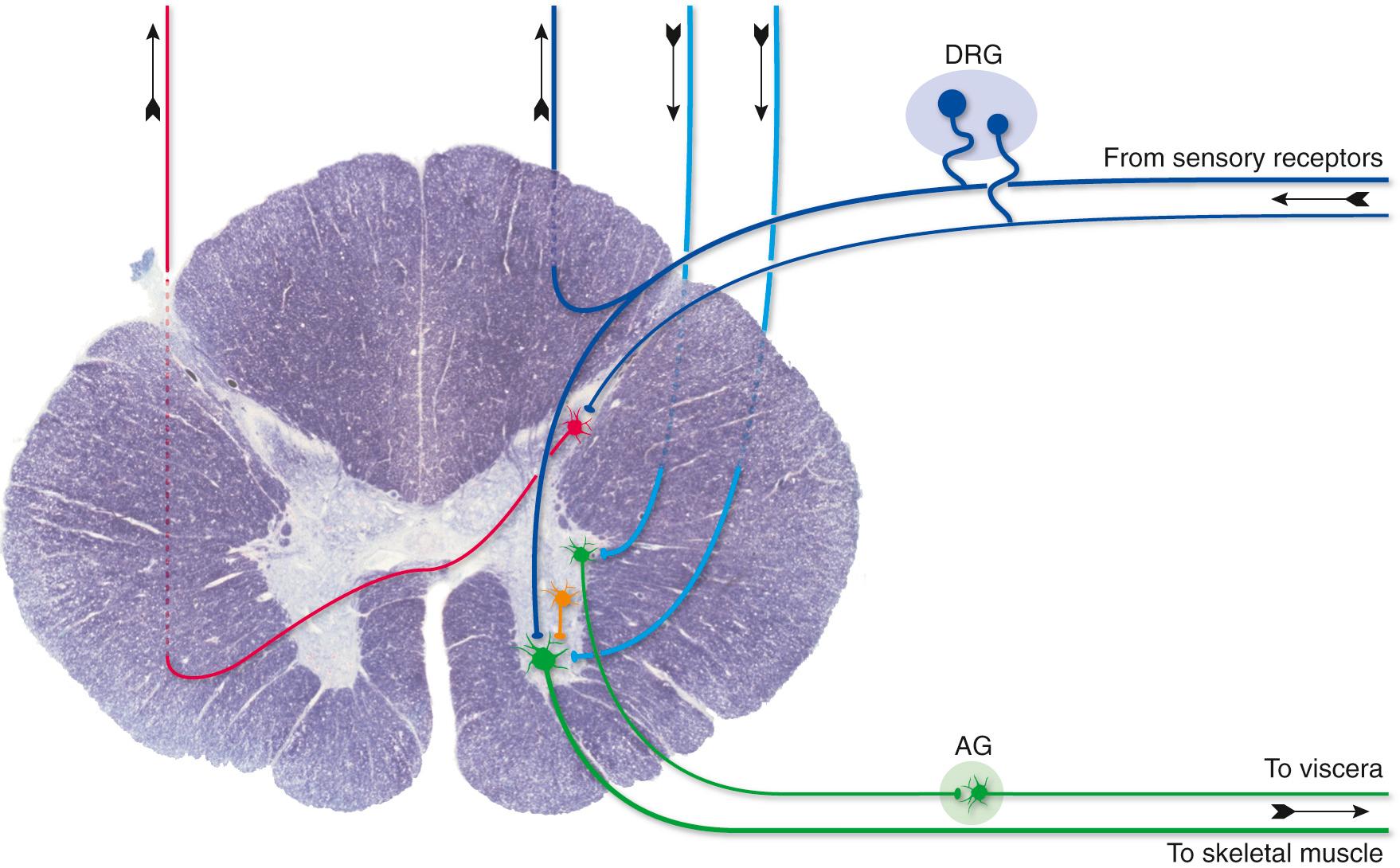
Peripheral nerves are, for most of their courses, collections of axons on their way to or from places such as skin, muscle, or internal organs, accompanied by glial and connective tissue sheaths (see Fig. 9.19 ). Many of these axons have cell bodies that also reside in the PNS, and these somata are typically clustered in ganglia (Greek for “swellings”) (e.g., dorsal root ganglia; DRG) at predictable sites along the nerve (see Fig. 1.10 ).
Neurons need mechanisms to deal not only with their electrical and chemical signaling functions but also with other consequences of their extended anatomy. A large neuron with a long axon (e.g., one of the neurons shown in Fig. 1.4D and E ) may have 99% of its cytoplasm in the axon, many centimeters away from the cell body; hence its single nucleus and associated synthetic apparatus must have efficient mechanisms for communicating with distant parts of its appendages. In addition, brains have no bones, but neurons have long, delicate processes, so there is a need for mechanical stabilization that can be met only partially by the external suspension mechanisms and fluid described in Chapters 4 and 5 . To address these issues, neurons, like other cells, contain a nucleus and an assortment of organelles—mitochondria, endoplasmic reticulum, Golgi apparatus, and cytoskeletal elements—but the abundance and configuration of these organelles in different parts of a neuron reflect the function of each of these parts.
The neuronal cell body is the site of synthesis of most of the neuron's enzymes, structural proteins, membrane components, and organelles, as well as some of its chemical messengers. Its structure ( Fig. 1.11 ) reflects this function. The nucleus is large and pale when stained for neurofibril, with most of its chromatin dispersed and available for transcription; it contains one or more prominent nucleoli, which are actively involved in the transcription of ribosomal RNA. The cytoplasm contains abundant rough endoplasmic reticulum and free ribosomes for protein synthesis, together with stacks of Golgi cisternae for further processing and packaging of synthesized proteins. Many mitochondria are also present, to meet the energy requirements of continuous, very active protein synthesis.
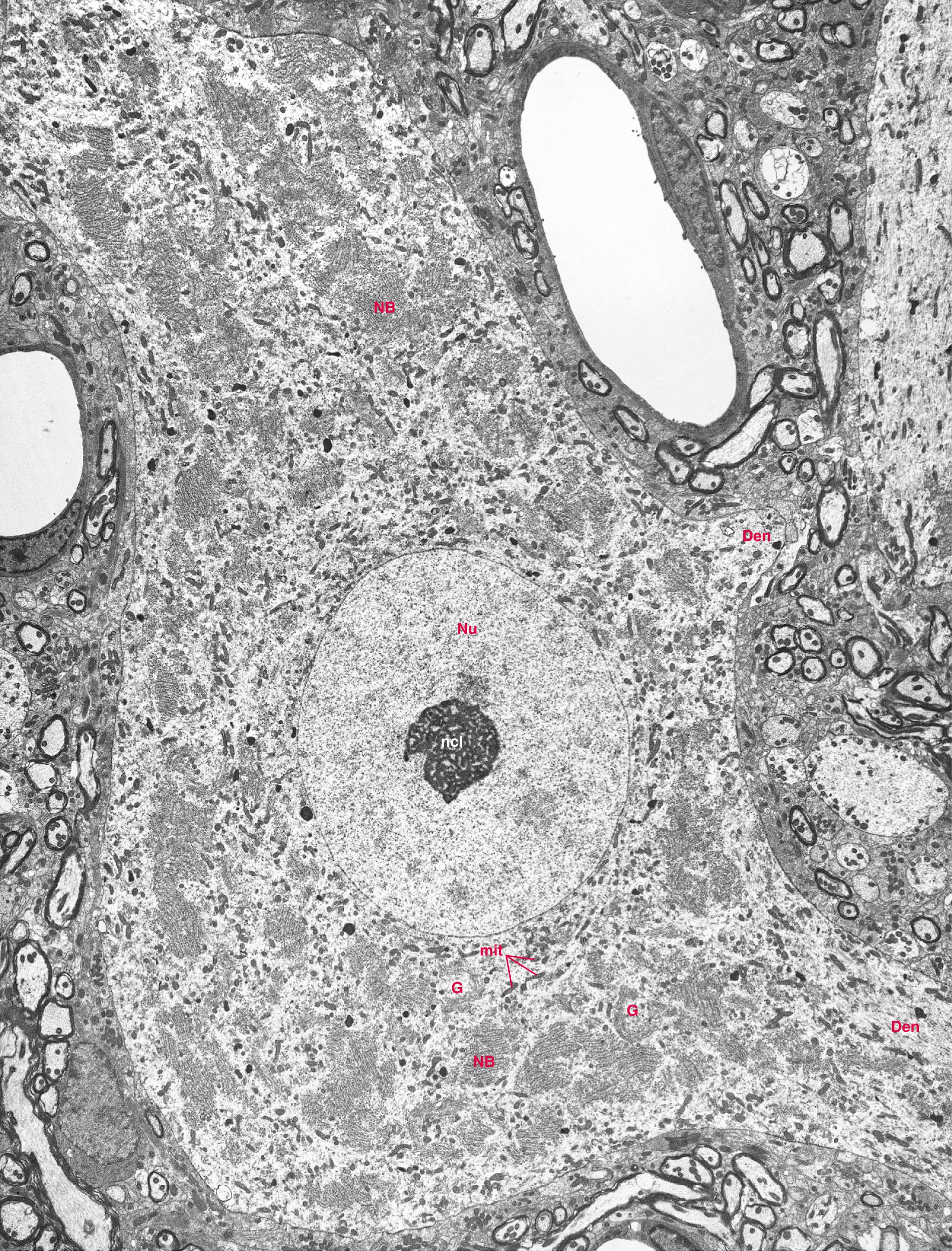
Ribosomes, whether studding the surface of the rough endoplasmic reticulum or free in the cytoplasm between the cisternae, are stained intensely by basic dyes, appearing light microscopically as clumps called Nissl bodies or Nissl substance ( Fig. 1.12 ). Nissl bodies are particularly prominent in large neurons, a consequence of the large total volume of cytoplasm contained in their processes, and appear in characteristic configurations in different neuronal types.
![Fig. 1.12, Nissl bodies in spinal cord motor neurons. At the light microscopic level (A and B), Nissl bodies (NB) appear as clumps of basophilic material distributed throughout the cell body and extending into dendrites (Den) but not axons (Ax) or their point of origin (the axon hillock, [*]). Electron microscopy (C and D) reveals that Nissl bodies are stacks of rough endoplasmic reticulum (ER) with interspersed clusters of free ribosomes (r), embedded in neuronal cytoplasm containing Golgi cisternae (G), mitochondria (mit), microtubules (m), and neurofilaments (nf). The actual size of the Nissl body in (C) is about 3 µm × 2 µm. Ncl, Nucleolus; Nuc, nucleus. Fig. 1.12, Nissl bodies in spinal cord motor neurons. At the light microscopic level (A and B), Nissl bodies (NB) appear as clumps of basophilic material distributed throughout the cell body and extending into dendrites (Den) but not axons (Ax) or their point of origin (the axon hillock, [*]). Electron microscopy (C and D) reveals that Nissl bodies are stacks of rough endoplasmic reticulum (ER) with interspersed clusters of free ribosomes (r), embedded in neuronal cytoplasm containing Golgi cisternae (G), mitochondria (mit), microtubules (m), and neurofilaments (nf). The actual size of the Nissl body in (C) is about 3 µm × 2 µm. Ncl, Nucleolus; Nuc, nucleus.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/IntroductiontotheNervousSystem/12_3s20B9780323653985000011.jpg)
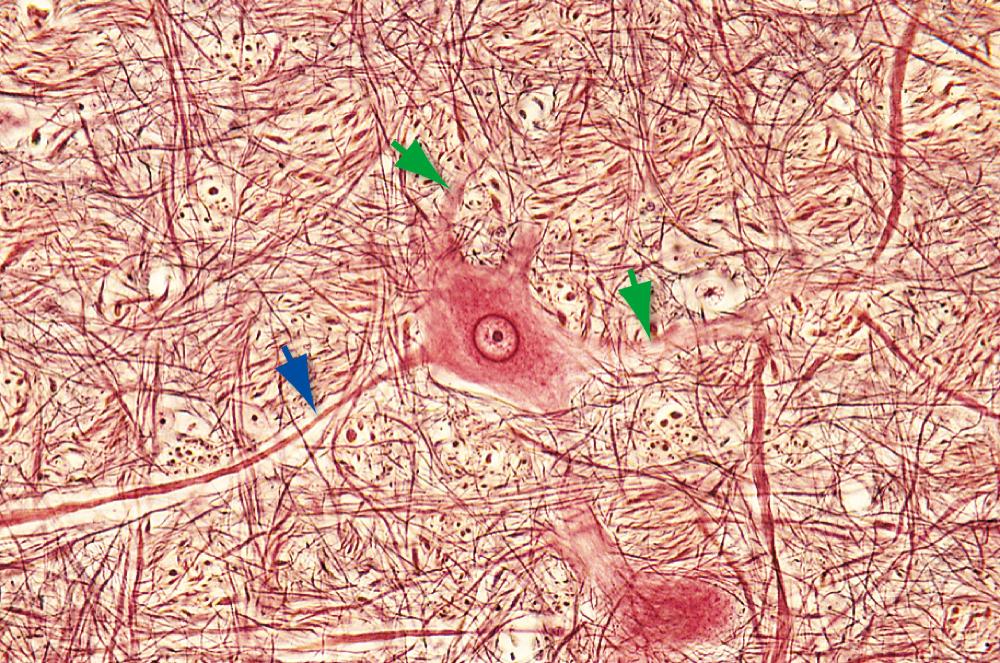
The organelles just described are embedded in a network of three kinds of filamentous protein polymers that extend throughout the neuron and its processes, collectively comprising the neuronal cytoskeleton. Microtubules are cylindrical assemblies, about 25 nm in diameter, of 13 strands (protofilaments) of protein arranged around a hollow core. Each protofilament is a polymer of the protein tubulin; an assortment of additional proteins associated with the microtubules links them to each other, to other cytoskeletal elements, and to various organelles as they travel toward or away from the cell body. Neurofilaments, the neuron's version of the intermediate filaments found in most cells, are ropelike assemblies of heteropolymers made up of 3 to 5 different proteins from the cytokeratin family providing structural support and regulating axon diameter. Neurofilaments often change in protein assembly over a neuron's lifetime, regulating the axonal growth not only during development but also in diseases and disorders. Recently, serum levels of neurofilament have been used to detect stages of diseases, including amyotrophic lateral sclerosis and Alzheimer's dementia. Neurofilaments are about 10 nm in diameter, much too small to be seen under the light microscope, but they aggregate in response to certain chemical fixatives. When silver stains are applied, such aggregates can be visualized as neurofibrils ( Fig. 1.13 ). Finally, microfilaments, the thinnest cytoskeletal element (7 nm), are twisted pairs of actin filaments. All three kinds of cytoskeletal elements contribute to maintaining the shape of the neuron. Microtubules also serve as the substrate along which organelles are transported through neuronal processes (as described in more detail later in the chapter). Microfilaments are important for anchoring membrane molecules in place (e.g., receptor molecules at synapses), for shuttling things to and from the cell membrane, and for movement of the advancing tip of growing axons.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here