Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
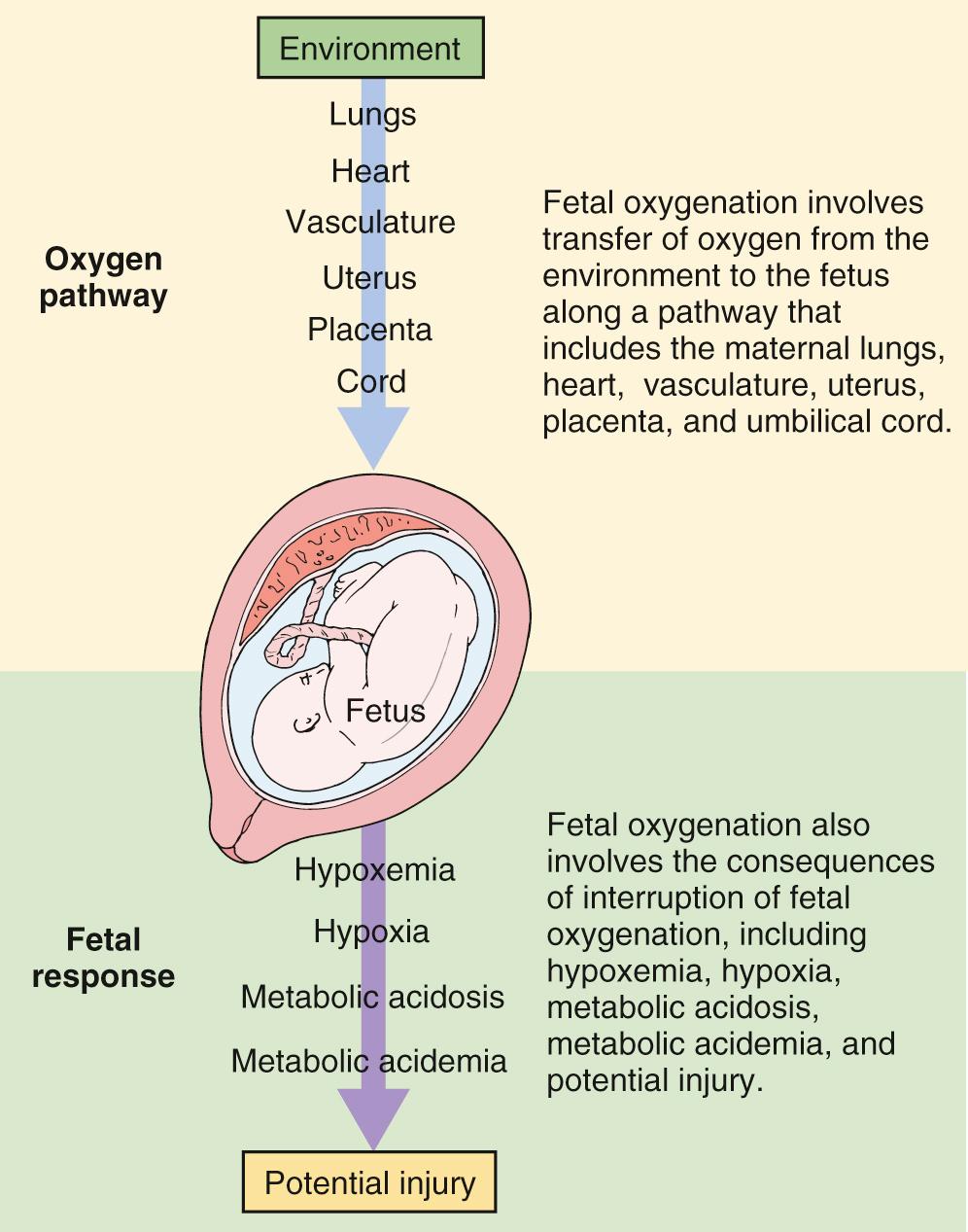
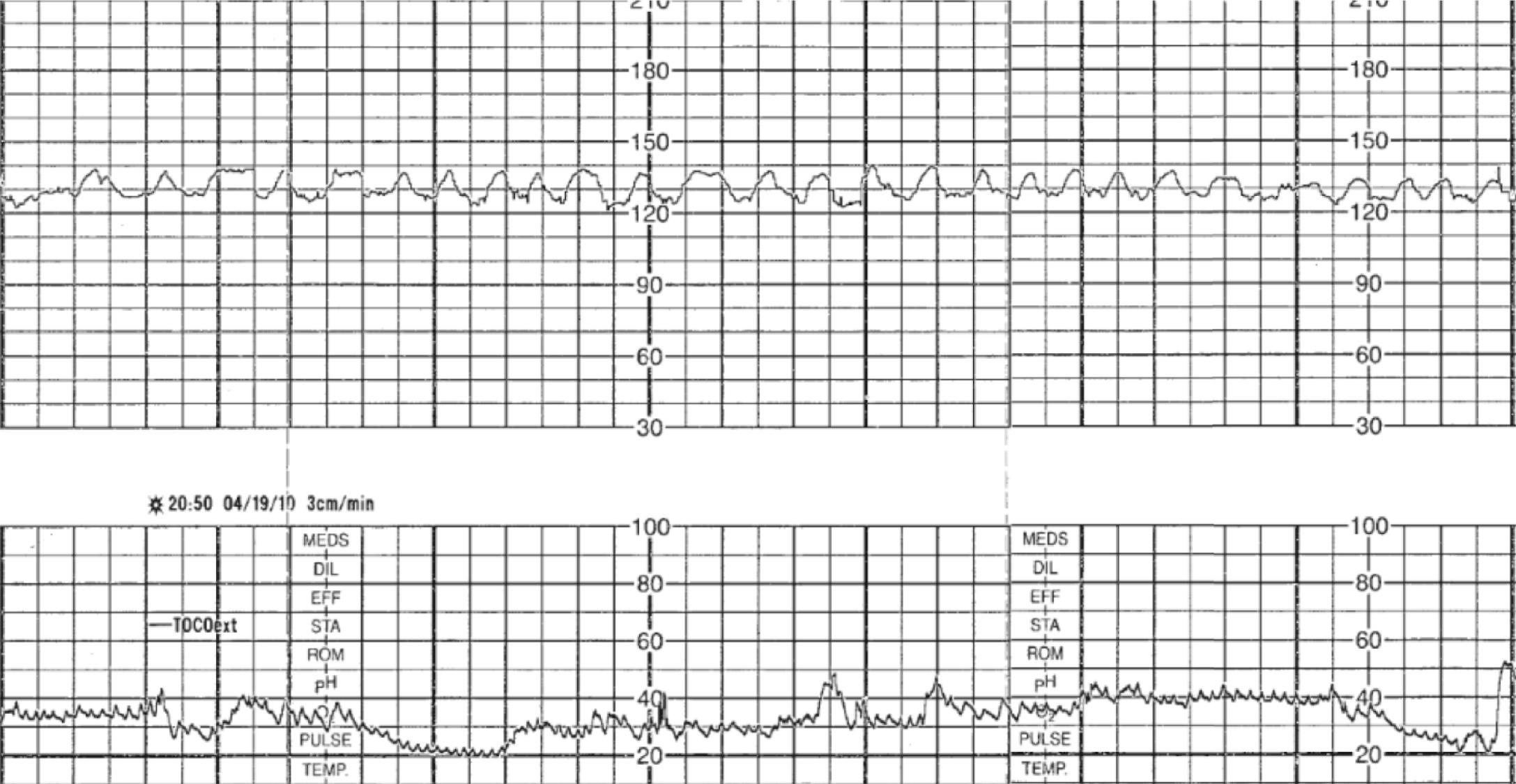
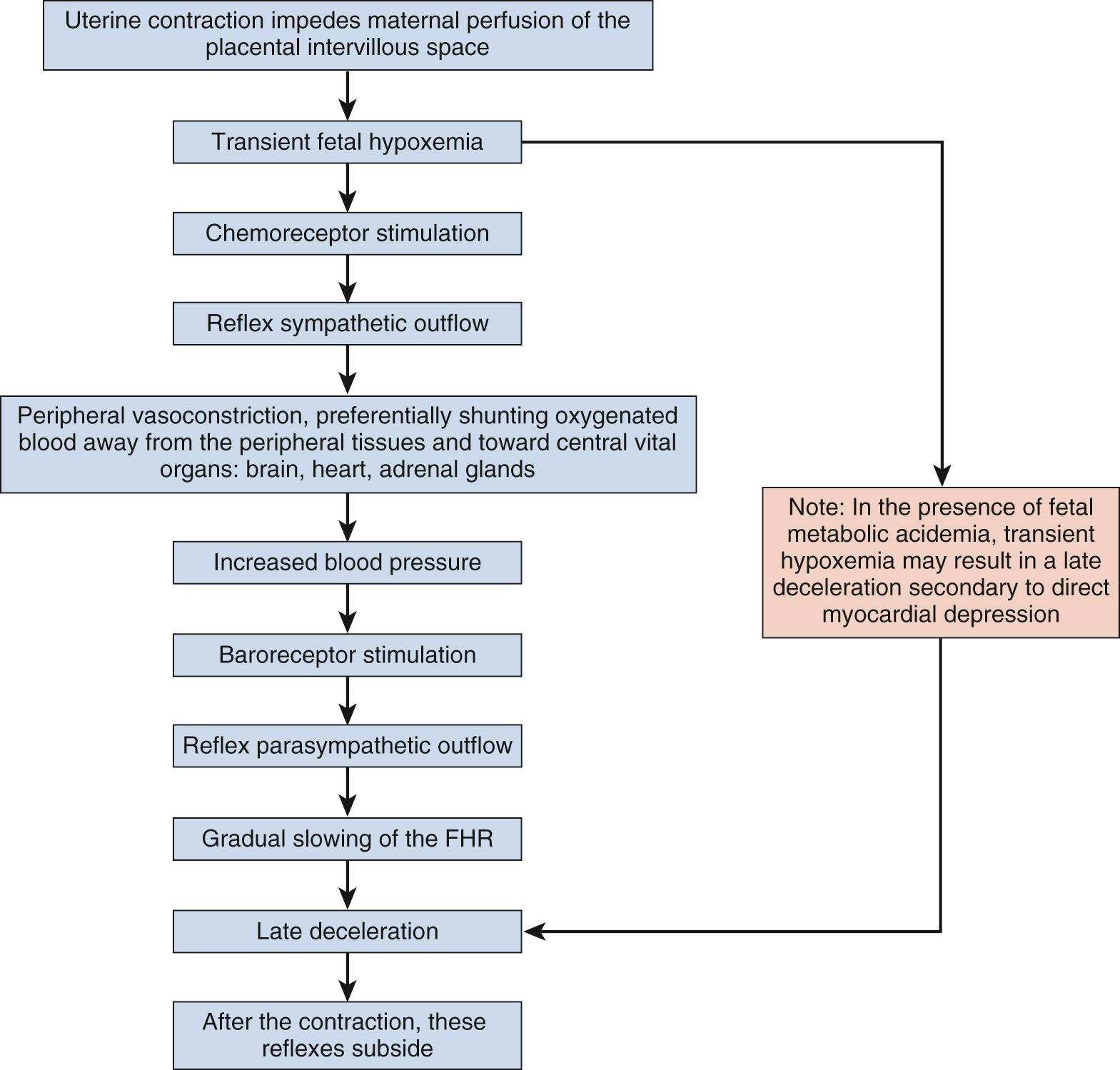
Intrapartum fetal monitoring to assess fetal well-being during labor and delivery is a key component of intrapartum management. In fact, over 80% of laboring patients in the United States undergo intrapartum electronic fetal monitoring (EFM) . “Nonreassuring” fetal status as interpreted on the basis of EFM accounts for nearly one-quarter of primary cesarean deliveries. This ubiquitous form of intrapartum fetal assessment has evolved from its origins as intermittent auscultation of fetal heart tones to what is now most commonly continuous EFM. Fetal heart rate (FHR) characteristics reflect the fetal response to intrauterine hypoxia. The theorized benefit of intrapartum EFM is to identify and intervene on fetal hypoxia secondary to the physiologic demands of normal and abnormal labor. Normal components of labor, such as contractions throughout the first and second stage and maternal expulsive efforts during the second stage, result in transient decreases in oxygen delivery and gas exchange for the fetus, which places the fetus at risk for interruption in oxygenation and hypoxia even in uncomplicated pregnancies ( Fig. 15.1 ). Prolonged, compromised oxygen delivery to the fetus may result in neonatal acidemia and associated neonatal morbidity. Although evidence for the prevention of such morbidity with continuous EFM is modest, intrapartum fetal monitoring remains an ingrained component of modern-day obstetrics.
The FHR and pattern are controlled by an interplay of the sympathetic and parasympathetic nervous systems, with central and peripheral influences. The first medical documentation of fetal heart tones was made in 1818 by the Swiss physician Dr. Francois-Isaac Mayor, who performed fetal auscultation by placing his ear next to the maternal abdomen. The introduction of the fetal stethoscope in the early 1900s allowed physicians to slowly link patterns recognized during intermittent auscultation with adverse neonatal outcomes, ultimately establishing criteria for fetal distress. The standard of care prior to the introduction of EFM was a combination of intermittent auscultation and fetal scalp pH sampling.
Intermittent auscultation was accomplished by placing a noninvasive device on the maternal abdomen and interpreting the FHR pattern by sound. A variety of devices were used over the years, including auscultative devices similar to the stethoscope as well as Doppler devices that detected movement of the fetal heart. These approaches offered the opportunity to intermittently interpret the FHR pattern but did not allow for continuous assessment. Given the ongoing, periodic, and often unpredictable nature of the physiologic challenges of labor, the inability to continuously monitor the fetus and the possibility that fetal distress could go undetected was the impetus for continuous fetal monitoring.
In 1958, Hon and Kubli published the first description of the use of EFM in humans. They reported that patterns observed in 80 laboring women were associated with fetal acidemia as measured with scalp sampling or umbilical cord assessment at delivery. The introduction of EFM in the 1960s led to the contemporary and commercially available monitoring systems that either use noninvasive Doppler-based technology for transabdominal FHR recognition or invasive fetal electrocardiography (ECG) via an electrode placed on the fetal scalp. Following the early descriptions and widespread availability of this modality, 13 randomized trials were conducted to compare the use of intrapartum EFM with intermittent auscultation aimed at reducing neonatal morbidity and improving obstetric outcomes ( Table 15.1 ). Among these efforts, a large trial of 12,964 women, each of whom was cared for by a single provider throughout labor either with intermittent auscultation or EFM, showed a reduction in neonatal seizures among the infants whose mothers were managed with EFM. However, after 4 years, there was no difference between the groups with regard to infant outcomes. There were no other neonatal differences between the groups, including no differences in the rates of cerebral palsy. The remainder of the trials provided no evidence for benefit of EFM over intermittent auscultation to improve neonatal outcomes. A systematic review of these trials that included over 37,000 women found no evidence for improved neonatal outcomes with EFM compared with intermittent auscultation, but it showed a significant increase in cesarean delivery among patients managed with EFM.
| Study | Year | N | Perinatal Morbidity | Perinatal Mortality | Cesarean Rate |
|---|---|---|---|---|---|
| Haverkamp | 1976 | 483 | No difference | No difference | Higher in EFM group |
| Renou | 1976 | 350 | See text | No difference | Higher in EFM group |
| Kelso | 1978 | 504 | No difference | No difference | Higher in EFM group |
| Haverkamp | 1979 | 690 | No difference | No difference | Higher in EFM group |
| Wood | 1981 | 989 | No difference | No difference | No difference |
| MacDonald | 1985 | 12,964 | Lower in EFM group a | No difference | No difference |
| Neldam | 1986 | 969 | No difference | No difference | No difference |
| Leveno | 1986 | 14,618 | No difference | No difference | Not reported b |
| Luthy | 1987 | 246 | No difference | No difference | No difference |
| Vintzileos | 1993 | 1428 | No difference | Lower in EFM group c | No difference |
| Herbst | 1994 | 4044 | No difference | No difference | No difference |
| Madaan | 2006 | 100 | No difference | No difference | No difference |
a Fewer neonatal seizures were reported in the electronic fetal monitoring (EFM) group, but no difference was found at 4 years of age.
b The cesarean rate for fetal indications was higher in the EFM group, but overall cesarean rates were not reported.
c The study ended after the third quarterly review because of a statistically significant fivefold decrease in perinatal mortality in the EFM group.
After the use of intrapartum EFM in the United States became widespread, Nelson and colleagues performed a nested case-control study among 150,000 live singleton births weighing over 2500 g to examine the effectiveness of EFM for the reduction of cerebral palsy. The patterns of 78 infants with cerebral palsy were compared with those of 300 randomly selected controls. The authors found that multiple late decelerations (odds ratio [OR], 3.9; 95% confidence interval [CI], 1.7 to 9.3) and decreased variability (OR, 2.7; 95% CI, 1.1 to 5.8) were associated with moderate to severe cerebral palsy but that most fetuses with those patterns did not develop cerebral palsy, with a false-positive rate of 99.8%. The authors also found that cesarean sections were often performed in the setting of these patterns and that, if this was common practice, many such surgeries would be performed without benefit to the fetus but increased maternal risks.
With the widespread use of intrapartum EFM, subsequent population-based observational studies have reported more optimistic outcomes. In 2004, Chen and colleagues examined the association between the use of intrapartum EFM and neonatal and infant deaths using the linked US birth and death data. They reported that 89% of the women studied underwent intrapartum EFM and that its use was associated with a significant reduction in infant mortality (adjusted RR [aRR], 0.75; 95% CI, 0.69 to 0.81), which was driven predominantly by a reduction in early neonatal death (aRR, 0.50; 95% CI, 0.44 to 0.57). Another population-based cohort study of over 55 million births of infants without anomalies between 24 and 44 weeks’ gestation examined the impact of intrapartum EFM on US trends in adverse perinatal outcomes between 1990 and 2004. These authors found that a 17% increase in the use of intrapartum EFM was associated with a 5% decrease in early neonatal deaths and a 2% decrease in late neonatal deaths as well as an increase over the same period of cesareans and operative vaginal deliveries performed for concerns of fetal well-being. Taken together, although the original trials and subsequent meta-analyses did not offer evidence that intrapartum EFM was superior to intermittent auscultation for preventing neonatal morbidity—perhaps due in part to the quality of some of the studies—the ubiquitous nature of intrapartum EFM use in the past two decades has enabled epidemiologic studies suggesting possible neonatal benefit. Nevertheless, studies have also demonstrated that the number of operative deliveries that would have to be performed to prevent one adverse perinatal outcome would be high, which is consistent with the pervasive findings that the use of intrapartum EFM is associated with an increase in cesarean and operative vaginal deliveries.
EFM is accomplished noninvasively with a device placed on the maternal abdomen kept in place by a flexible strap ( Fig. 15.2 ). The device contains a fetal Doppler transducer that detects fetal cardiac motion or the motion of a major vessel. Because the monitor captures movement and is on the maternal surface, several factors—such as fetal or maternal movement or maternal body wall thickness—can interrupt the signal.
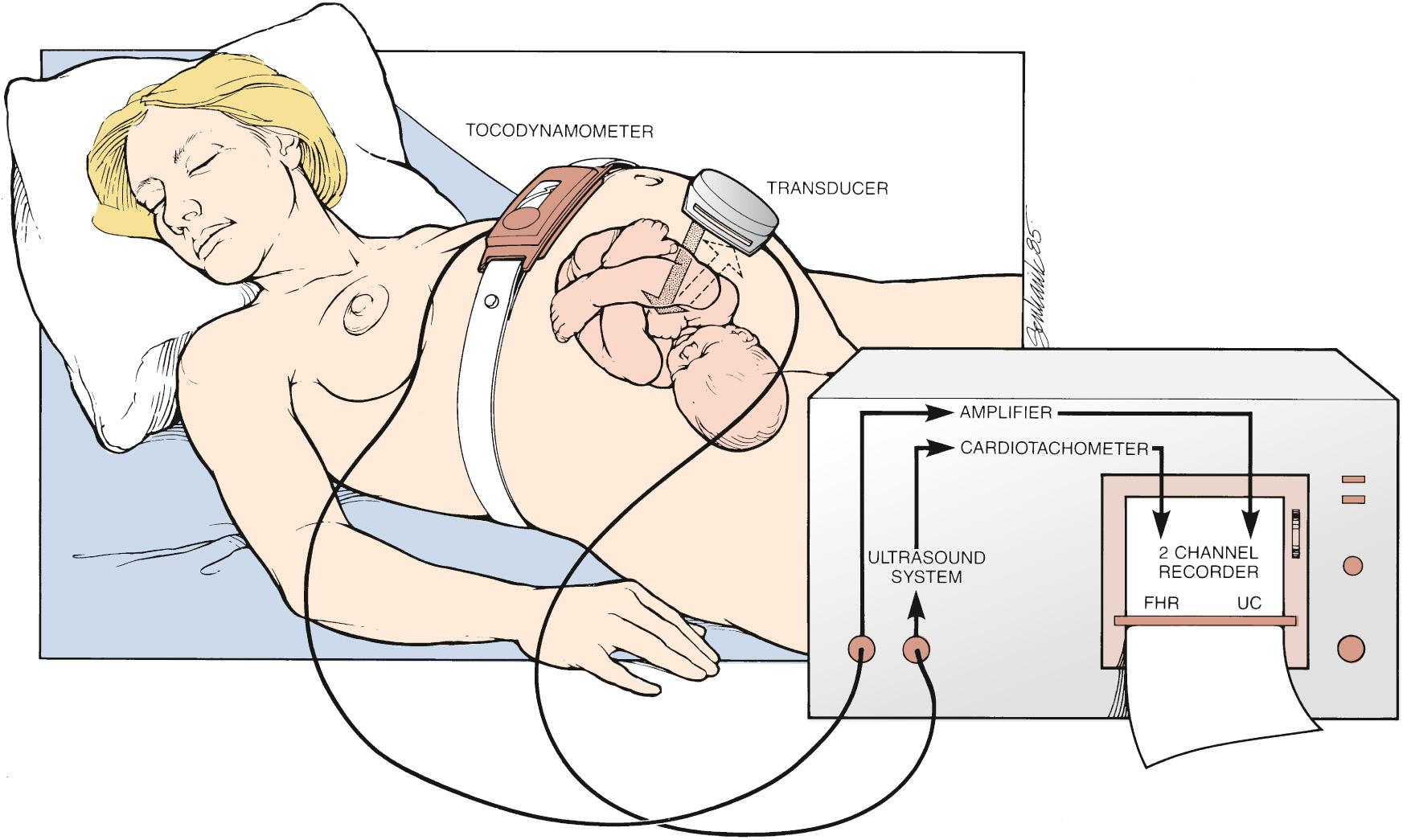
Simultaneously, a second similar Doppler device, called a tocodynamometer, monitors uterine activity. Importantly, the tocodynamometer can demonstrate the presence of contractions, including when they begin and end. But it cannot measure the strength of the contractions. Both the FHR monitor and the tocodynamometer are connected to a bedside terminal that plots the heart rate patterns in real time on EFM paper (moving at 3 cm/min in the United States and 1 cm/min in Europe and other parts of the world) and/or plotted virtually on an electronic display, allowing providers to see and interpret the patterns at bedside.
Sometimes acquisition of the FHR and/or contraction patterns by internal monitors becomes important for clinical interpretation. This can occur frequently when the maternal body habitus limits the ability of external monitors to capture a signal reliably because the monitors are based on ultrasound technology. The FHR can be internally monitored with a fetal scalp electrode (FSE), a small metal corked electrode that is placed on the skin of the fetal scalp. The other end of the cord connects to the same monitor used for external monitoring, generating the same output ( Fig. 15.3 ). The input, as opposed to fetal heart motion that is captured and transmitted by external monitors, is the electrical activity of the fetal heart measured by the interval between beats (R-R interval). The advantage of internal fetal monitors is that they remove the possibility of interference with the signal from maternal motion, body habitus, and so on. Thus the signal is more accurate and continuous. Because use of the scalp electrode is invasive, it is not appropriate for all clinical scenarios and is not without risk. In the setting of maternal infections, such as human immunodeficiency virus or hepatitis, FSEs are avoided to reduce the risk of vertical transmission. In addition, though relatively rare, there have been reports of neonatal complications such as hematomas and scalp abscesses.
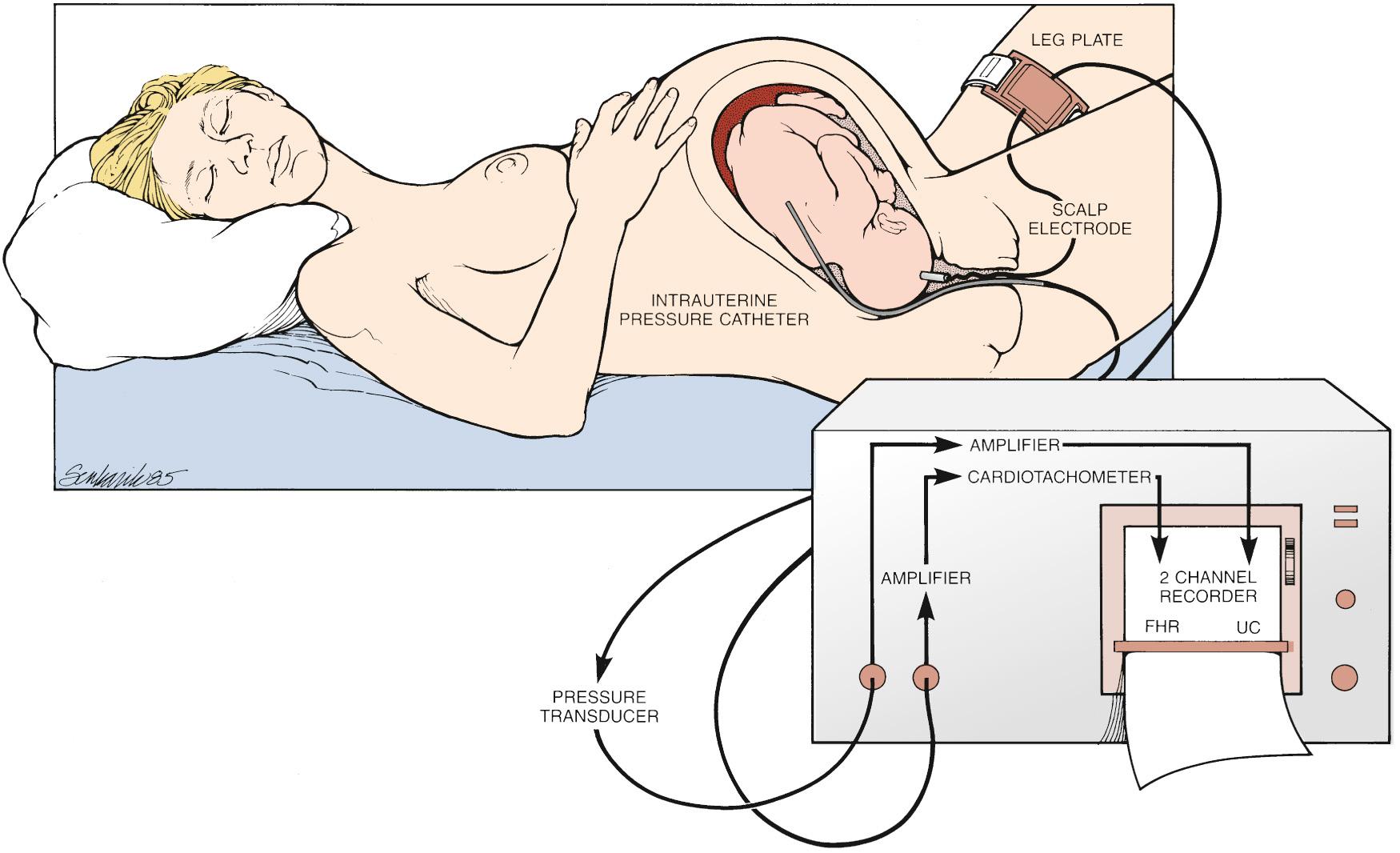
An invasive approach to uterine contraction monitoring is also clinically available. An intrauterine pressure catheter (IUPC), which is a flexible catheter with a pressure transducer at the tip, can be advanced through the cervix into the uterus provided that the membranes are ruptured. Instead of the ultrasound-based technology used for the external tocodynamometer, the catheter measures the uterine pressure directly. The advantage of this approach is that it allows the clinician to gauge both the frequency of contractions and their strength. As with the difference between external and internal monitors for gauging the FHR, use of an IUPC overcomes the maternal factors that could interrupt the external signal. However, risks associated with the use of IUPCs have also been demonstrated. Harper and colleagues, in a retrospective cohort study comparing the use of IUPCs with external monitoring, found that women monitored with IUPCs had a higher risk of maternal fever and infection, which was similar to the findings reported by Soper et al. FSEs and IUPCs are commonly used, relatively safe clinical tools to assist in the assessment of FHR patterns and contractions; however, given the rare but possible associated complications, they should not be used without clinical indications.
In 2008, a consensus conference was convened by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD); the American College of Obstetrics and Gynecology (ACOG); the Society for Maternal-Fetal Medicine (SMFM); the Association of Women's Health, Obstetric, and Neonatal Nurses (AWONN); other relevant stakeholders; and scientists in the field to discuss nomenclature for EFM patterns as well as the state of the science. Historically, despite the fact that EFM was widely used in clinical practice and interpreted at the bedside by clinicians and nurses alike, the nomenclature and documentation were different, creating significant confusion. At the 2008 consensus conference, it was acknowledged that a single common nomenclature endorsed by all parties and used at the bedside was critical. It was also acknowledged that the prior two-tiered designation of EFM patterns as either “reassuring” or “nonreassuring” was inadequate and did not fully capture the range of patterns seen at the bedside or reflect the existing evidence of association. Therefore a new three-tiered nomenclature system was designated and endorsed by all of the stakeholders shortly thereafter. Since then all EFM patterns have been designated by the category system as category I (normal), category II (indeterminate), or category III (abnormal) ( Table 15.2 ).
| Pattern | Definition |
|---|---|
| Baseline | Mean FHR rounded to increments of 5 beats/min in a 10-min window excluding accelerations, decelerations, and periods of marked FHR variability (>25 beats/min). There must be at least 2 min of identifiable baseline segments (not necessarily contiguous) in any 10-min window or the baseline for that period is indeterminate.
|
| Variability | Fluctuations in the FHR baseline are irregular in amplitude and frequency and are visually quantitated as the amplitude of the peak to the trough in beats per minute.
|
| Accelerations | Abrupt increase (onset to peak <30 s) in the FHR from the most recently calculated baseline. At ≥32 weeks, an acceleration peaks ≥15 beats/min above baseline and lasts ≥15 s but <2 min. At <32 weeks, acceleration peaks ≥10 beats/min above baseline and lasts ≥10 s but <2 min. Prolonged acceleration lasts ≥2 min but <10 min. Acceleration ≥10 min is a baseline change. |
| Early | A gradual (onset to nadir ≥30 s) decrease in FHR during a uterine contraction. Onset, nadir, and recovery of the deceleration occur at the same time as the beginning, peak, and end of the contraction, respectively. |
| Late | A decrease in FHR is gradual (onset to nadir ≥30 s) during a uterine contraction. Onset, nadir, and recovery of the deceleration occur after the beginning, peak, and end of the contraction, respectively. |
| Variable | Decrease in the FHR is abrupt (onset to nadir <30 s); it is ≥15 beats/min below the baseline and lasts ≥15 s but less than 2 min. |
| Prolonged | Deceleration is ≥15 beats/min below baseline and lasts ≥2 min or more but <10 min. Deceleration ≥10 min is a baseline change. |
| Sinusoidal pattern | Pattern in FHR baseline is smooth, sine wave–like, and undulating with a cycle frequency of 3–5/min that persists for at least 20 min. |
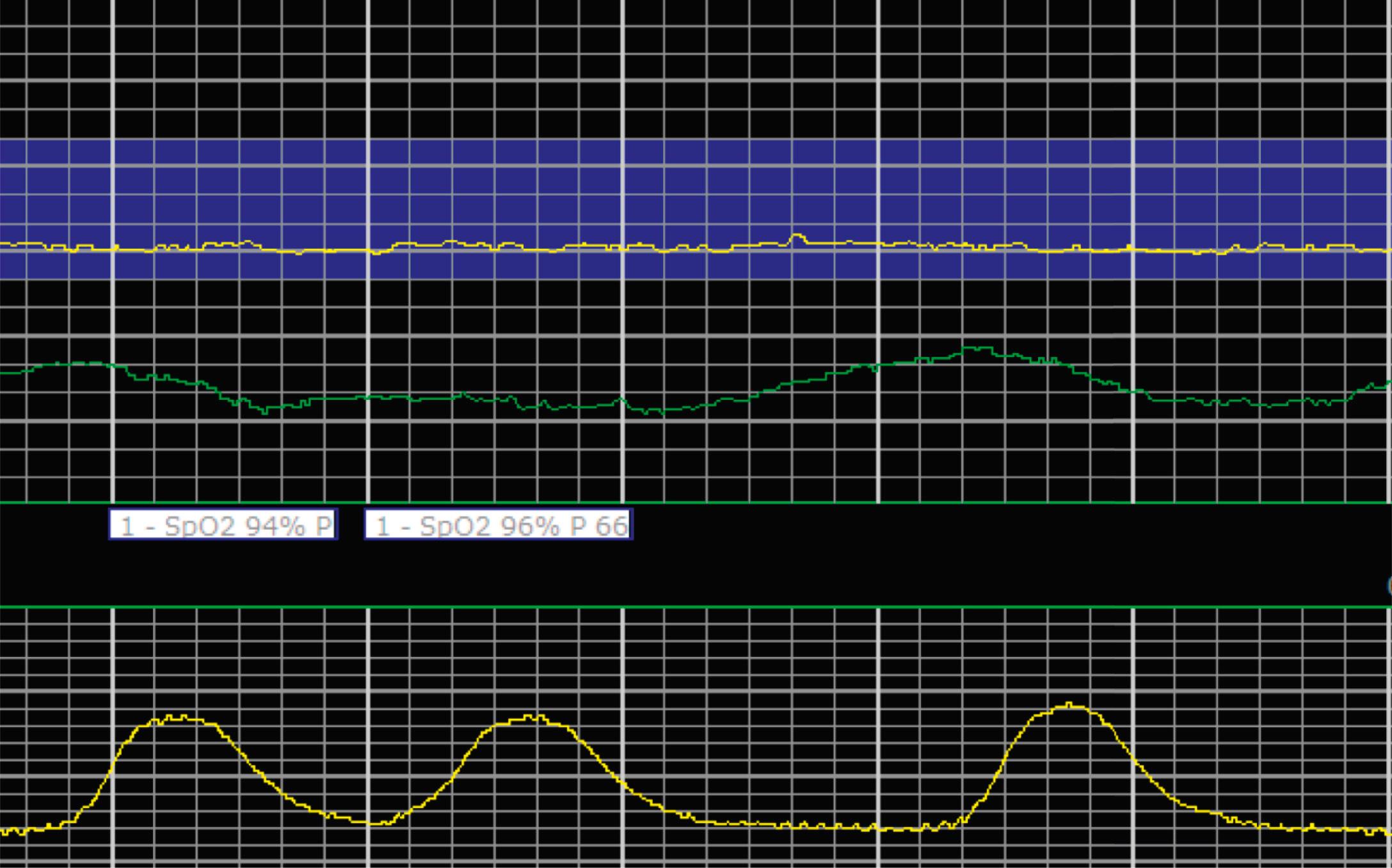
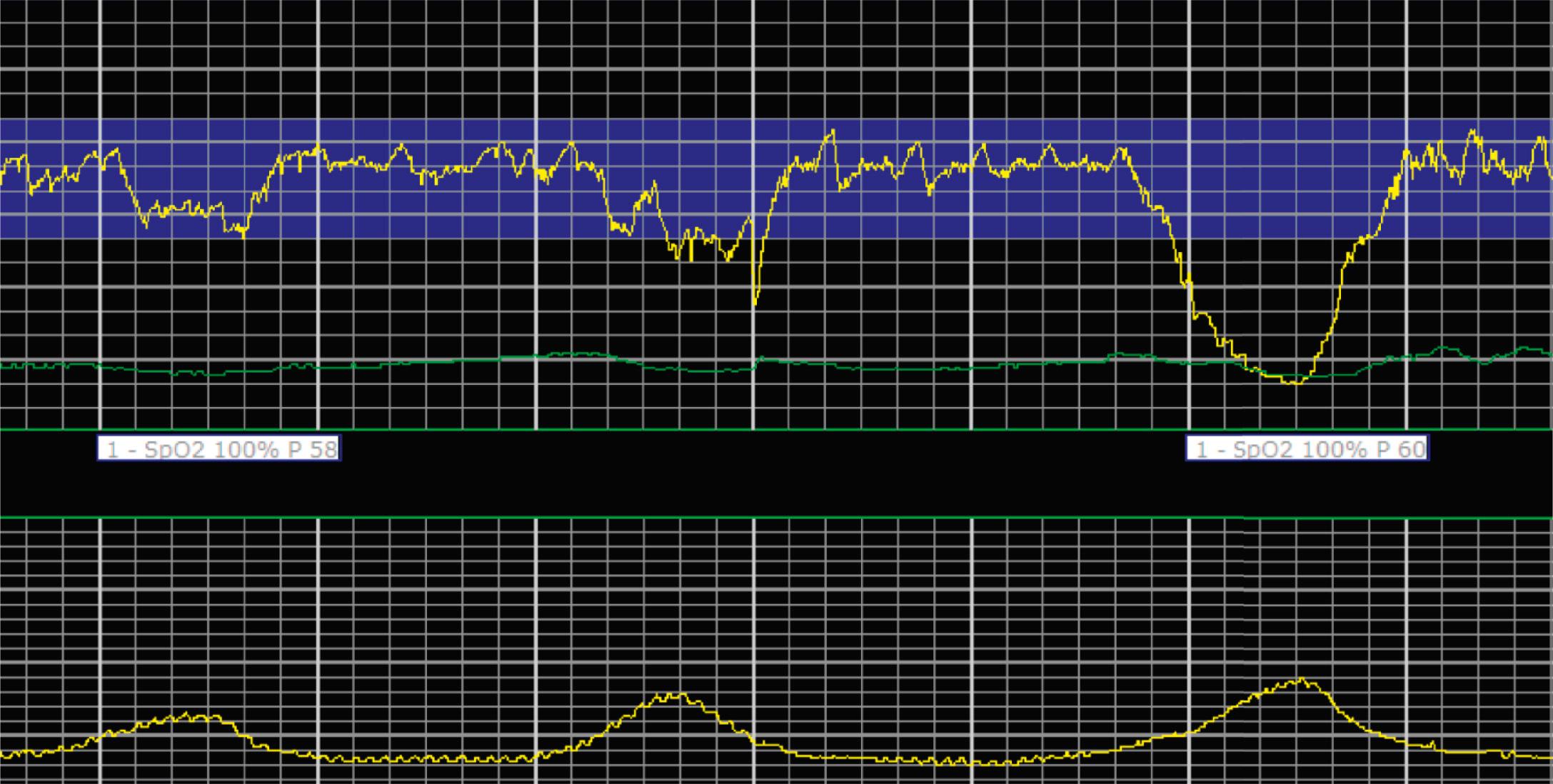
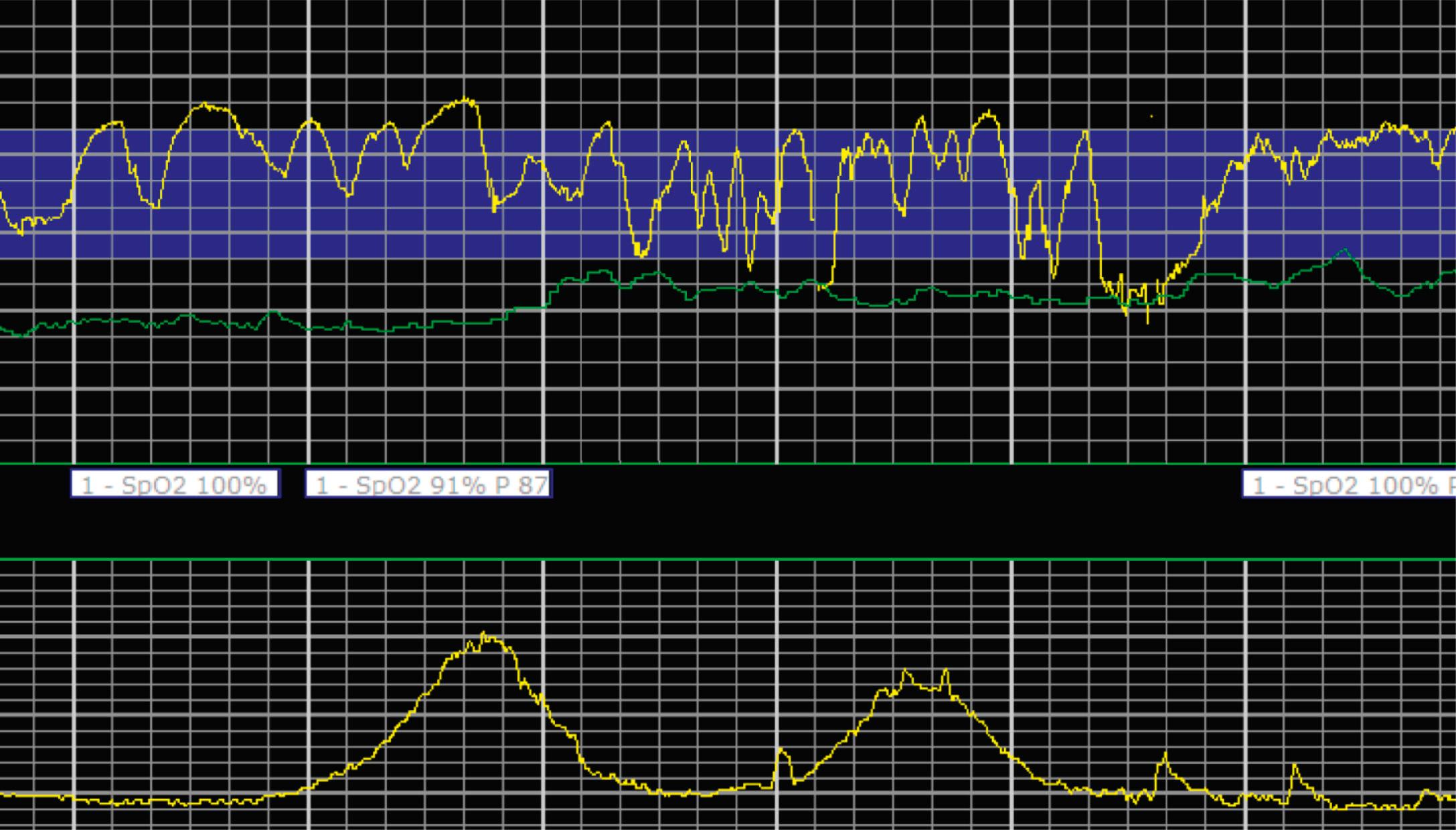
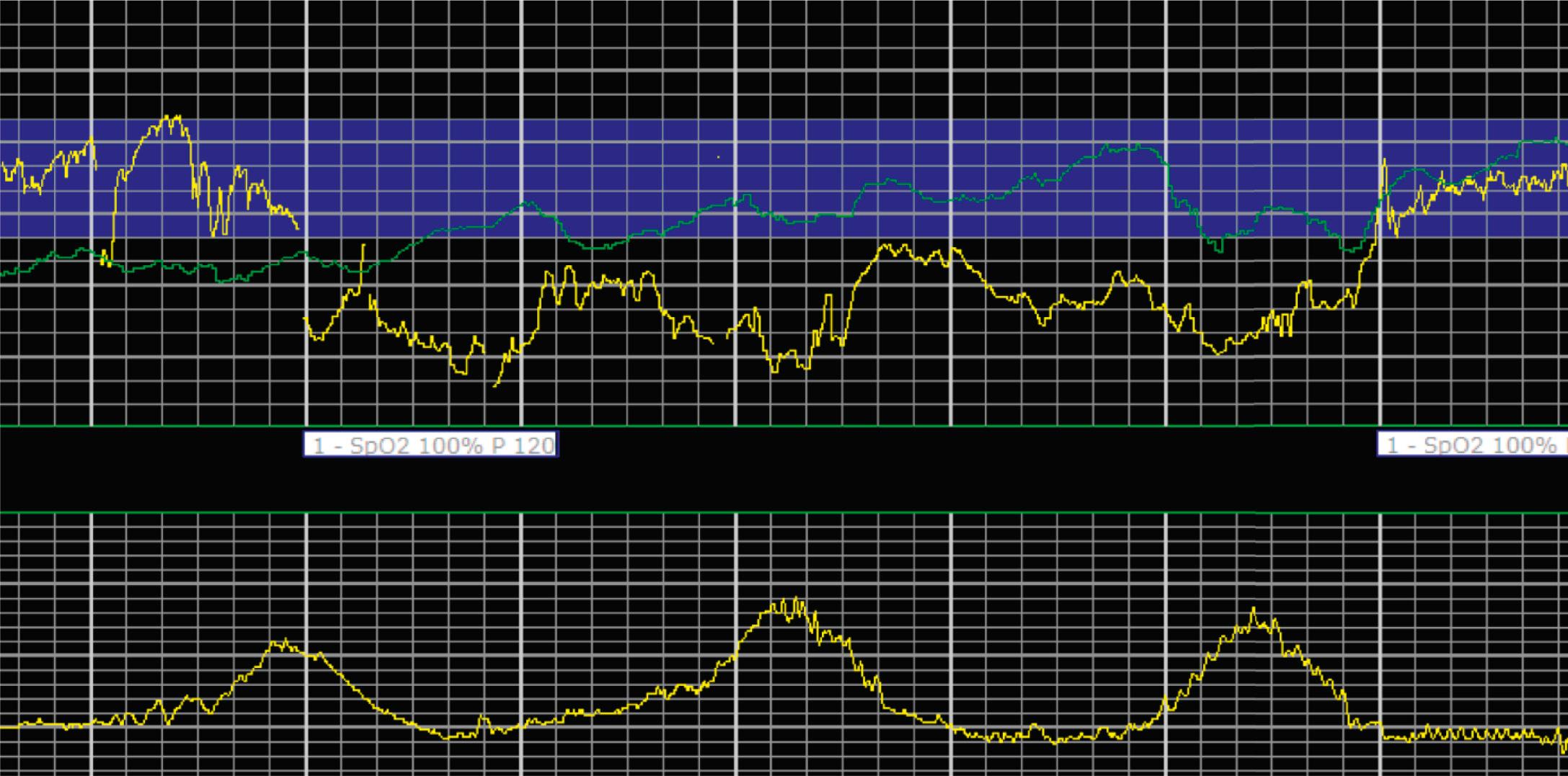
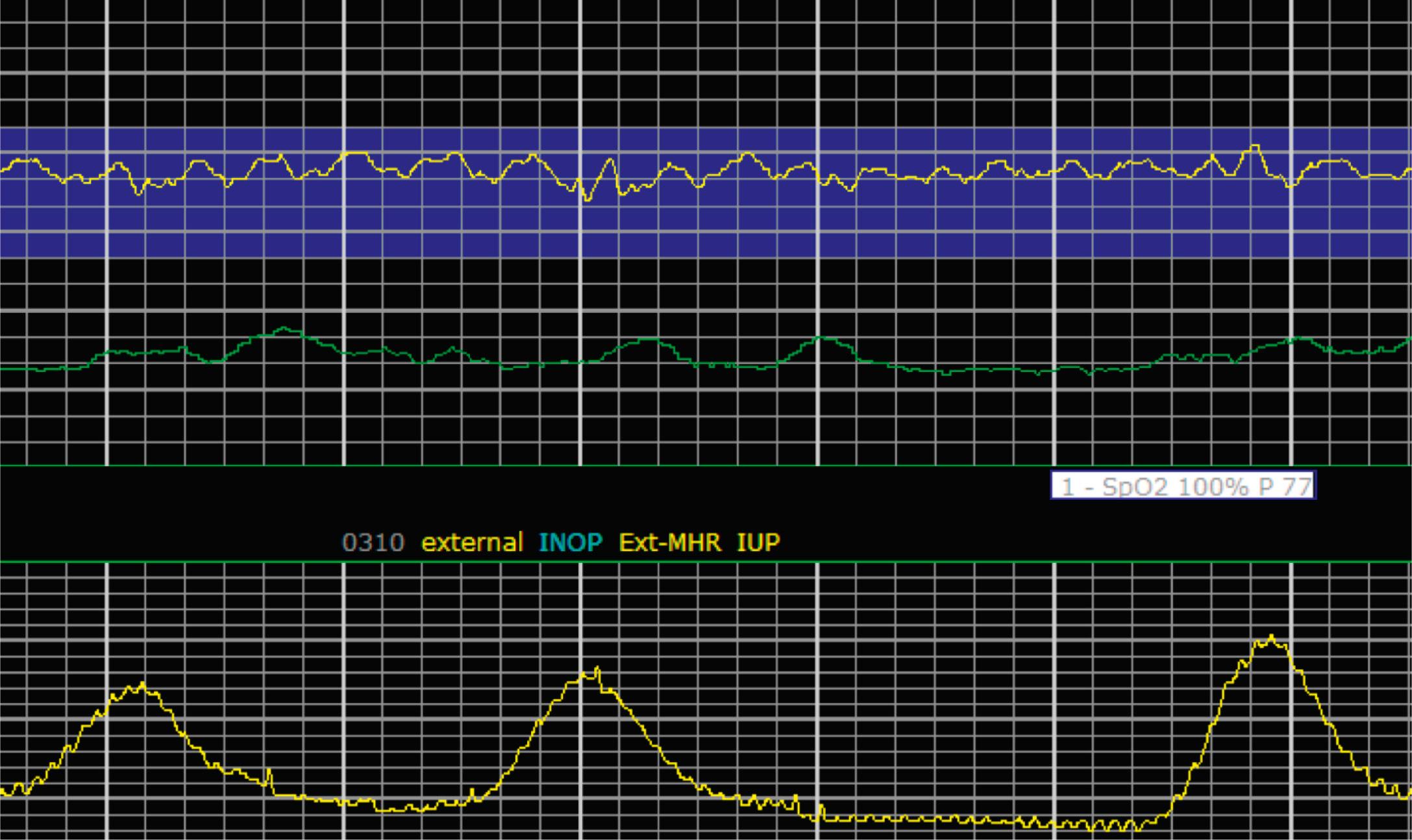
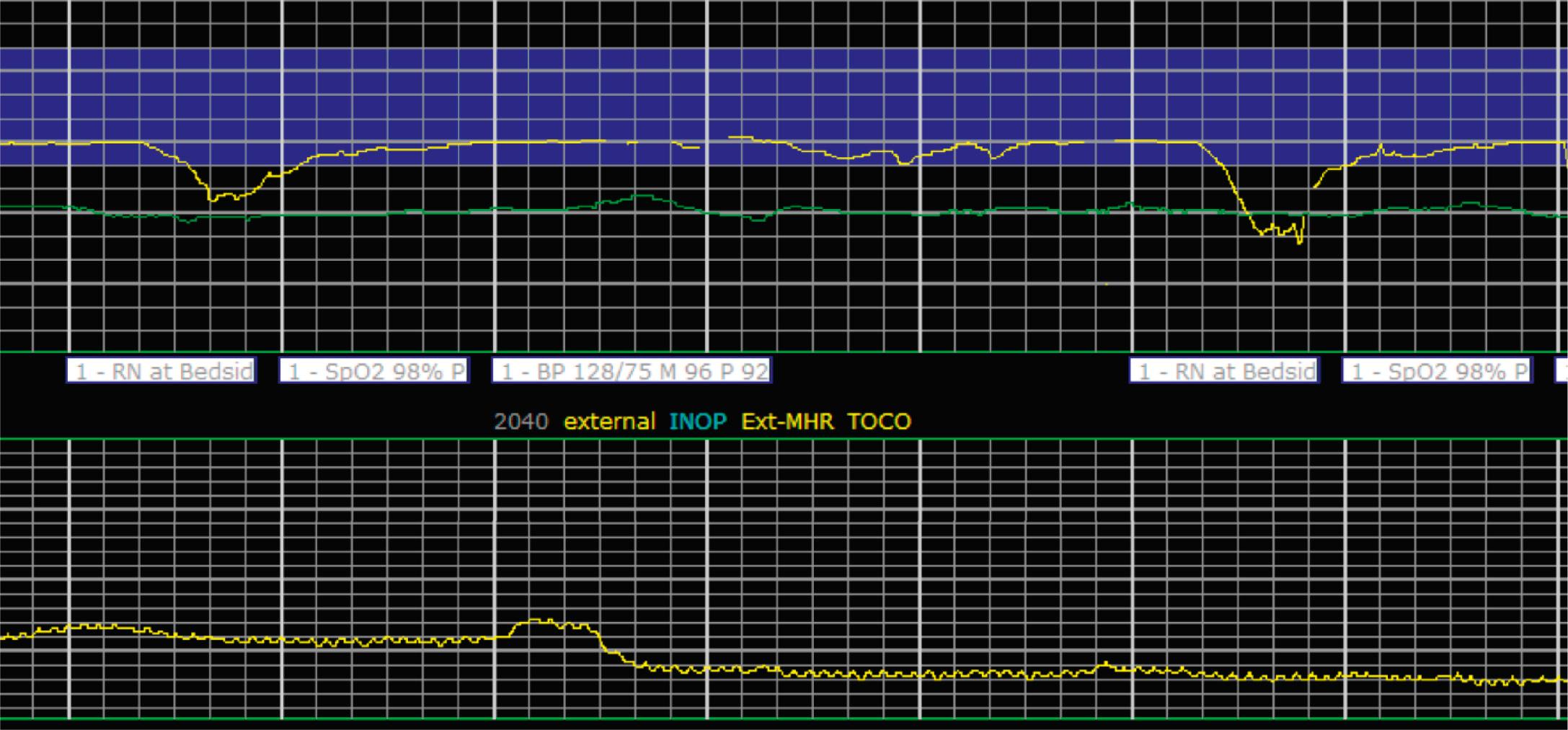
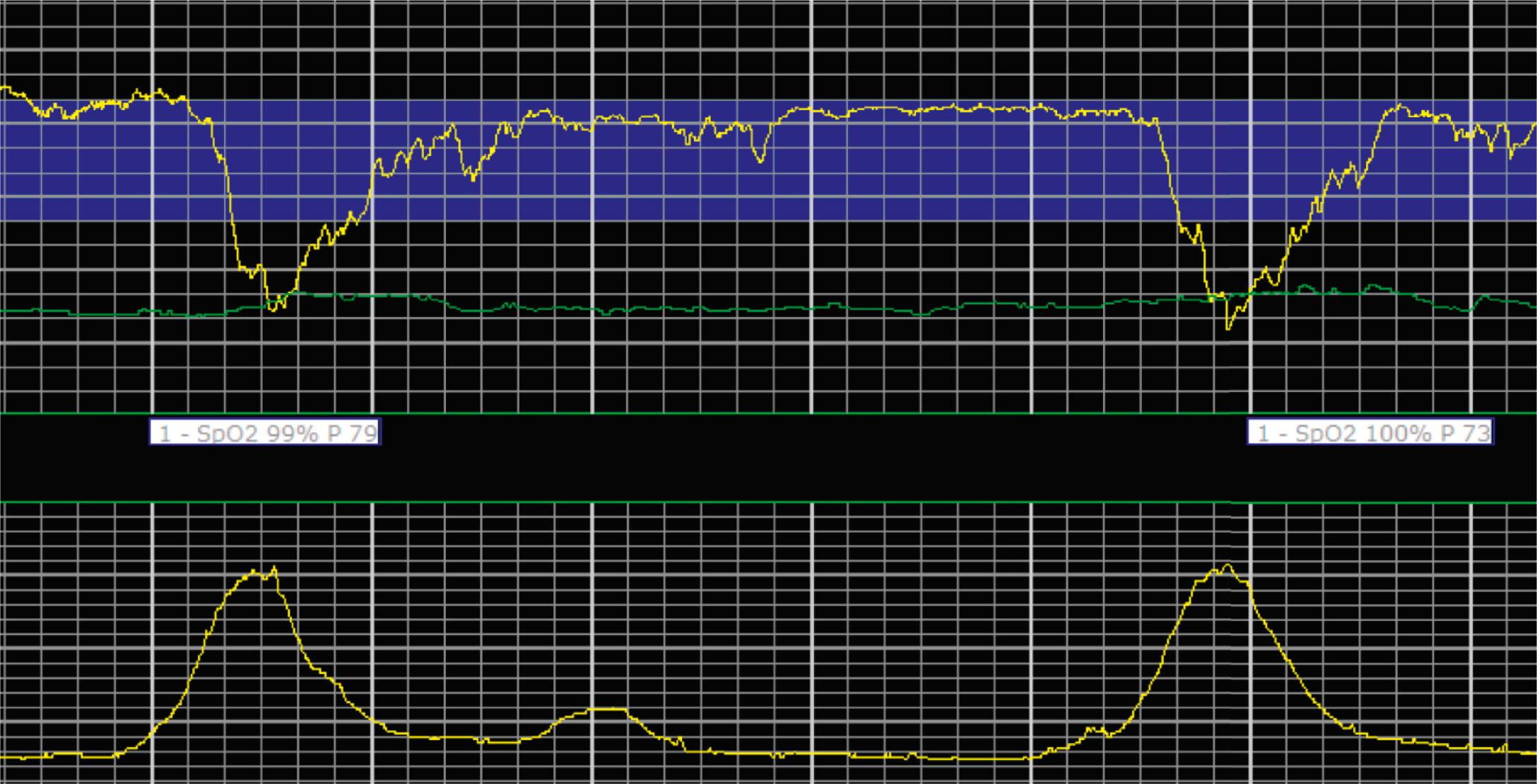
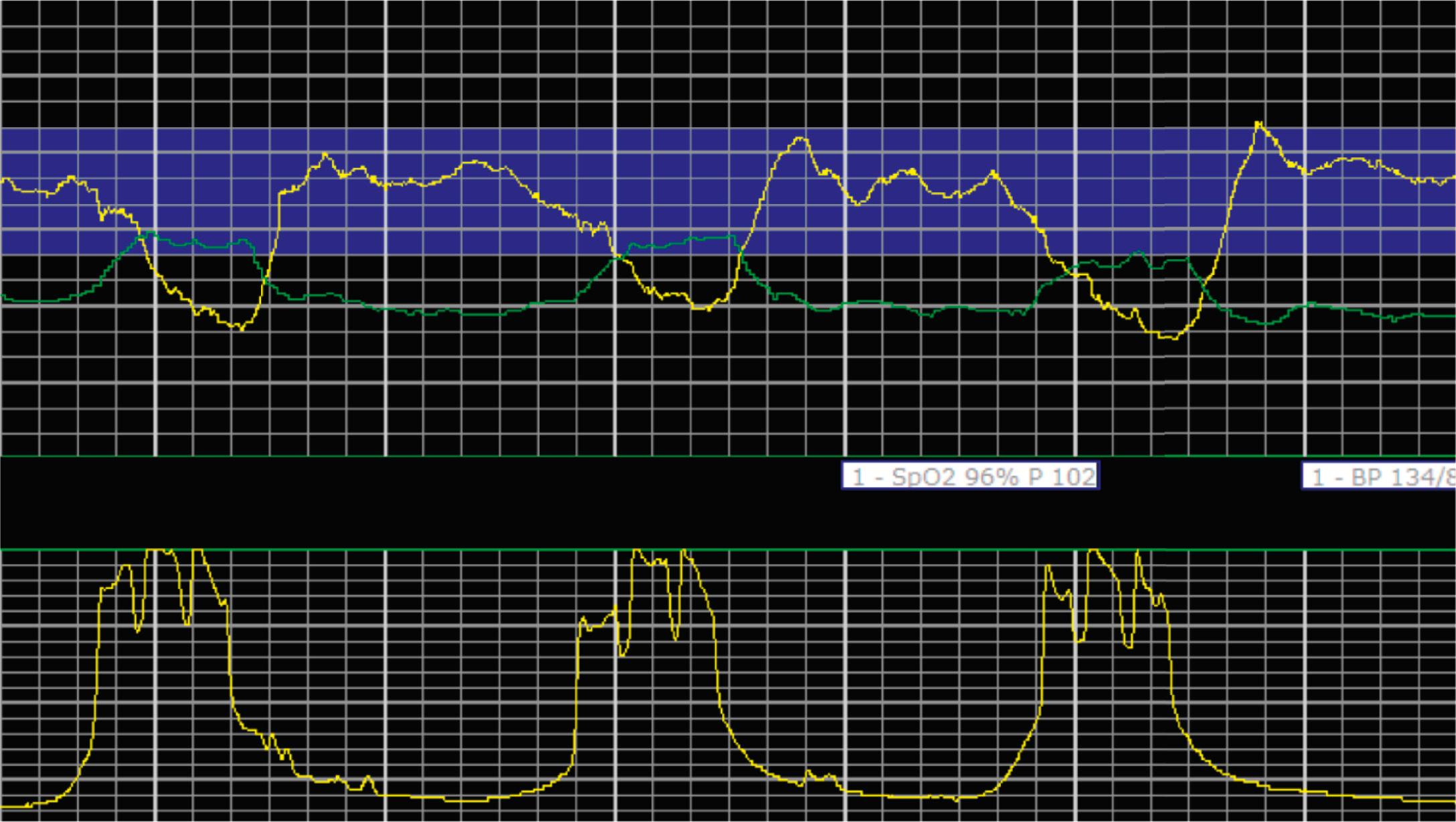
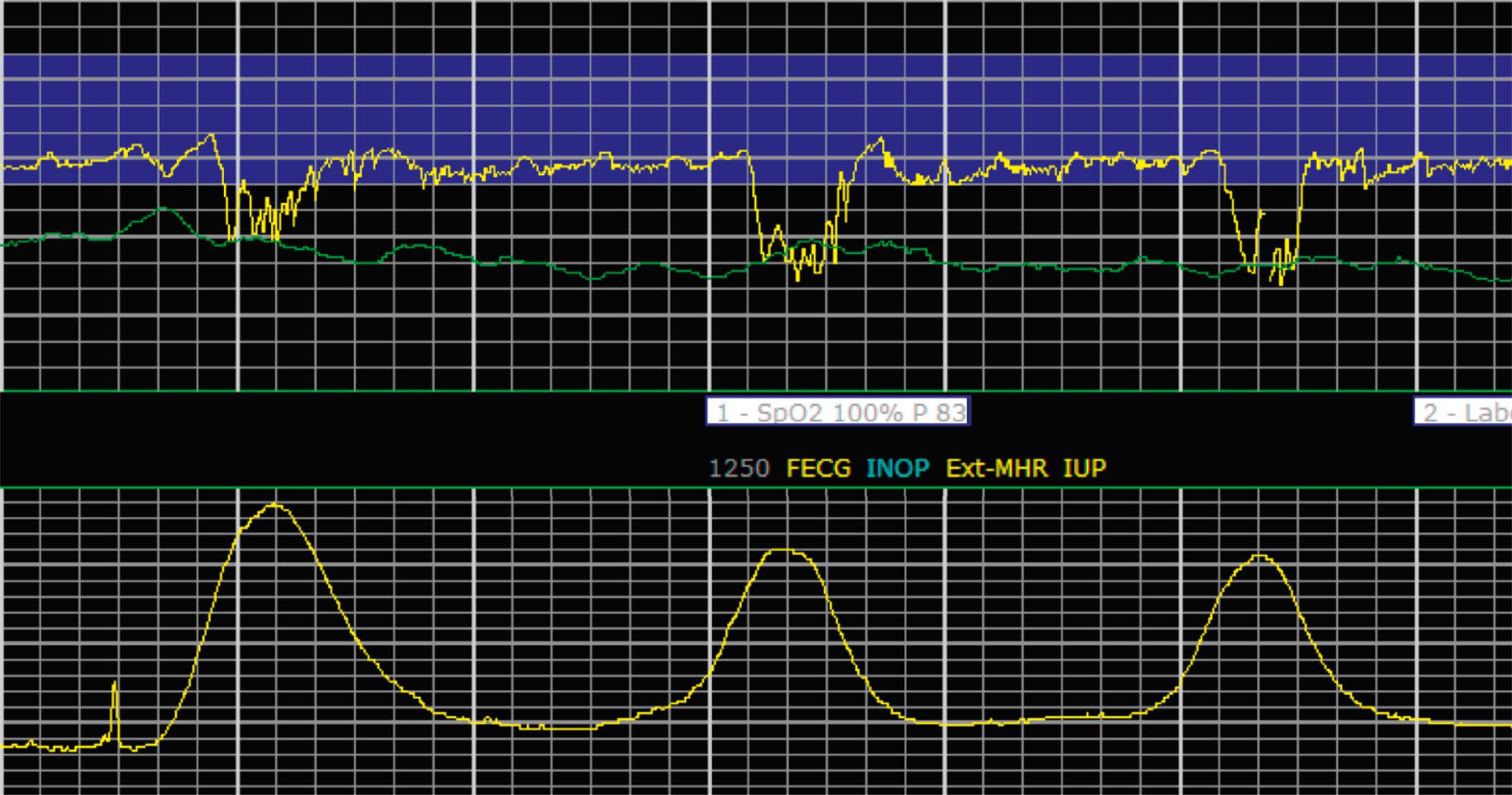
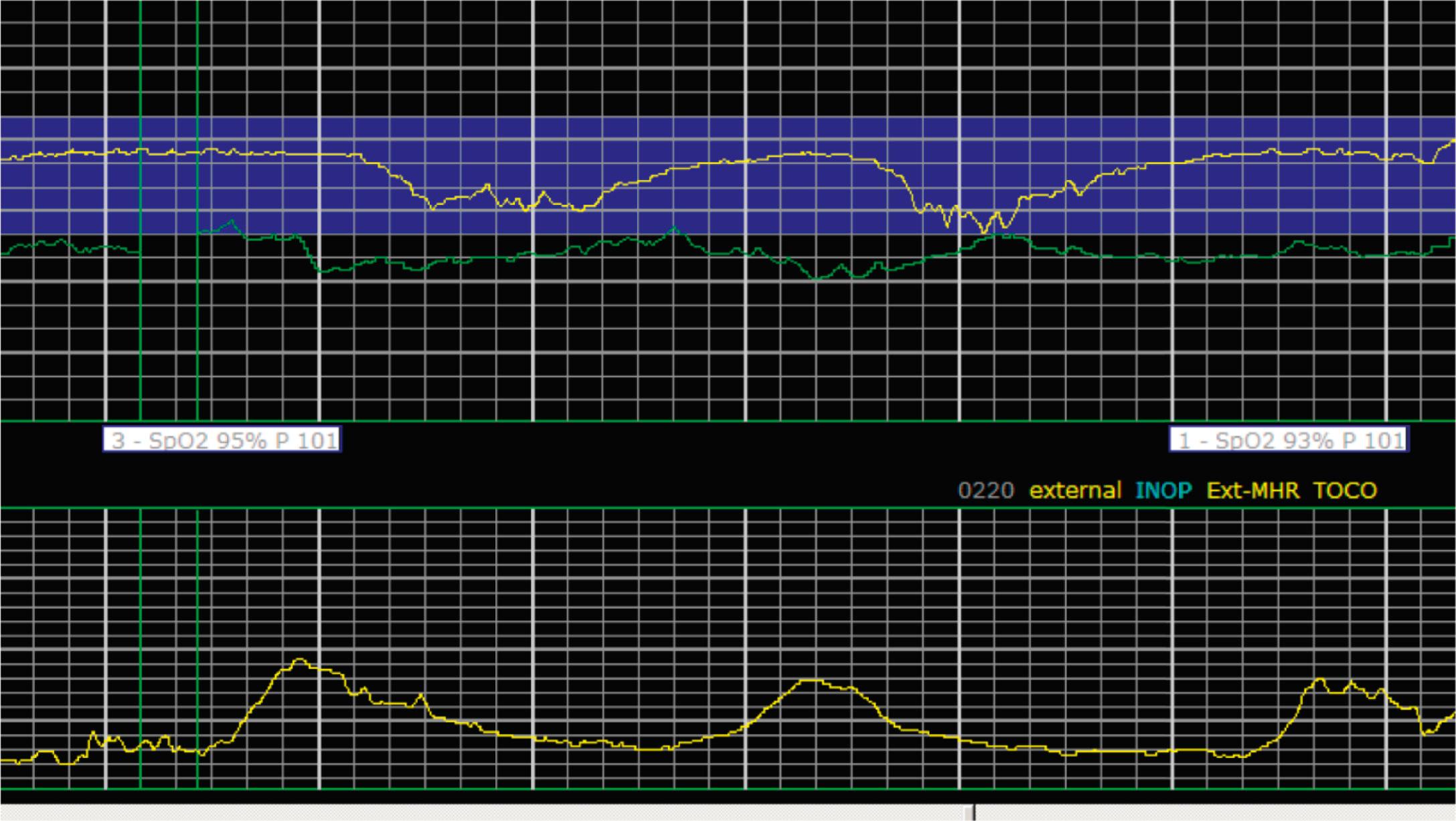
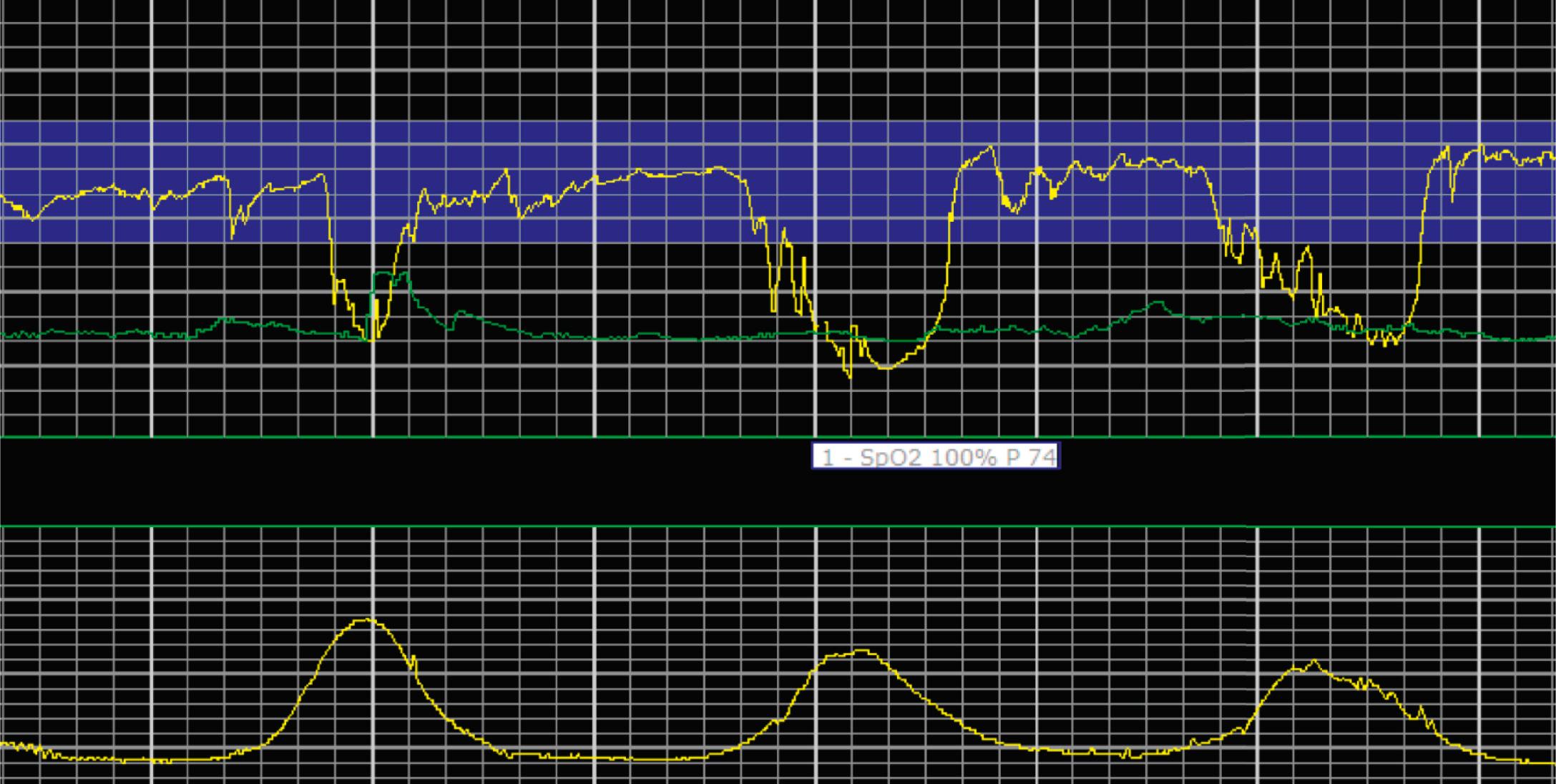
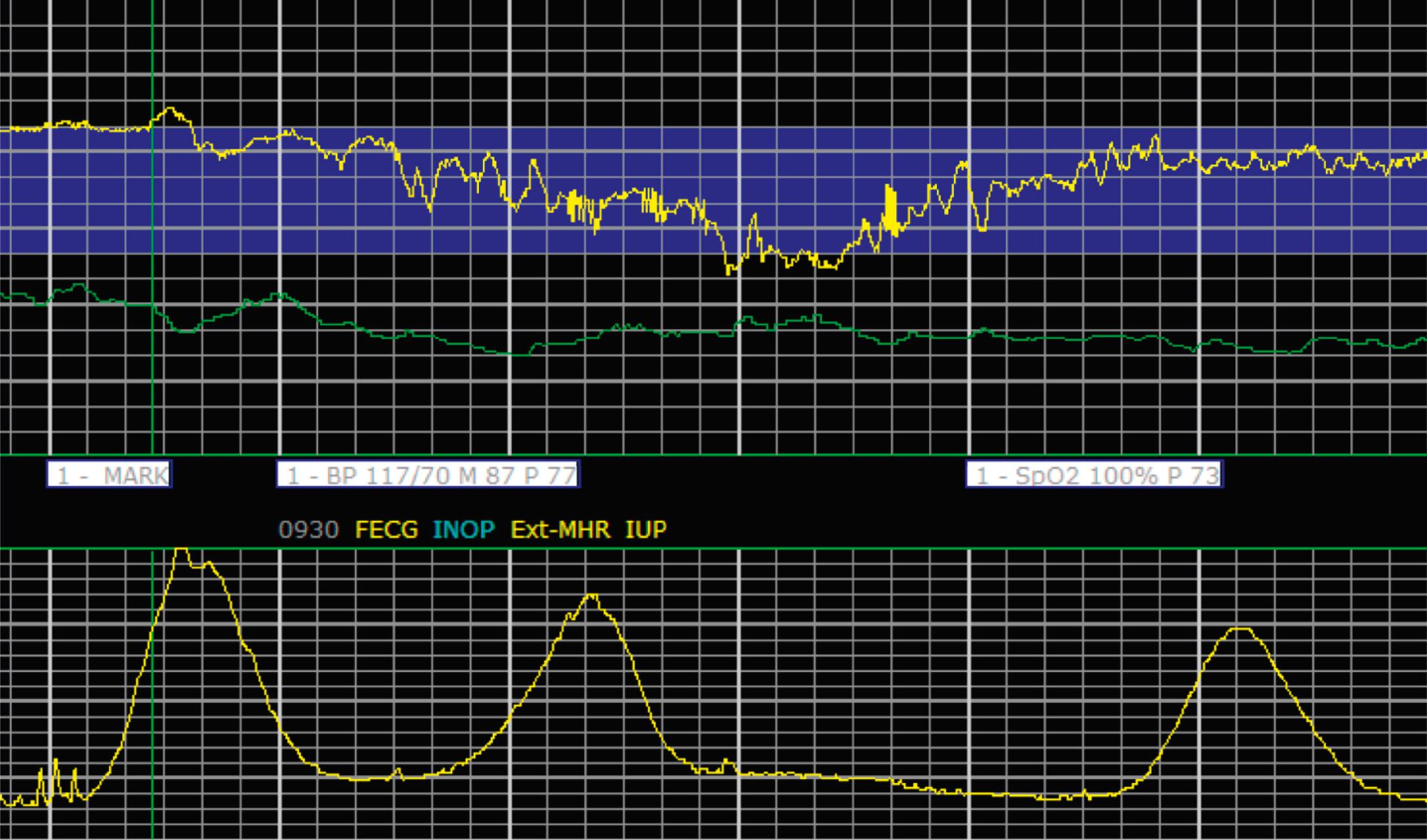
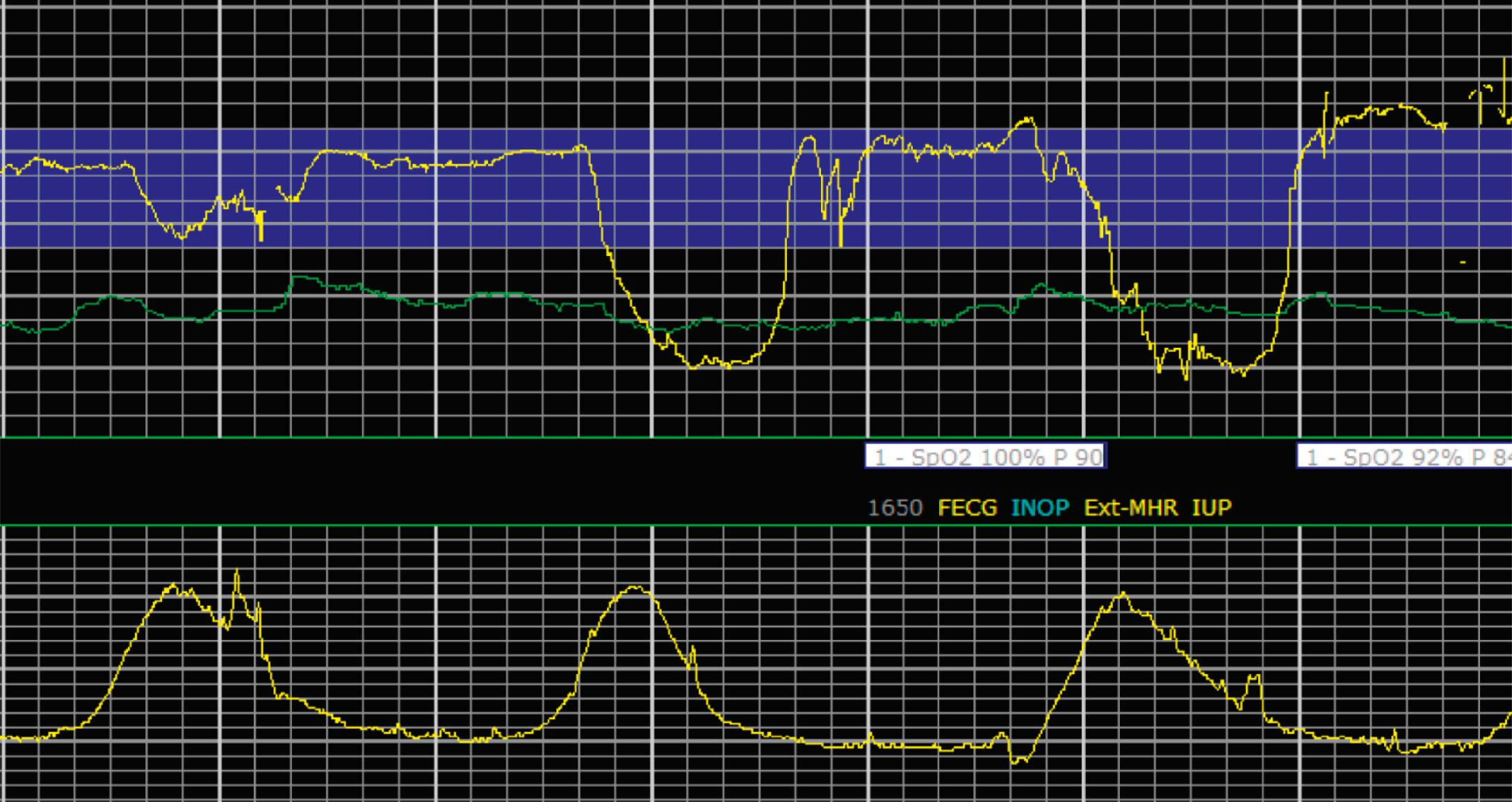
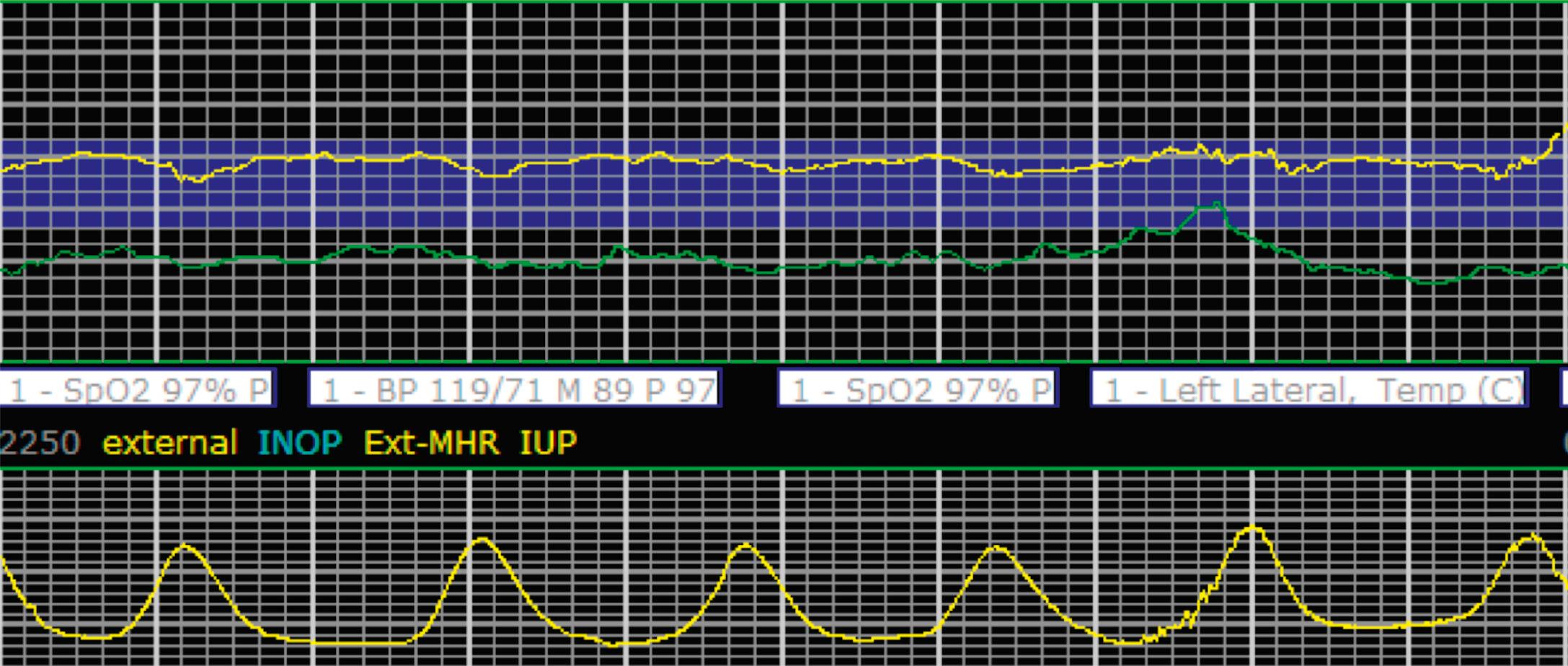
EFM patterns are interpreted clinically with visual observation in a stepwise approach. Several observed components are then used to make an overall category designation. The first step is determination of the baseline heart rate, which can be defined by at least 2 minutes in any given 10-minute period. A normal range is 110 to 160 beats/min. A baseline heart rate greater than 160 beats/min defines tachycardia and one below 110 beats/min defines bradycardia. Next, at the baseline, the magnitude of variability is determined, which is the amplitude of the oscillation around the baseline. There are four variability definitions: absent (no oscillation) ( Fig. 15.4 ), minimal ( Fig. 15.5 ), moderate (6 to 25 beats/min) ( Fig. 15.6 ), and marked (>25 beats/min) ( Fig. 15.7 ). A specific pattern of repetitive oscillation is referred to as sinusoidal ( Fig. 15.8 ). Though rare, it is important to identify as it has been associated with fetal pathology such as anemia.
Accelerations are rises in the FHR above the baseline or at least 15 beats/min and last at least 15 seconds before returning to the baseline ( Fig. 15.9 ). At less than 32 0/7 weeks, accelerations are defined less stringently, as an increase of at least 10 beats/min that lasts at least 10 seconds before returning to the baseline. Physiologically, accelerations are thought to be due to fetal movement.
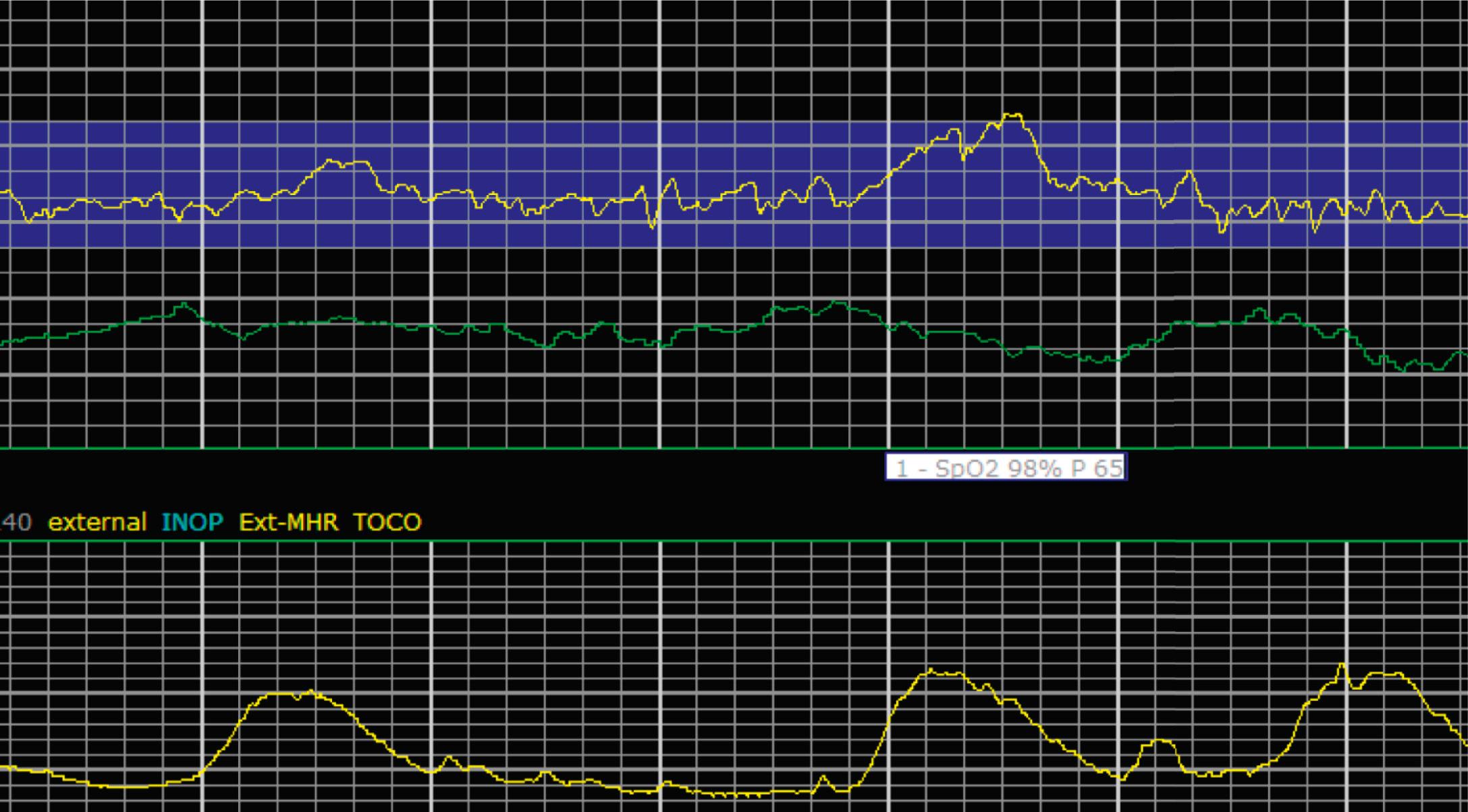
It is important to determine the presence or absence of decelerations. Decelerations are decreases in the FHR below the baseline that then return to baseline. There are four different types of decelerations. Because their temporal relationship to contractions helps to define the deceleration type for some, accurate assessment of contraction timing is prudent. Early decelerations are marked by a gradual decrease followed by a gradual return to baseline, which is concluded by the time the contraction ends; it lasts less than 2 minutes and generally is a mirror image of the contraction in terms of onset, nadir, and resolution. Early decelerations are thought to be a physiologic response to fetal head compression and thus are seen most commonly during active periods of fetal descent in labor.
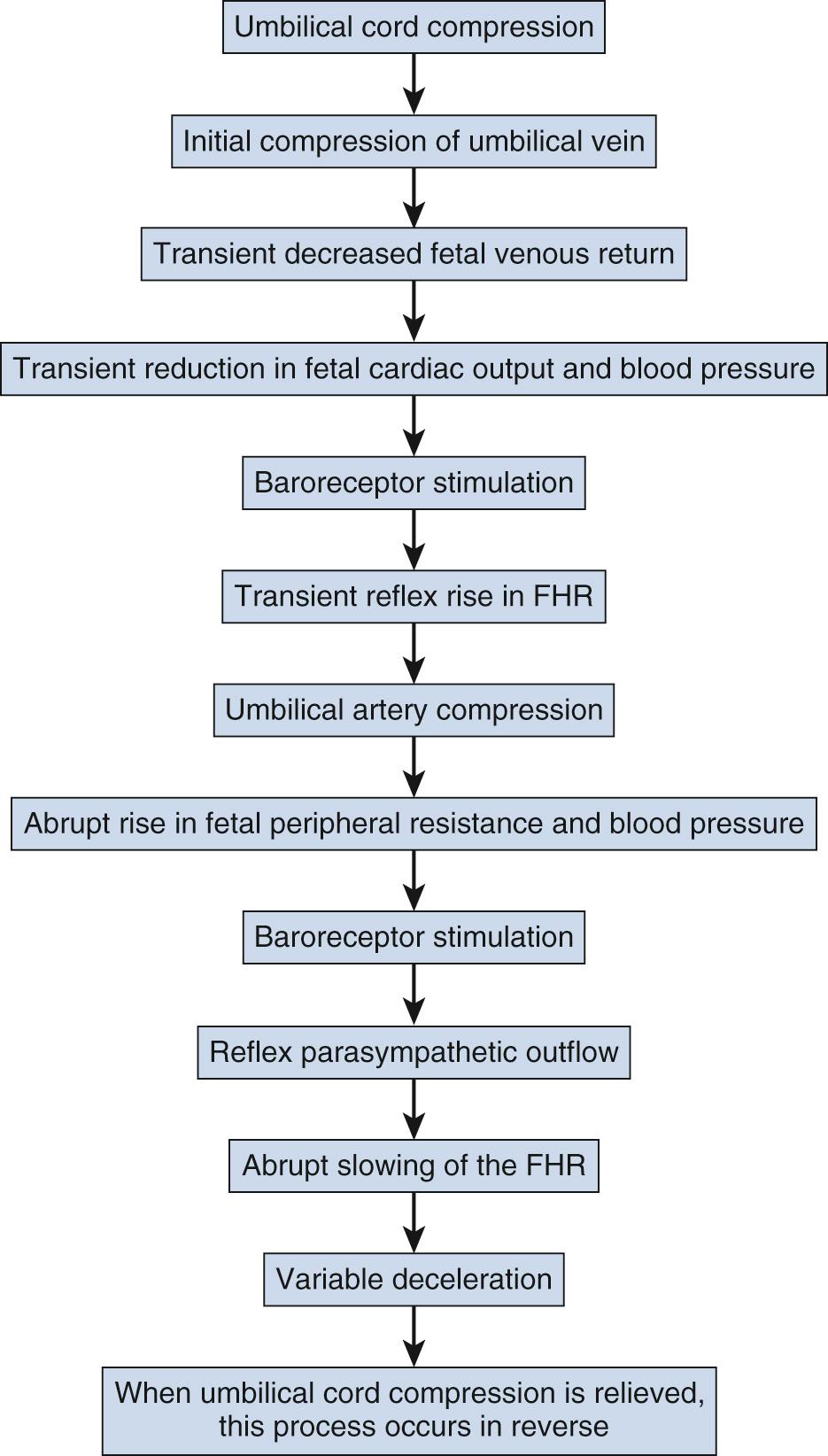
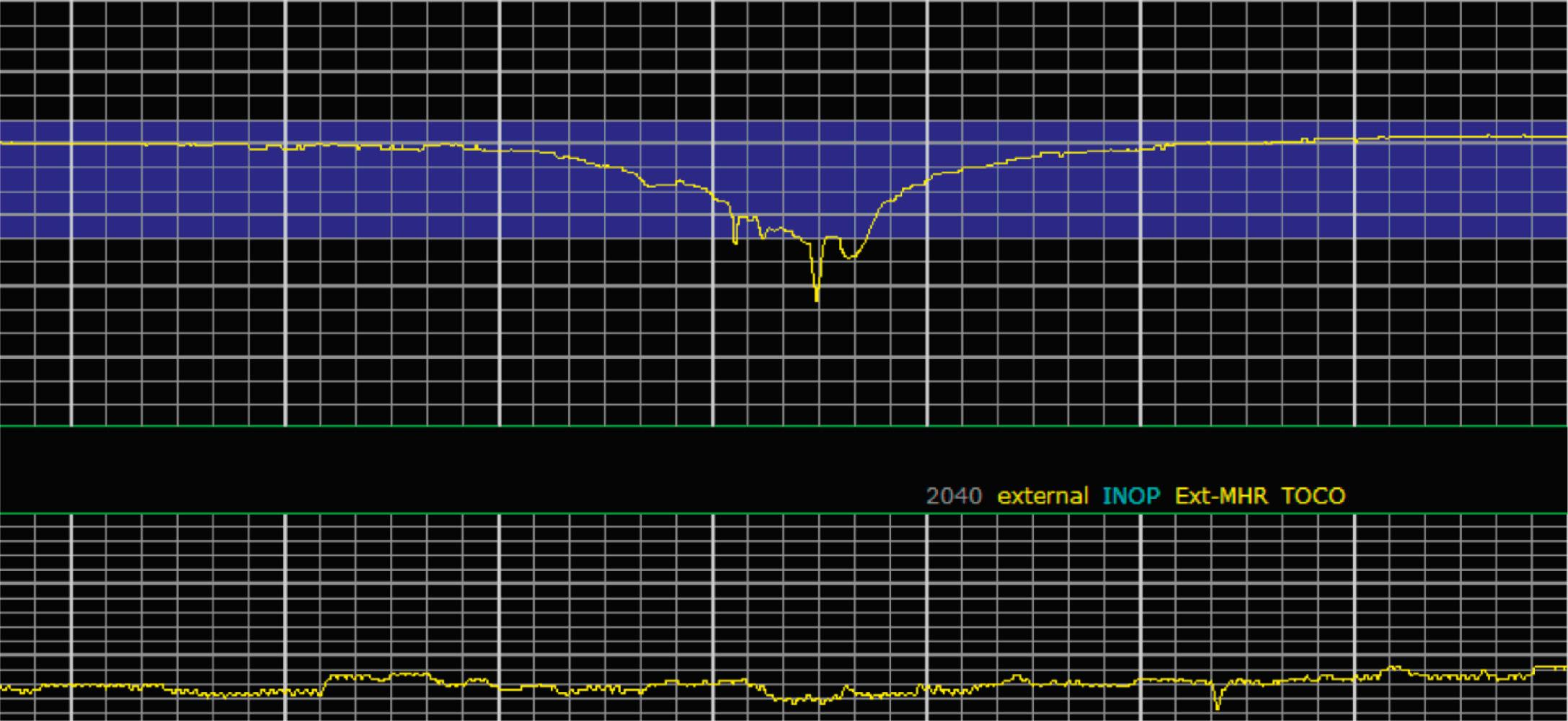
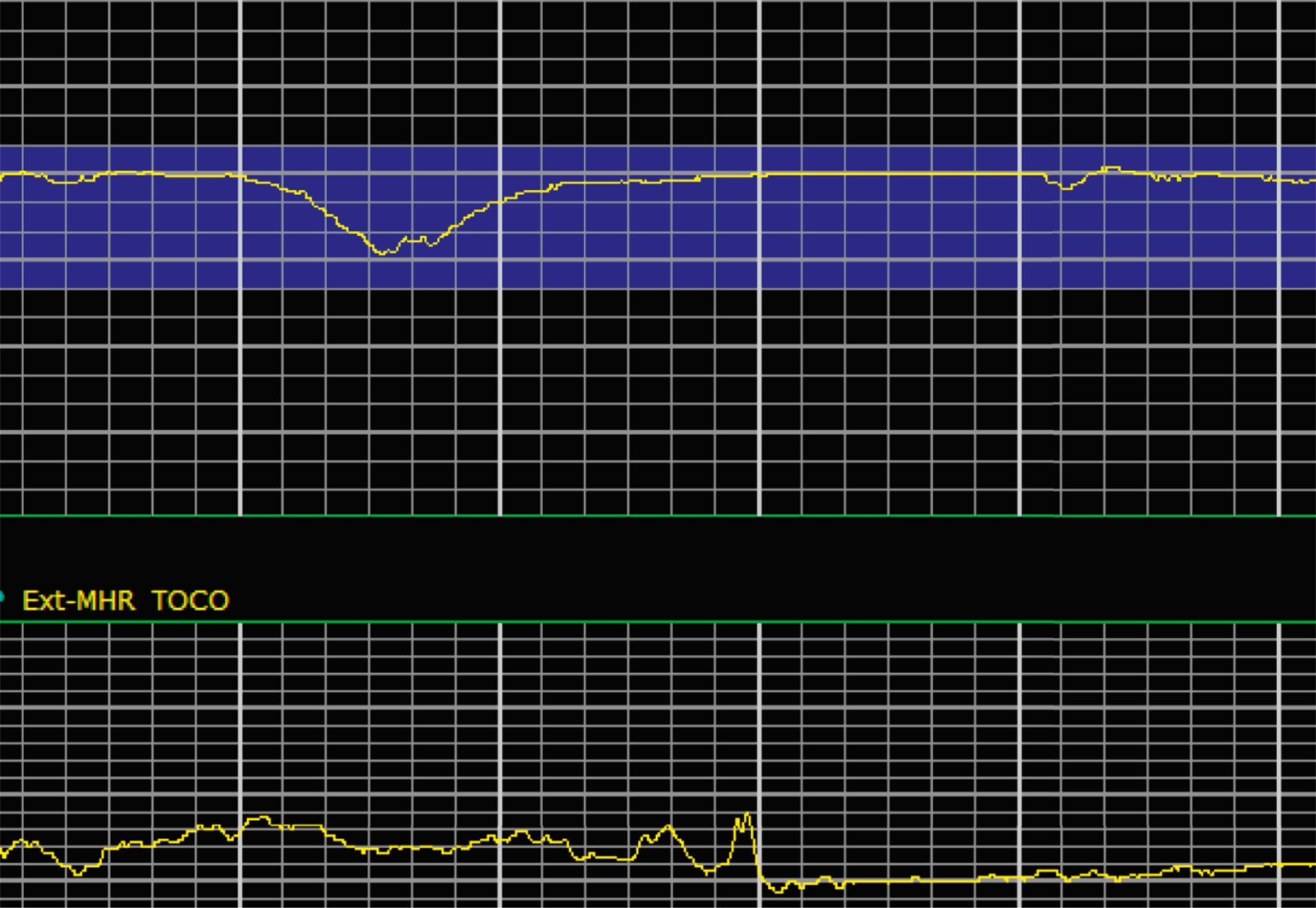
Variable decelerations are characterized by an abrupt descent and an abrupt return to baseline of at least 15 beats/min lasting less than 2 minutes ( Fig. 15.10 ). The nadir of the deceleration is reached in less than 30 seconds, and the shape is often described as a “V” or “U.” Variable decelerations are caused by umbilical cord compression, which can happen at any time due to the relative movements and positions of mother and fetus and particularly during labor contractions and the cardinal movements. When the umbilical cord is compressed, the thin-walled umbilical vein is typically compressed first, which reduces fetal preload and can produce a short, transient compensatory increase in FHR that immediately precedes the variable deceleration. When the umbilical arteries are compressed as the cord is fully occluded, baroreflexes and chemoreflexes mediate a decrease in heart rate. As compression of the umbilical cord resolves, the thick-walled arteries regain flow first, followed by the vein, often creating the symmetric appearance of variable decelerations ( Fig. 15.11 ).
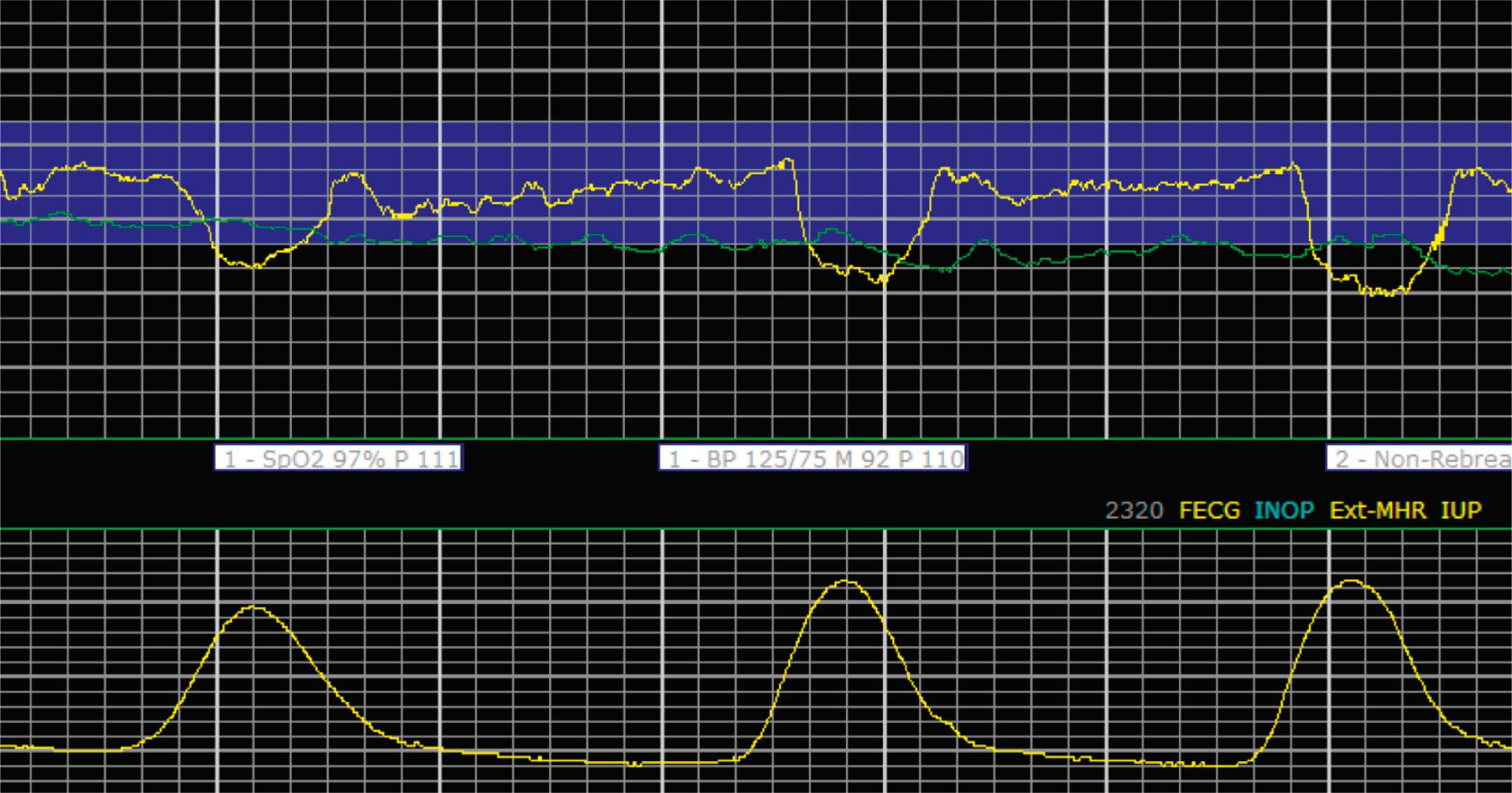
Late decelerations are characterized by a slow descent and slow return to baseline ( Fig. 15.12 ). The timing of the nadir of late decelerations is after the peak of the contraction, and the deceleration resolves after the contraction has concluded. Late decelerations are thought to be mediated by baroreflexes and chemoreceptor responses. The late timing has been associated with hypoxia and acidemia ( Fig. 15.13 ).
Prolonged decelerations are characterized not by shape or timing relative to contractions but by duration ( Fig. 15.14 ). Any deceleration of at least 15 beats/min regardless of shape that lasts more than 2 minutes but less than 10 minutes is a prolonged deceleration. Decelerations that occur with more than 50% of contractions during any given period are considered recurrent, regardless of type.
The combination of features over any 10-minute time interval are considered together and a category is assigned to inform communication, documentation, and management. Category I is considered a normal EFM pattern (see Figs. 15.15 and 15.16 ), whereas category III is abnormal (see Figs. 15.17, 15.18, and 15.19 ). The majority of patterns seen during labor are category II, encompassing a wide spectrum of patterns ( Figs. 15.20 to 15.27 ).
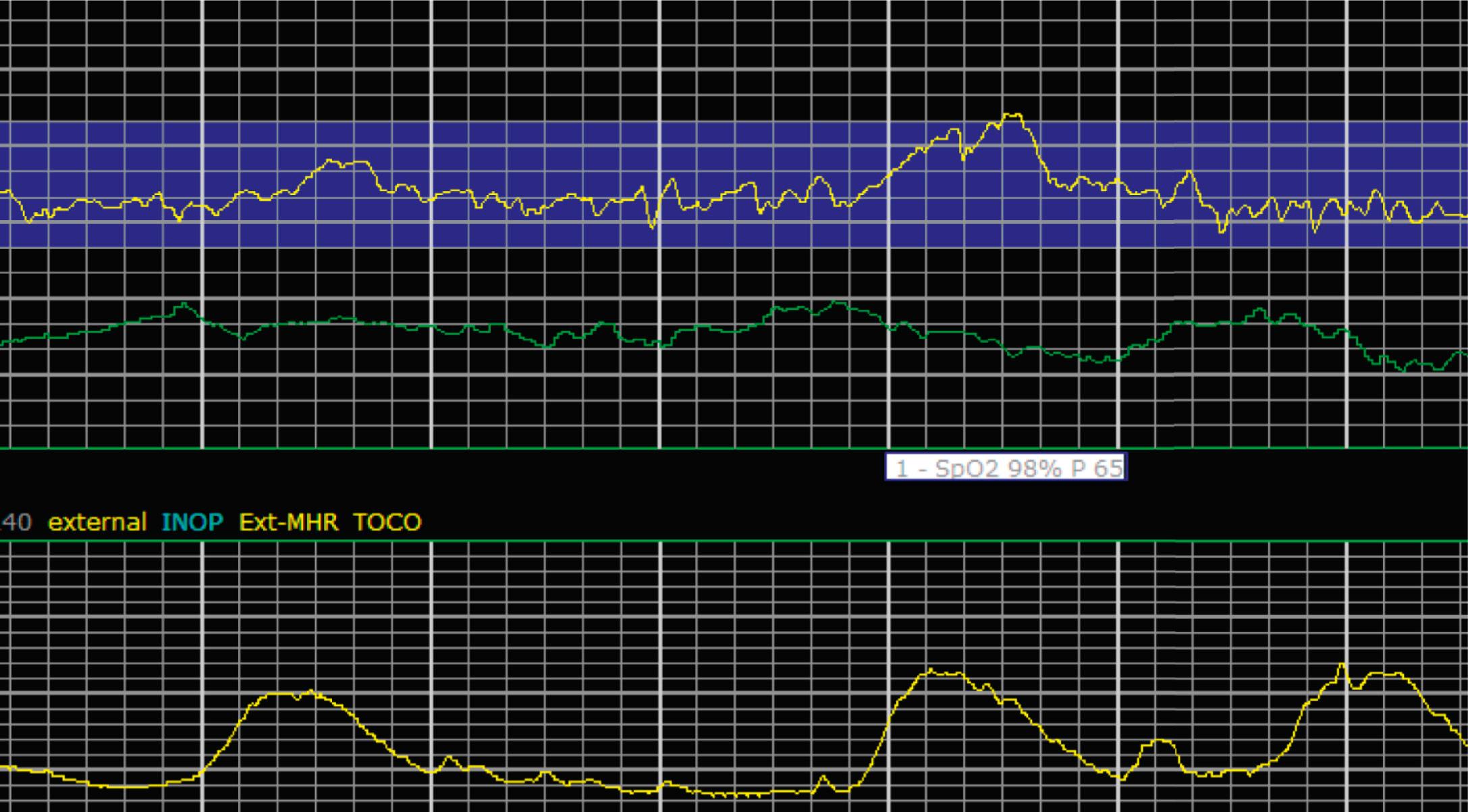
A few historically described EFM features were not included in the 2008 nomenclature system because—although they were once thought to be clinically informative—no evidence has demonstrated an association with those patterns and clinically meaningful outcomes. In terms of variability, historically there was a distinction between short-term and long-term variability. These terms attempted to distinguish between beat-to-beat variation at the baseline (short-term variability) and periodic variation around the baseline (long-term variability). However, despite the hypothesis that decreased short-term variability was associated with acidemia, larger studies of higher quality did not demonstrate any association with meaningful clinical outcomes, including acidemia. Thus this distinction is no longer used clinically and accordingly not included in the clinical nomenclature standard set forth by NICHD. Similarly, it was historically thought that the variability within a deceleration, particularly at the nadir, had an association with acidemia. However, no studies have provided evidence for that, and the current guidelines for determining variability say to do so when the fetus is at the baseline heart rate.
Two features of decelerations were also historically thought to be important for clinical interpretation. The term shoulder referred to the brief increase in FHR often seen preceding and/or after the deceleration. The term overshoot referred to the FHR following a deceleration, which would rise above the established baseline before returning to it. All of these features were thought to be clinically informative. However, they have never been shown to be associated with acidemia and/or adverse neonatal outcomes and thus were appropriately removed from the nomenclature system. These descriptions should not be included in clinical communication or documentation unless new evidence emerges and the recommendations change.
Uterine monitoring is an important part of intrapartum fetal monitoring because it enables the interpretation of EFM patterns in the context of uterine activity . There are two main reasons why specific uterine activity is important apart from interpreting fetal well-being in the context of labor progress. First, the FHR decelerations should be interpreted timed with uterine contractions to establish whether or not late decelerations are occurring. Decelerations that nadir after the apex of the contractions and resolve after the resolution of the contractions are late decelerations that require clinical evaluation. Second, tachysystole is an important consideration because it has been suggested that decreased oxygenation during excessive contractions could lead to fetal hypoxia over time.
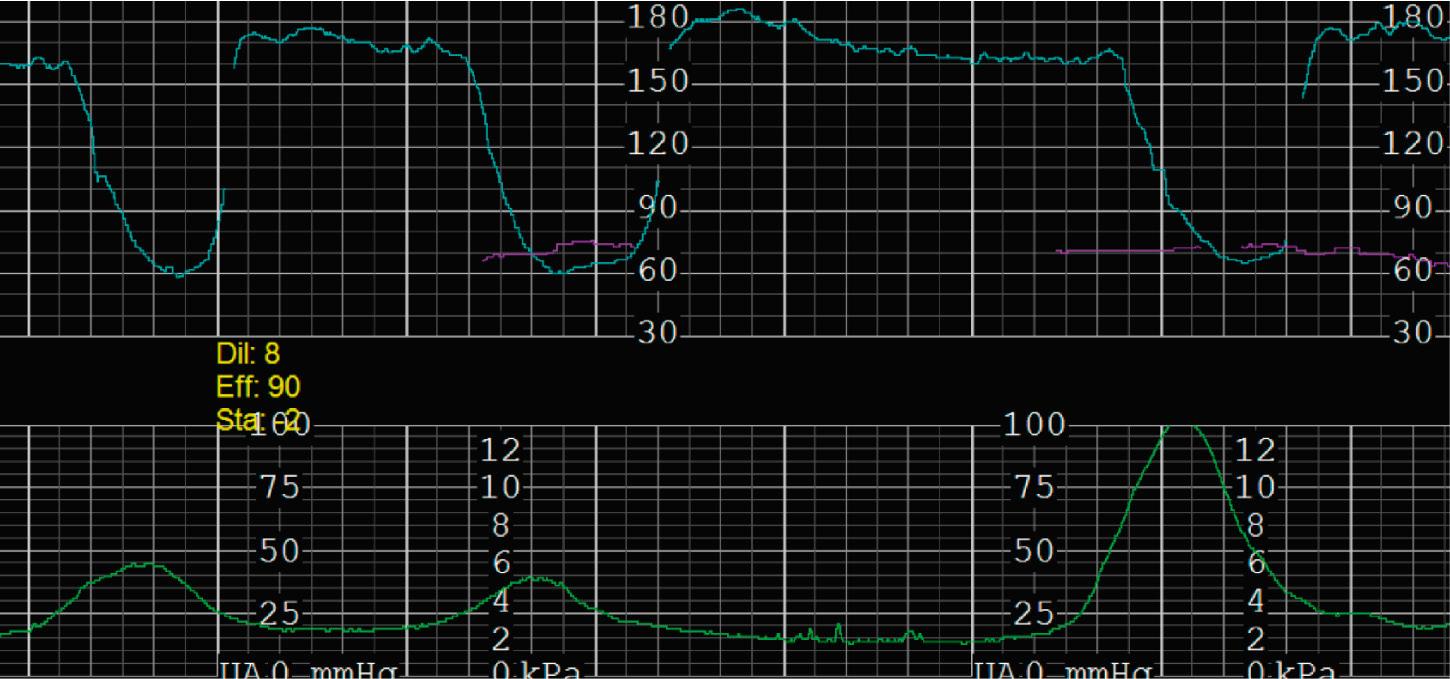
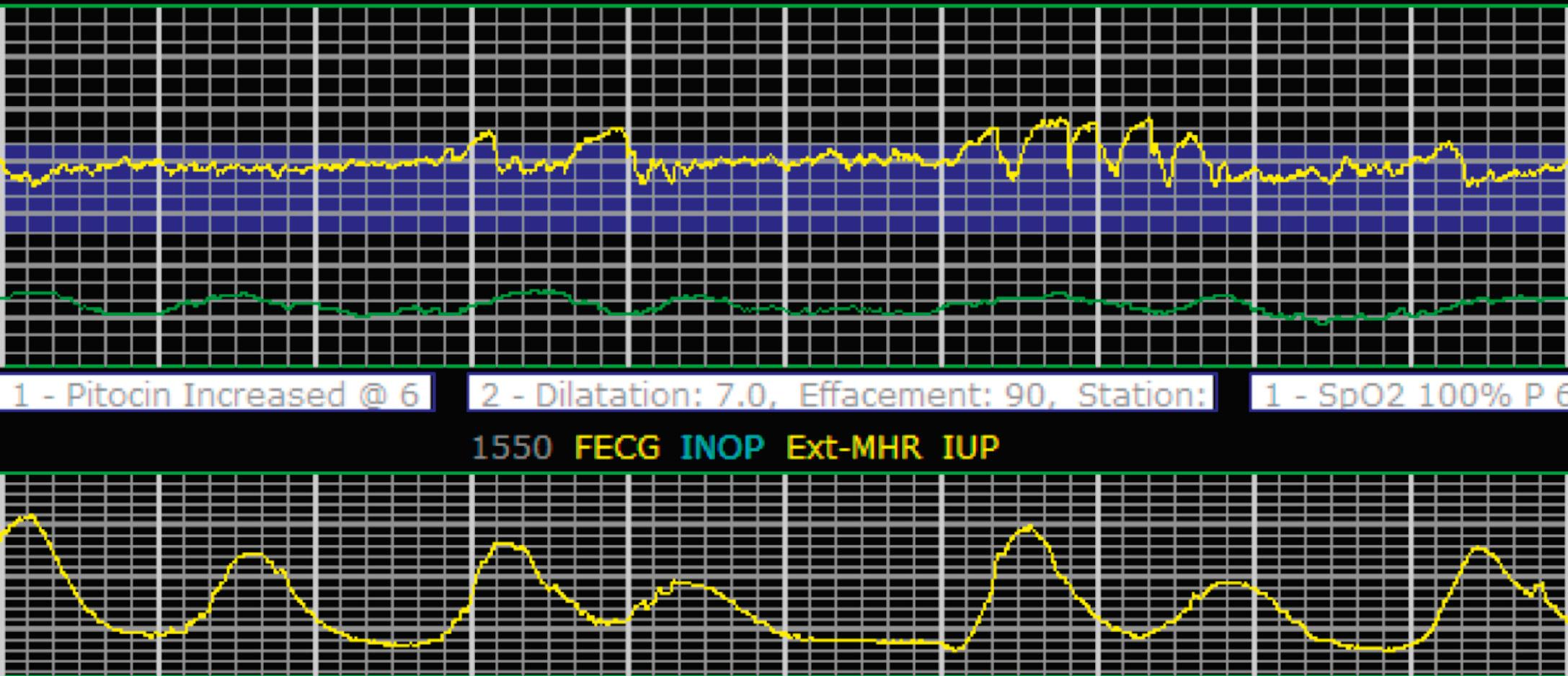
Tachysystole is defined as greater than 5 contractions in a 10-minute period averaged over 30 minutes. In the presence of tachysystole, the accompanying FHR pattern is important to evaluate. A prospective study of 584 women at term undergoing misoprostol labor induction examined the association between tachysystole in the first 4 hours after the initial dose of misoprostol and adverse infant outcomes. It found that tachysystole was associated with fetal decelerations but that there was no association with neonatal morbidity. In contrast, a retrospective cohort study of over 50,000 deliveries examined the association between tachysystole and neonatal morbidity. It found that tachysystole was associated with adverse neonatal outcomes, specifically the number of episodes of tachysystole and composite neonatal morbidity (RR, 1.15; 95% CI, 1.07 to 1.23). It identified risk factors for tachysystole that included oxytocin or misoprostol use and epidural anesthesia. Because tachysystole may be associated with adverse infant outcomes, NICHD nomenclature recommends making a clinical distinction between tachysystole with ( Fig. 15.28 ) or without FHR abnormalities ( Fig. 15.29 ). When tachysystole occurs during spontaneous labor without FHR abnormalities, no intervention is needed and continued observation is appropriate. But when category II or III EFM patterns occur in the setting of tachysystole, measures should be taken to resolve the tachysystole to resolve the EFM patterns. It is also reasonable to make efforts to resolve tachysystole that occurs in the setting of labor induction or augmentation whenever possible to prevent FHR abnormalities from developing.
Other nomenclature systems are used outside the United States. For example, the Federation for Gynecology has its own nomenclature system, as does the National Institute for Health and Care Excellence, although both share many similarities with the category system used in the United States. A study comparing the interobserver variability in using the three different systems to identify pathologic EFM patterns in labor found them to have a high level of agreement.
Intrapartum EFM is used in a continuous fashion from the onset of labor in the majority of clinical circumstances. However, ACOG continues to hold, largely because the data for the effectiveness of continuous EFM are inconclusive, that intermittent EFM during labor is reasonable for some low-risk patients. If intermittent monitoring is elected in selected patients, a 1 : 1 nurse to patient ratio is required. ACOG further recommends that fetal monitoring be accomplished for 30 minutes during every hour of the active phase of labor and every 15 minutes during the second stage of labor based on expert opinion when intermittent monitoring is employed for selected low-risk women.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here