Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
Subdural grid and strip electrodes have been used in the presurgical evaluation for epilepsy surgery for more than half a century.
They are useful for patients requiring intracranial electroencephalographic (EEG) monitoring for seizure localization through both interictal and ictal zone mapping and functional mapping.
The implantation of subdural electrodes requires an initial working hypothesis of the epileptogenic zone location based on noninvasive evaluation.
Compared with the scalp EEG, the intracranial EEG has the ability to record a broad range of frequencies with greater spatiotemporal resolution.
Subdural electrodes are used in the setting of chronic and/or intraoperative intracranial EEG monitoring. They are also used for direct recording of the epileptiform discharges in a chronically ambulatory setting as a component of a neuromodulatory device that treats epilepsy.
Although some complications can occur, such as cerebrospinal fluid leakage or infection, the use of subdural electrodes is relatively safe.
Subdural electrodes provide unique access to the intracranial EEG for researchers interested in studying various brain networks by passive event-related potential recordings using cognitive tasks as well as perturbation experiments with direct electrical cortical stimulation.
Similar to other forms of intracranial EEG recordings (depth or stereo-electroencephalography [SEEG]), subdural recordings have limitations of spatial resolution because only a limited area of the brain can be mapped in an individual patient.
Selecting the method and regions of coverage using intracranial EEG recording is dependent upon the patient’s suspected type of epilepsy and the importance of functional brain mapping.
Intracranial electroencephalographic (iEEG) monitoring is an important tool that allows direct in vivo recordings of the human brain. iEEG enables localization of the epileptogenic cortex for the purposes of refining surgical strategies in patients who suffer from medically refractory focal epilepsy. Subdural electrodes first came into use for iEEG monitoring in the presurgical evaluation near the middle of the 20th century. Their initial use in epilepsy surgery was to identify resective boundaries in patients with mesial temporal lobe epilepsy for what is now called the standard temporal lobectomy. More recently, it has been noted that noninvasive data, including scalp electroencephalographic (EEG) recordings, cannot adequately localize the epileptogenic zone in about 25% of patients with medically refractory seizures. These patients may be candidates to undergo iEEG monitoring.
iEEG is used to further investigate the regions of epileptogenicity in patients in whom scalp video-EEG monitoring, supported by anatomic and functional imaging tests, has failed to adequately localize the epileptogenic zone. It is also used in those patients who are in need of functional eloquent cortex mapping. Subdural electrodes (in grids or strips) are placed directly on the surface of the cerebral cortex. The electrodes are implanted in a silicone mesh that comes in various sizes and electrode configurations. These electrodes allow for sampling from the gyral crowns but have limited access to activity arising from the deeper sulci and mesial cortices. Placement depends on the ability to access the surface of the brain; therefore regions such as the mesial aspect of the cerebral hemisphere, the mesial temporal lobe (including the hippocampus), and the insula are problematic. Subdural electrodes can record at much higher spatial resolution than scalp electrodes because they are much closer to the signal source. However, only a finite number of subdural electrodes can be implanted and so only a limited region of the brain surface can be accessible to iEEG recordings in any one patient. In this way, by means of electrocorticography (ECoG), subdural electrodes can view iEEG activity arising from more restricted regions of the cortex that would be blind to scalp-recorded EEG. Using subdural grid electrodes allows for sampling contiguous areas, which can help delineate the boundaries of regions of abnormal epileptic activity and adjacent normal functional areas. Direct cortical electrical stimulation using subdural electrodes is considered the “gold standard” method for mapping the eloquent cortex in patients undergoing epilepsy or brain tumor surgery. Recording from subdural electrodes can be performed either acutely in the intraoperative setting or chronically over several days using implanted multielectrode grids in video-EEG monitoring units. More recently, responsive neurostimulation provided by a neuromodulatory device called the RNS System (NeuroPace, Inc.) allows for chronic ambulatory ECoG over several years using subdural and/or depth electrodes.
Intraoperative subdural EEG recordings were first performed by Wilder Penfield and Herbert Jasper during the late 1930s and early 1940s. Penfield and Jasper analyzed results of direct cortical stimulation and interictal spikes to help guide epilepsy surgery. Fischer-Williams and Cooper reported on the use of subdural electrodes in patients undergoing epilepsy surgery, showing that they could effectively record from the orbitofrontal cortex, amygdala, and hippocampus using subdural electrodes. The routine use of subdural recording in the evaluation of epilepsy surgery did not begin until the mid-1970s. By the 1980s, our institute, the Cleveland Clinic, had begun to report the use of larger implanted subdural grids. Other institutions also began reporting on use of epidural electrodes and subdural recordings for epilepsy surgery around the same time.
Despite the widespread use of subdural electrodes at many epilepsy centers, there exists some variability in terms of indications, techniques, combined use of depths, the number of implanted electrodes, and the areas of the cortex that are sampled. Implantation of subdural electrodes requires a large craniotomy with significant postoperative pain and longer recovery. Because subdural electrodes record primarily from superficial regions of the cortex, they are best suited for epilepsies not involving deeper brain structures, or when there is a need for functional eloquent cortex mapping. To address recording from deeper regions of the cortex, stereo-electroencephalography (SEEG) has been employed primarily at our center since 2009. SEEG methodology is discussed in more detail in Chapter 90 .
The generator source for EEG is intracellular laminar currents of apical pyramidal cell dendrites in the outer layer of the cerebral cortex. These currents generate electric dipoles or sheets of dipoles from excitatory and inhibitory postsynaptic potentials. The activity can be recorded from a distance as a result of linear summation of the electric dipoles when the populations of neurons are more or less synchronously activated for a sufficient period.
The electric field instantaneously spreads over an infinite number of tracks between the positive and negative ends of the dipole. Outside the neuron, the circuit is completed by the current flowing through the extracellular fluid in a direction opposite to the intracellular current. Volume conduction of the electrical activity can occur when there is spread of current through a conductive medium that is then picked up by a remote recording electrode.
Ictal and interictal epileptiform abnormalities are recorded by scalp EEG when a relatively large and stable cortical focus, typically with greater than 10 cm 2 surface area, is involved in generating the epileptiform activity. Scalp EEG is also predisposed to preferentially recording dipoles that are oriented radially. Interictal spikes circumscribe a more restricted focus than ictal activity, which often has propagated beyond the onset region by the time the recordings can be seen at the scalp. Therefore scalp EEG tends to provide more localization value with interictal spikes than with ictal patterns.
There are a number of circumstances that complicate the interpretation of scalp EEG recordings. The scalp electrodes record primarily the positive or the negative pole of a radially oriented dipole. Scalp EEG waveforms are mostly influenced by dipoles at the apex of a gyrus, because the generators lie perpendicular to the scalp and create vertical dipoles visible to the scalp electrodes. Sulcal dipoles can also contribute to the scalp EEG but to a lesser extent than vertically oriented dipoles. Only sources that extend over multiple cortical layers comprising several square centimeters will have sufficient energy to generate detectable discharges from scalp electrodes. , Approximately 70% of the cortical surface lies within the depth of sulci, and also many brain areas, including medial surfaces and other deep structures such as the insula, are not optimally recorded by scalp EEG. Hence a focal epileptogenic discharge arising from a deep source or within a sulcus may not produce a sufficient field to be detected by the scalp electrodes, despite the synchronous activation of a large population of neurons. Epileptiform activity from these deeper sources may appear so small in amplitude at the scalp electrode that it may be obscured by background activity. In addition, conduction of the brain electrical activity to the scalp is impeded by the poor conductivity of the skull. Passage through the multiple layers (cerebrospinal fluid [CSF], dura, skull, and scalp), causes a smearing effect on the surface potential distribution, causing the potential from localized foci to appear in a much wider scalp area. As a result of many of these limitations of the scalp EEG, iEEG recording is considered in selected patients so as to more accurately locate the source of epileptiform activity prior to recommending a cortical resection.
There are several noninvasive tools other than scalp EEG to evaluate the epileptic focus. These include magnetoencephalography, brain magnetic resonance imaging (MRI), voxel-based morphometry, 18 F-fluorodeoxyglucose–positron emission tomography, single-photon emission computed tomography (SPECT), or subtraction ictal SPECT coregistered to MRI (SISCOM). Each has advantages and limitations, but discussion of these modalities is beyond the scope of this chapter.
Compared with the scalp EEG, subdural electrodes are much closer to the source of cerebral activity. They are separated from the cortex by relatively high-conductivity media (CSF and brain parenchyma), thus the amplitudes of signals are considerably higher. Most of the artifacts seen in a scalp EEG are not present in subdural recordings or are present at a much lower relative amplitude (e.g., alternating current interference), yielding a much better signal-to-noise ratio. Subdural electrodes rapidly achieve a low impedance, which usually remains stable for the duration of the chronic recording.
Subdural recording is also less sensitive to the orientation of the dipole generators than is scalp EEG. For instance, a sheet of dipoles folded in on each other results in a net cancellation of electrical signals and thereby produce a “closed” field such as that often seen in the hippocampus. As a result, activity from these closed fields is not visible at the level of the scalp EEG. Spikes or sharp waves are recorded by scalp EEG only when a relatively large region of cortex has been involved in the epileptic activity. It had been initially reported that a minimum of 6 cm 2 of cortex discharging simultaneously is needed to reflect a visible potential on the scalp surface. Experience in recordings with subdural and other invasive electrodes has demonstrated that much of the epileptiform activity cannot be visualized with scalp, nasopharyngeal, or sphenoidal electrodes. In studies comparing simultaneous scalp EEGs and iEEGs in patients with temporal lobe epilepsy, 90% of interictal spikes with a source area of >10 cm 2 had associated scalp-recorded spikes, whereas only 10% of cortical spikes that covered an estimated source area of <10 cm 2 had an accompanying scalp-recorded spike. The difficulty in recording these lower amplitude spikes may involve the much higher electrical impedance of the skull as compared to the brain and CSF, but also results from the low-pass properties of these layers, which attenuate higher frequencies even further. , Additionally, high-frequency responses likely become desynchronized over the cortex, resulting in the attenuation of the response seen at the scalp electrode. Therefore use of subdural electrode recordings provides the opportunity to detect focal EEG seizure onsets well before any changes are detected at the scalp electrode by decreasing the distance between the recording electrode and the cortical surface. As a result, activity that would be visualized on the scalp only as a diffuse or nonlocalizable ictal onset may be seen as a more focal ictal onset using subdural recordings.
Subdural electrodes can provide wide coverage over various surfaces of the cortex with contiguously spaced electrodes. Depth electrodes can allow for a better view of dipoles that are deep-seated or whose orientation is tangential to the plane of the subdural electrodes, but lack the contiguous coverage seen with subdural electrodes. Deep-seated sources are more easily localized using depth electrodes. These electrodes, combined with the anatomo-electro-clinical methodology of SEEG, are particularly suited for epileptogenic zone hypothesis–driven presurgical evaluation of the neural network in nonlesional or neocortical epilepsy. The details of differences between subdural electrodes and depth electrodes, including the technique of SEEG, are described under “Comparison With Depth Electrodes” later in the chapter. Advantages of iEEG recordings using subdural electrodes include better functional mapping. However, there may be a greater risk for symptomatic hemorrhage, infections, and permanent neurological sequelae when using subdural electrodes as compared to SEEG.
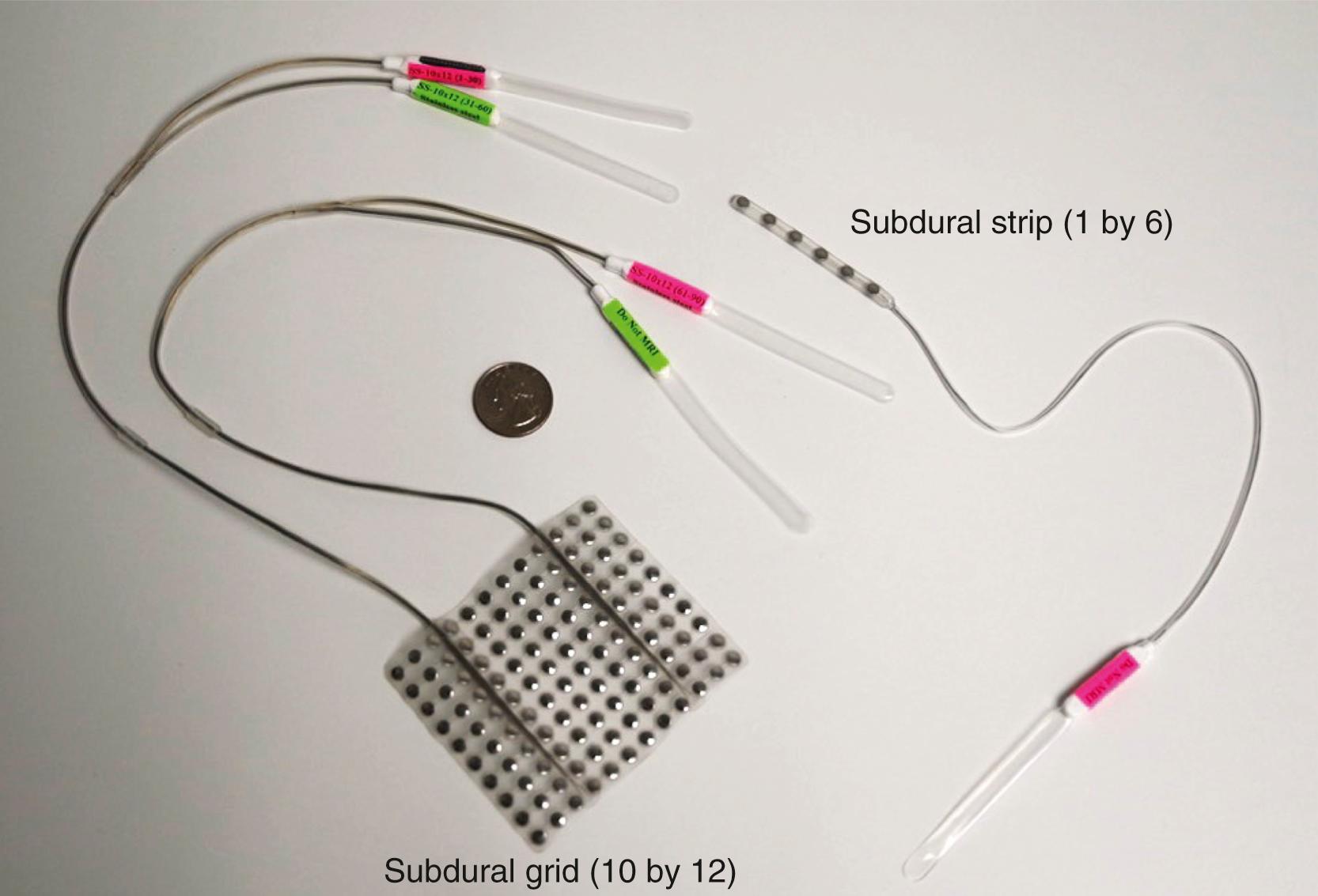
There are generally two types of subdural electrodes arrays: grids and strips. Linear subdural strip electrodes and rectangular subdural grid electrodes can be used for intracranial monitoring depending on the location that needs to be sampled. All invasive electrodes, including depth electrodes, have the advantage of recording the signals from smaller populations of neurons that are not detectable by scalp EEG, as described previously. ,
Subdural electrodes consist of several conductive disks held together by a flexible matter. A variety of materials have been used to make the subdural electrode contacts, including platinum, silver, and stainless steel. These small disk electrodes are embedded in a thin sheet of Silastic (Dow Corning Corporation), with a variable amount of their surface area exposed to the cortex. The composition of subdural recording grids and the techniques for their manufacture have undergone significant development in the past four decades in order to address several key issues: (1) biocompatibility and biostability (i.e., chemical inertness), (2) sufficient flexibility, (3) insolubility in body fluids, (4) impermeability to ionic species, (5) relatively low dielectric constant to reduce interelectrode crosstalk and capacitive leakage, and (6) freedom from pinholes and cracks. These grids were made to order at some epilepsy centers in the past but are now available commercially from several companies that produce high-quality subdural electrode configurations.
The standard dimensions of subdural electrodes previously used in our institution are a 0.127-mm-thick platinum electrode with a diameter measuring 3.97 mm and center-to-center interelectrode distance of 1 cm. The electrodes are embedded in a mold of flexible Silastic ( Fig. 89.1 ). The shape and size of the subdural electrodes can vary from a single row (i.e., a strip electrode) to various grid arrays comprising from 12 to 120 (e.g., rectangular array of 12 × 10 contacts with 7-mm center-to-center spacing) electrodes. The flexible nature of the electrode array allows it to be molded over various cortical regions. For example, the EEG activity from the frontoparietal lateral convexity can be recorded using a larger 8 × 8 electrode array, whereas the evaluation of EEG activity over mesial and basal surfaces of the cortex usually consists of smaller arrays or strip electrodes. Interhemispheric arrays may be curved to follow the convexity and can have contacts on both cerebral hemispheres.
Subdural grid or strip electrodes are implanted in the operating room under general anesthesia. The insertion of subdural electrodes can be accomplished using burr holes for subdural strip electrodes or via craniotomy for larger electrode arrays, which are usually placed on the cortical areas likely to undergo resection surgery ( Fig. 89.2B ). The incision and craniotomy should be planned to allow access to a large cortical area, to enable proper tunneling of the electrode wires to allow a longer tunnel to reduce infection, and to allow for a resection at the time of grid removal.
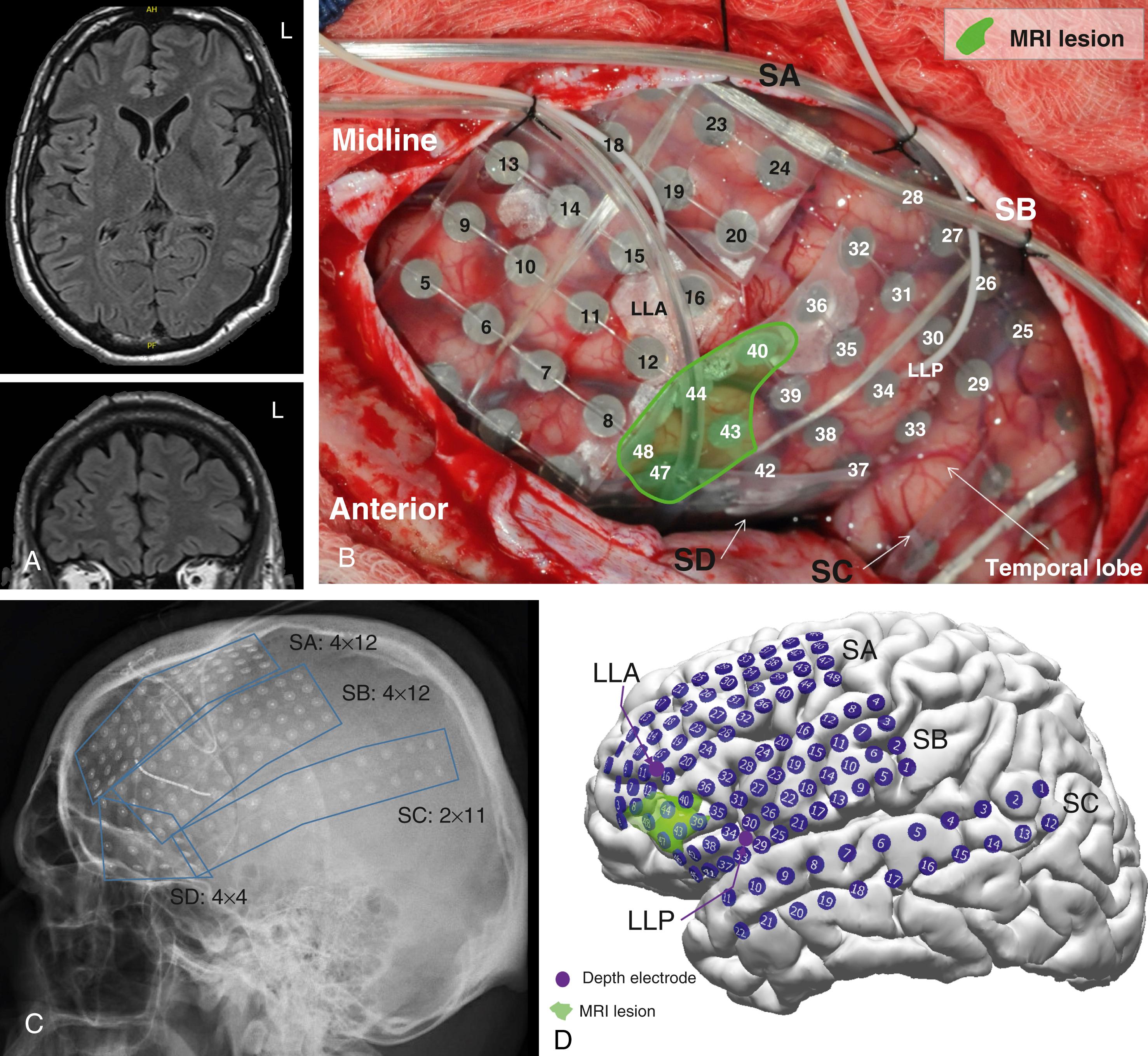
Most subdural grids are placed under direct visualization to avoid bleeding from bridging veins or bony malformations. Smaller grids or strips are often slid over or under the brain without direct visualization, especially at orbitofrontal, inferior-temporal, and interhemispheric locations. The grid cables are kept in a bundle and sutured to the dural edge to help prevent movement after closure. The dura is closed with interrupted sutures and the bone flap is loosely approximated to accommodate increased mass effect postoperatively. The cables are carefully tunneled under the skin to exit from a separate stab incision a few centimeters away from the original incision margin so as to minimize the chance for infection. A separate purse-string suture is usually placed around the cables as they exit to anchor them and to provide strain relief. Subdural electrodes are typically left implanted for a period of 5–10 days. The total duration of monitoring depends on how quickly seizures are recorded and in some cases the institution’s preferences. In rare instances, subdural electrodes have been kept in place longer in situations in which seizures had not been recorded.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here