Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Destructive cerebral lesions are not embryonic malformations, but rather the result of an insult to a normally developed fetal brain. The most common causes are hemorrhage, hypoxia-ischemia, and infections, but the pathophysiology is unclear in many cases. The prognosis is usually poor. This chapter describes the main features of fetal intracranial destructive lesions, including intracranial hemorrhages, porencephaly, hydranencephaly, and schizencephaly. Other intracranial lesions that develop late in gestation, including intracranial cysts and intracranial tumors, are also described. Fetal cerebral infections are discussed in Chapter 165, Chapter 166, Chapter 167, Chapter 168 .
An intracranial hemorrhage (ICH) is a collection of extravasated blood occurring in the fetal brain, usually affecting the lateral ventricles, although it can occasionally be found in other parts of the brain. Synonyms include germinal matrix hemorrhage, intraventricular hemorrhage, intraparenchymal hemorrhage, and subdural hematoma .
Postnatal ICH is common in preterm neonates. In neonates weighing less than 1500 g or younger than 32 weeks' gestational age, the incidence is 40%. ICH has been reported rarely in utero, with an overall estimated incidence of 1 : 10,000 pregnancies.
There are numerous well-demonstrated and postulated causes of in utero ICH, including maternal hemorrhagic disorders, coagulopathy, maternal hypotension, preeclampsia, severe fetal growth restriction, placental abruption, infections, nonimmune hydrops, fetomaternal hemorrhage, complications of monochorionic twin pregnancies, and maternal cocaine abuse. Platelet disorders (immune thrombocytopenic purpura, alloimmune thrombocytopenia, and hidden antiplatelet autoantibodies) can cause fetal platelet destruction and have been associated with ICH before the onset of labor. Fetal alloimmune thrombocytopenia is caused in most cases by maternal alloimmunization against the human antigen HPA-1a and may cause ICH in the third trimester. A fetal platelet count is difficult to obtain, because invasive procedures are normally avoided in these cases. A recent report suggests that the number of previous gestations, which relates with the grade of exposure and immunization to the antigen, is the best indicator to predict ICH. Fetal subdural hematomas can be caused by trauma. At least one predisposing factor can be identified in about half of cases.
Postnatally, most intraventricular hemorrhages originate in the subependymal germinal matrix region. In the premature brain, the germinal matrix contains thin-walled friable vessels supported by a delicate matrix that is easily injured by any elevation of the cerebral blood pressure, as in fetal hypoxia or thrombosis. It is unclear whether the pathophysiology is the same in intrauterine hemorrhage.
ICH is more frequently detected in the third trimester, but it can be detected as early as 20 weeks. Only major ICH can be easily recognized prenatally. Small hemorrhages may resolve or leave a small residual cyst. In massive ICH, blood initially accumulates in the lateral ventricles and migrates into the third ventricle or the aqueduct of Sylvius, causing hydrocephalus. Finally, a porencephalic cyst may originate from the destructive process of the periventricular white matter.
ICH is commonly classified by severity into four grades:
Grade I: Limited to the subependymal matrix
Grade II: Clear spillover to the ventricles but filling less than 50% of the lateral ventricle (without acute ventriculomegaly)
Grade III: Spillover to the ventricles with filling more than 50% of the lateral ventricle (with acute ventriculomegaly)
Grade IV: Characteristics of grades I to III with destruction of periventricular parenchyma
Most ICH is intraventricular and appears as an irregular echogenic mass on ultrasound (US) ( Fig. 40.1 ). The lateral ventricles frequently dilate after hemorrhage (grade III) ( Fig. 40.2 ), and in some cases an infarct may develop in the periventricular cortex (grade IV) ( Fig. 40.3 ). Subdural hematomas appear as echogenic collections displacing brain tissue, but a specific diagnosis is frequently difficult ( Fig. 40.4 ).
Bilateral ventriculomegaly with irregular echogenic areas within the ventricles
Echogenic lesions developing a hypoechogenic core over 1 to 2 weeks
Four clinical phases can be observed in the ultrasound features of ICH. The time for the development of each phase after the initial event is variable.
Fresh hemorrhagic phase: presence of echogenic blood filling the lateral ventricles (3–8 days)
Liquefying phase: presence of a mass with external echogenic lining and internal anechoic core (3–8 days)
Complete liquefying phase: presence of a cystic hypoechoic mass (7–28 days)
Resolution phase: disappearance of ventriculomegaly and blood clots (7–105 days).
Doppler velocimetry of the middle cerebral artery (MCA) is a useful additional tool in the diagnosis of ICH. An increased velocity in the MCA can document fetal anemia in case of massive hemorrhage, whereas the increased intracranial pressure impairs cerebral blood flow with increased resistance to flow in cerebral vessels.
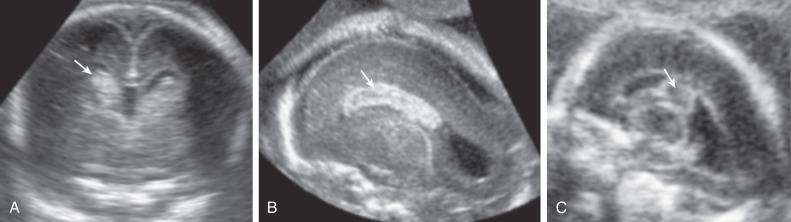
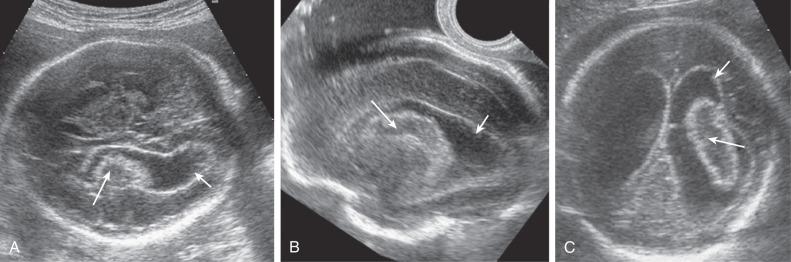
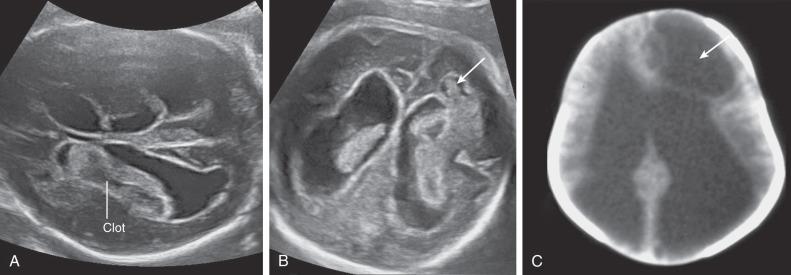
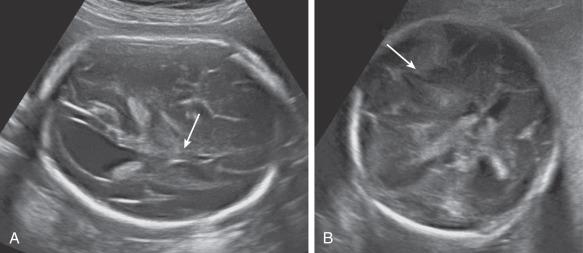
In massive ICH, there may be a mass effect that can be mistaken for a brain tumor. A hint for differential diagnosis is that fetal tumors grow or remain stable, whereas blood clots shrink and change appearance during pregnancy.
Other causes of ventriculomegaly (infections, brain structural abnormalities, genetic conditions) must be considered in the differential diagnosis. In unclear cases, serial US scans and fetal magnetic resonance imaging (MRI) should be performed.
Prenatal treatment considerations include the following:
Identification of the cause (thorough maternal history, maternal platelet count, testing for maternal alloimmunization and isoimmunization)
Maternal administration of steroids or intravenous immunoglobulin, fetal platelet transfusion if there is alloimmune fetal thrombocytopenia
US monitoring
Mode of delivery independent of the presence of platelet disorders
Offer of termination of pregnancy if appropriate
The prognosis for ICH largely depends on the severity of the condition, which ranges from mild neurologic deficits to neonatal death. A 50% perinatal death rate and 50% neurologic compromise in survivors have been reported. The grade of hemorrhage correlates with the severity of the prognosis. The risk of recurrence depends on the underlying cause. Patients with alloimmune thrombocytopenia carry a very high (85%–90%) recurrence risk of ICH.
ICH usually affects lateral ventricles.
ICH is common in premature newborns; it is rarely found prenatally.
Fetuses with alloimmune thrombocytopenia have a high incidence of fetal hemorrhage, and there is a very high risk of recurrence in subsequent pregnancies.
Fetal ICH has a poor prognosis.
Porencephaly is defined as the presence of cystic cavities within the brain matter. The cavities usually communicate with the ventricular system, the subarachnoid space, or both. In contrast to schizencephaly, the lining of the cavity usually contains white matter. Synonyms include encephaloclastic porencephaly and porencephalic cyst .
Porencephaly is very rare with only isolated case reports in utero.
An ischemic insult is thought to cause this type of brain damage. It can be considered the result of a fetal ischemic stroke. Middle cerebral artery occlusion is postulated to be the main cause of porencephaly. Birth trauma, inflammatory diseases, amniocentesis complications, and twin-to-twin transfusion syndrome are also recognized as possible causes. In addition, familial and chromosomal types of porencephaly have been described.
The neuropathologic feature of porencephaly is a usually unilateral intrahemispheric cleft. The cleft is a fluid-filled cavity that usually communicates with the cerebral ventricles internally and the subarachnoid space externally and usually is lined with white matter. In contrast to schizencephaly, porencephaly is not associated with neuronal migration abnormalities.
Porencephaly is a lateralized lesion that appears as a cystic cavity within the brain, usually communicating with the lateral ventricle, the subarachnoid space, or both ( Fig. 40.5A ).
Unilateral cystic cavity in the brain communicating with the ipsilateral ventricle
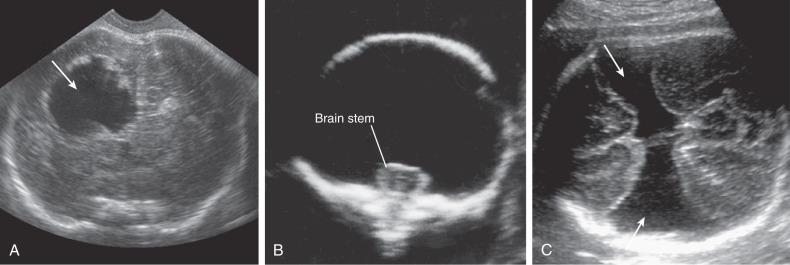
Distinction from unilateral schizencephaly may be difficult. In schizencephaly, clefts usually show smooth contours, whereas in porencephaly, the cavities tend to be rounder and with jagged contour. Fetal MRI can be useful in the third trimester by showing whether the defect is lined with white matter (porencephaly) or gray matter (schizencephaly).
Arachnoid cysts are usually smooth-walled, do not communicate with the ventricular system, and, in contrast to porencephaly, have a mass effect on the adjacent brain.
Treatment considerations include the following:
Similar diagnostic work-up as for ICH
Offer of termination of pregnancy if appropriate, or conservative management
Porencephaly is a rare destructive lesion of the fetal brain probably correlated with vascular occlusion and infarction. The prognosis is poor, and termination of pregnancy should be offered before the fetus is viable. Seizures, developmental delay, hemiparesis, and intellectual impairment are usually present in patients with this kind of lesion.
Porencephaly is a rare destructive lesion of the fetal brain, probably secondary to vascular occlusion.
It appears as a unilateral, white matter–lined cystic cavity communicating with the ipsilateral ventricle, with no mass effect.
The prognosis is very poor, and termination of pregnancy should be offered.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here