Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Since their discovery > 60 years ago, tuft cells have intrigued and mystified researchers. Early studies focused on the unique morphology of tuft cells and their distribution pattern across mucosal surfaces, but their function remained unknown. The last 10 years have finally brought rapid advances in our understanding of intestinal tuft cells, including: (1) a detailed characterization of tuft cell ontogeny and associated lineage-specific transcriptional regulators in the small intestine; (2) accumulating evidence for a possible contribution of tuft cells to tumorigenesis; (3) identification of tuft cells as critical regulators of type 2 immune responses in the small intestine; and (4) experimental support for the long-standing hypothesis of chemosensing by tuft cells. Nonetheless, the homeostatic function of intestinal tuft cells remains unknown.
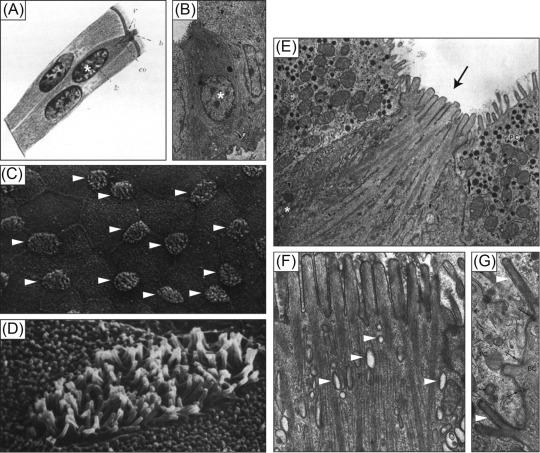
In the late 1920s, Dr. Chlopkow, a Russian scientist working at the state university in Tomsk, undertook a project to map out the developmental stages of intestinal goblet cells. Peering through his microscope, Dr. Chlopkow spotted a cell with a bundle of unusually long and thick microvilli protruding into the intestinal lumen. He sketched the cell in his notebook and included an illustration when writing up his work for publication ( Fig. 31.1 A ). Dr. Chlopkow concluded that he had found an early-stage goblet cell, and that the microvilli were lost during development. In fact, Dr. Chlopkow had provided the first report of a novel epithelial lineage now known as the tuft cell, but the “discovery” of these cells would take another 30 years.
With the emergence of electron microscopy came a more careful classification of epithelial cells based on their ultrastructural features. In 1956, Rhodin and Dalhamn described cells in the rat trachea marked by a cluster of long microvilli at their apical surface, and later that year Järvi and Keyriläinen found similar cells in the mouse stomach. It soon became clear that these cells constituted a distinct lineage that has been variously referred to as multivesicular, caveolated, fibrillo-caveolated, brush, and tuft cells. Here we will use the term tuft cell, which has emerged as the preferred nomenclature for these cells in the gastrointestinal tract.
Generally, tuft cells are found in the columnar epithelium of endoderm-derived hollow organs. In rodents, they have been definitively identified in the trachea, the auditory tube, the thymus, the excretory ducts of salivary glands, the glandular stomach, the gall bladder and bile duct, the pancreatic duct, the small intestine, the cecum, the colon, and the urethra. Cells with tuft cell-like morphological features have also been described in the rat and perhaps human alveolar epithelium, but appear to be absent in mice, and a full characterization of these cells is still needed.
Besides rodents, tuft cells have also been described in various endoderm-derived tissues of fish, frogs, dogs, humans, and numerous other placental mammals. In this chapter we focus primarily on tuft cells of the rodent gastrointestinal tract, although many morphological features, cellular markers, and perhaps even functions appear to be shared across all anatomical sites and species.
In most cases tuft cells appear as isolated cells and constitute < 1% of the epithelium, but there are notable exceptions. In the mouse gall bladder and the rat bile and pancreatic ducts, tuft cells still appear as isolated cells but are far more abundant. Additionally, tuft cells have been shown to cluster around sphincters, such as the esophageal sphincter and the sphincter of Oddi, and in the mouse stomach tuft cells make up ~ 30% of the cells at the gastric groove that divides the forestomach and corpus. However, this gastric groove does not exist in humans.
The ultrastructure of tuft cells, as revealed by transmission electron microscopy, freeze-fracture microscopy, and scanning electron microscopy, is remarkably similar across all tissues and species examined to date. The iconic feature of tuft cells is the cluster of microvilli at the apical surface ( Fig. 31.1 C–E). These microvilli are thicker and longer than those of neighboring cells and give tuft cells their name. Other consistent features of tuft cells include: (1) a relatively narrow apical membrane, which leads to the impression that tuft cells are pinched at the top ( Fig. 31.1 B and E); (2) prominent actin microfilaments that begin at the tips of the villi and extend into the cell, terminating just above the nucleus ( Fig. 31.1 E and F); (3) abundant but largely empty apical vesicles that form a complex tubulovesicular network ( Fig. 31.1 F); (4) a network of microtubules that runs among the microfilaments and the tubulovesicular structures; (5) a Golgi apparatus on the apical side of the nucleus; (6) a lack of rough endoplasmic reticulum and secretory vesicles, and (7) desmosomes and tight junctions that anchor tuft cells to their neighboring cells ( Fig. 31.1 G). Collectively, these features define the prototypical tuft cell depicted in Fig. 31.2 .
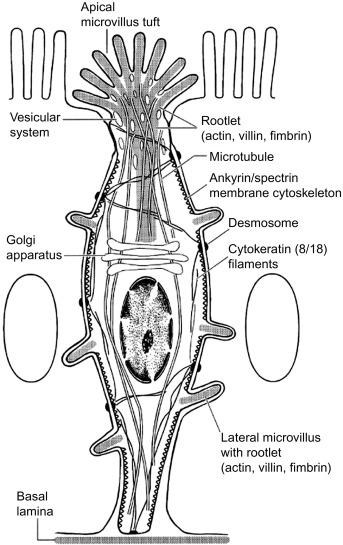
While there is widespread agreement about the features described above, other aspects of tuft cell morphology remain in dispute or have only been described once or twice. For example, some tuft cells have lateral microvilli that extend into neighboring cells and contain the same microfilaments as those at the cell surface ( Fig. 31.1 G). Several papers also described abundant glycogen deposits in tuft cells, but this was not universally noted. Additionally, one study found that the membrane of tuft cell microvilli is particularly cholesterol-rich compared to nontuft epithelium, and staining with the lectin UEA-1 demonstrated that the apical glycocalyx of tuft cells, which is generally thinner than on surrounding cells, contains abundant fucose residues. The functional relevance of these observations remains to be determined.
There are also areas of disagreement or discrepancy. For example, while most studies noted the elongated appearance of mitochondria, one study noted an abundance of mitochondria, while another commented on their paucity. The precise nature of the apical tubulovesicular network also remains in dispute. Early studies using isolated thin sections described only abundant vesicles, but serial sectioning and 3D imaging later revealed the complex interconnected network of hollow structures ( Fig. 31.3 ). The presence of this tubulovesicular network is now widely accepted, but it remains unclear whether it is continuous with the intestinal lumen. Nabeyama and Leblond argued that the invaginations (or caveolae) between microvilli at the apical surface connect to the tubulovesicular network, but this has been challenged by others.
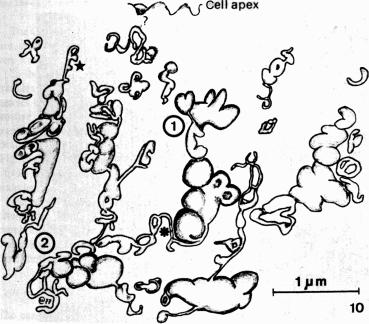
How do we explain the discrepancies noted above? It is possible that different fixation protocols could reveal or mask certain structures or even give rise to artifacts. More interestingly, these differences may reflect the underlying biology of tuft cells, such as tissue-specific specialization and/or dynamic changes in tuft cells that occur following a meal or other shift in intestinal physiology. It has been suggested, for example, that a dynamic tubulovesicular network would allow tuft cells to rapidly alter their luminally exposed surface area. Perhaps live-cell imaging of tuft cells could provide additional insights.
While electron microscopy facilitated the initial discovery and characterization of tuft cells, several excellent tuft cell markers are now available for antibody-based immunofluorescence and immunohistochemistry ( Table 31.1 ).
| Expression in Tuft Cells | Expression in Other Epithelial Cells | References | |
|---|---|---|---|
| Structural | |||
| acTUB | All (apical accumulation) | None reported | |
| CK18 | All | Some weak staining | |
| DCLK1 | All | Rare enteroendocrine and stem cells; induced in tumors | |
| UEA-1 | All | Most cells in ileum | |
| VIL1 | All (apical accumulation) | All (no apical accumulation) | |
| Chemosensing | |||
| CHAT | All | None reported | |
| GNAT3 | Some | Some enteroendocrine cells | |
| PLCB2 | Some | Some enteroendocrine cells | |
| Taste receptors | Some | Some enteroendocrine cells | |
| TRPM5 | All | Rare enteroendocrine cells | |
| Other | |||
| GFI1B | All | None reported | |
| HPGDS | All/most | None reported | |
| POU2F3 | All | None reported | |
| PTGS1 | All | None reported | |
| SIGLECF | All/most | None reported | |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here