Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
More than 1 billion people are infected with intestinal nematodes worldwide. Also referred to as soil-transmitted helminths (STHs), intestinal nematodes are complex, nonsegmented, multicellular worms. Each organism is surrounded by a species-specific acellular cuticle composed of structural proteins, enzymes, and lipids that permit tissue migration through the host and evasion of the host immune response. Under the cuticle lies a muscular ring of tissue and entire gastrointestinal and reproductive tracts. There are more than a dozen species of intestinal nematodes that cause human disease, led by the roundworm (Ascaris lumbricoides), whipworm (Trichuris trichiura), hookworms ( Necator americanus, Ancylostoma duodenale, and Ancylostoma ceylanicum ) and threadworm (Strongyloides stercoralis). Intestinal nematode infections are found commonly in people living in extreme poverty in tropic and subtropic regions of the Americas, sub-Saharan Africa, and Asia. Although these infections most commonly occur in low- and middle-income countries, vulnerable populations living in high-income countries are also at risk, with evidence showing that hookworm and other STH infections are present among the poor in the southern United States. Persons living in conditions with poor sanitation, overcrowding, unsafe water supply, poor health care infrastructure, and low income potential (earning less than $2 per day) are at the highest risk of repetitive exposure and infection. In addition, because these organisms have overlapping geographic endemicity and affect similar at-risk groups, polyparasitism is common (e.g., a single individual may be simultaneously infected with Ascaris roundworms, Trichuris whipworms, and hookworms), as are STH coinfections with malaria and other tropical diseases.
Intestinal nematodes infect all age groups from neonates to adults; however, only a small proportion of the community will harbor the greatest disease intensity. The age of peak prevalence and peak worm intensity varies based on the specific organism, development of partial immunity after repeated infection, and changes in behavior-associated risk factors. Intestinal nematodes are not a major cause of death, but instead result in severe chronic disability, causing more than 3 million disability-adjusted life years (DALYs) worldwide. However, alternative estimates indicate that the global disability from STH infections may be much higher than previously realized. Malnutrition; weight loss; gastrointestinal symptoms; increased bacterial, viral, and parasitic infections such as human immunodeficiency virus (HIV), malaria, and tuberculosis; and cognitive and growth stunting are well-recognized features of intestinal nematode infection. These organisms impair physical and mental growth, impede educational advancement, decrease workforce productivity, and suppress economic development, creating a sustained cycle of poverty in endemic regions.
High prevalence and heavy worm burden can be devastating to a community. Annual or biannual mass preventive chemotherapy programs have been established in endemic areas worldwide. Administration of a single dose of albendazole or mebendazole is recommended for all preschool and school-aged children, nonpregnant adolescent girls (10–19 years old), and nonpregnant women of reproductive age (15–49 years old) living in areas with disease prevalence >20% to reduce worm burden and to reduce morbidity. In addition, pregnant women in their second or third trimester living in areas with hookworm or Trichuris prevalence >20% and in areas where >40% of pregnant women have anemia should also be routinely treated. The major goal of these programs is improvements in STH-associated morbidities including growth stunting, cognitive disabilities, and pregnancy outcome, although some investigators have questioned the actual and sustainable benefits of global deworming programs. Interruption of intestinal nematode transmission within a community using mass preventive chemotherapy programs is also feasible in some areas with low worm prevalence, a robust health system, and in-country financial support to maintain control programs. However, global elimination of these diseases will not be feasible without prioritizing reduction in poverty, making changes in infrastructure, investing in health education, and improving hygiene and sanitation, alongside the expansion of preventive chemotherapy programs across broader age classes ( Table 286.1 ). There is also a need for improvements in the efficacy of single-dose anthelmintic drugs, especially for trichuriasis and hookworm, prompting the search for new-generation anthelmintic drugs and vaccines.
| MAJOR HUMAN SPECIES | COMMON NAME | ESTIMATED NO. OF CASES (MILLIONS) |
DURATION OF INFECTION | MAJOR CLINICAL SYNDROME | TREATMENT |
|---|---|---|---|---|---|
| Ascaris lumbricoides | Roundworm | 800 | 1–2 years | Vitamin A malabsorption Intestinal obstruction Asthma |
Albendazole 400 mg PO once or mebendazole 500 mg PO once |
| Trichuris trichiura | Whipworm | 435 | 1–3 years | Colitis Trichuris dysentery syndrome Rectal prolapse |
Albendazole 400 mg PO × 3–7 days or mebendazole 500 mg daily or 100 mg PO twice × 3–7 days Also combined with ivermectin (200 µg/kg daily) |
| Necator americanus Ancylostoma duodenale/ceylanicum |
Hookworm | 450 | 3–5 years 1 year |
Anemia Ground itch Abdominal pain |
Albendazole 400 mg PO once or mebendazole 100 mg twice daily x 3 days |
| Strongyloides stercoralis | Threadworm | 100 | Lifelong | Larva currens Eosinophilia Abdominal pain Dissemination to other organs including skin and brain |
Ivermectin (200 µg/kg daily ×2) Disseminated: ivermectin (200 µg/kg daily ×14) |
| Enterobius vermicularis | Pinworm | Estimated at 30% world population | 1 month | Pruritus ani Vulvovaginitis |
Pyrantel pamoate (11 mg/kg ×1) Albendazole (400 mg ×1) |
A. lumbricoides , human roundworm, is the most common helminthic infection in the world, chronically infecting 800 million individuals. Year-round transmission is standard in humid tropical and subtropical regions of the Americas, sub-Saharan Africa, and Asia. In more arid environments, where eggs can remain viable in the soil for months, seasonal transmission occurs. Ascaris prevalence and worm intensity increase from infancy to preschool and school-aged children. Young children disproportionately harbor the highest amount of worms within a community, leading to the most significant morbidity. Mortality secondary to ascariasis is low, accounting for only 2700 deaths per year, but ascariasis is a significant cause of severe morbidity, causing 1.27 million DALYs owing to the effects of intestinal parasitism on child growth and development. In addition, the larval stages of Ascaris worms migrating through the lungs have been shown to cause asthma and other lung disorders.
Ascaris suum , swine roundworm, is morphologically indistinguishable from A. lumbricoides and can cause human disease. A. suum and A. lumbricoides have minimal genetic differences, with greater than 98% similarity of the mitochondrial genome, with known nucleotide differences commonly resulting in synonymous mutations. In addition, the life cycles of both species are identical and can be completed in both humans and pigs, causing similar clinical manifestations. As with A. lumbricoides, A. suum infection has a high prevalence in pigs worldwide, including in the United States, with few farms free of infection. Humans become infected by ingesting pig manure or soil contaminated with A. suum eggs.
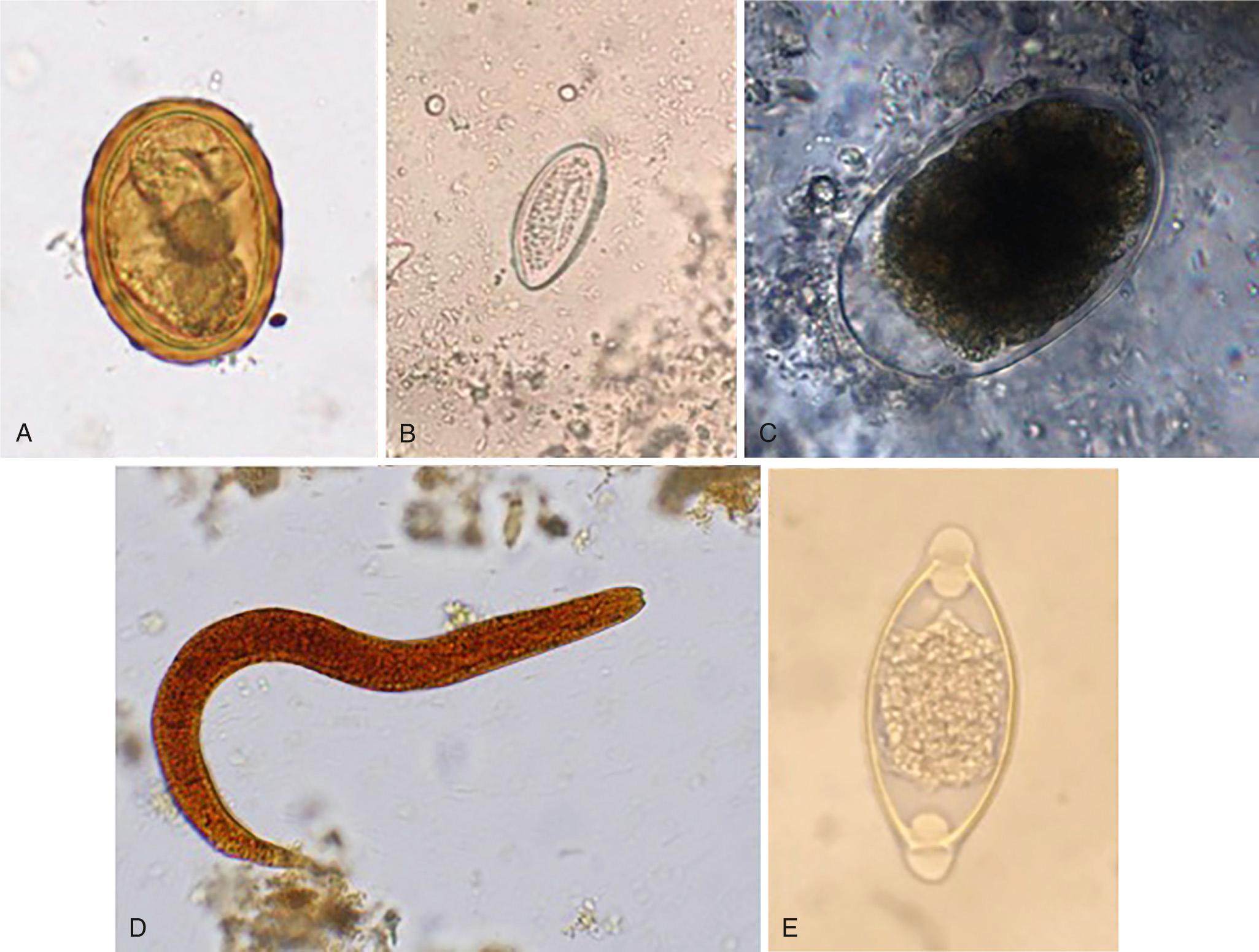
Adult Ascaris worms live in the small intestine of the host and can survive in the presence of host digestive hydrolases owing to the presence of a resistant outer cuticle. Adult worms are large and can grow up to 30 cm in length and live up to 1 to 2 years within the host. Female adult worms release over 200,000 eggs per day, or 3540 eggs per gram of stool ( Table 268.2 ). Ascaris eggs are durable; they are resistant to extreme environmental conditions and survive up to 10 years in ideal soil conditions and up to 2 months without soil. The ability to survive in extreme conditions promotes transmission of ascariasis in both moist and arid environments and in rural and urban communities. Infection occurs by fecal-oral transmission when embryonated eggs from the environment are ingested. After ingestion, stage 2 larvae (L2) hatch in the host small intestines, penetrate the intestinal wall, and are carried by the portal vasculature system to the liver. From the liver, the L2 larvae are transported in the blood to the lung parenchyma, where the larvae molt from stage L2 to L3 larvae. The L3 larvae migrate up the bronchotracheal tree, across the epiglottis, and are swallowed back into the intestinal lumen of the small intestine, where they develop into adult worms ( Fig. 286.1 ). Adult worms generally live from 12 to 24 months. During the migratory process, Ascaris larvae release excretory/secretory products (ES) that elicit a strong host inflammatory response represented by visceral eosinophilic infiltrates, peripheral eosinophilia, and high levels of immunoglobulin E (IgE). Egg production can be detected in the host stool for 2 to 3 months after embryonated eggs are ingested ( Fig. 286.2 ).
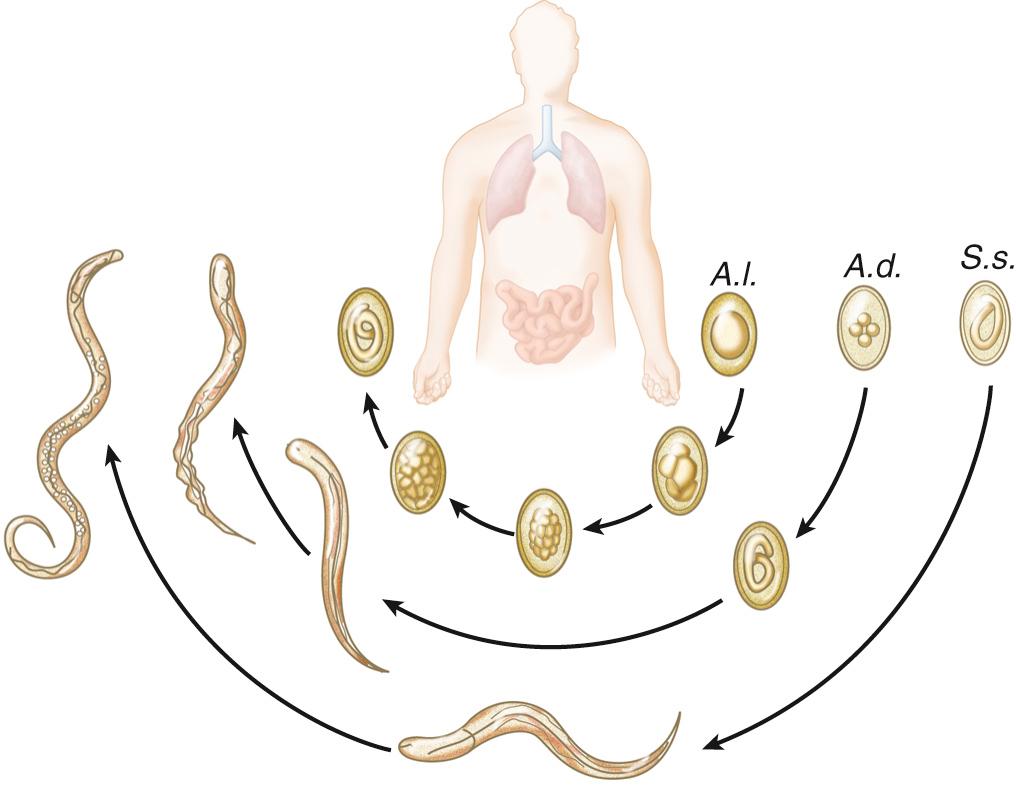
The symptoms of ascariasis vary based on the phase of the disease—that is, acute migratory phase versus chronic intestinal phase. Symptoms associated with the acute migratory phase are a result of the intense host type 2 immune response, with very high levels of IgE and eosinophilia, that occurs as the larvae migrate through visceral organs. Infected people may have abdominal pain, nausea, and vomiting as the larvae migrate through the intestines and liver, and symptoms of acute lung inflammation known as Loeffler syndrome, dyspnea, bronchohyperreactivity, and eosinophilic infiltrate as the larvae migrate through the lung parenchyma. Chronic intestinal infection is commonly asymptomatic or associated with mild symptoms of abdominal distention, pain, and nausea. However, people harboring a moderate-to-heavy worm burden can have significant morbidity. Ascariasis can have a severe impact on the host nutritional status, specifically protein loss and vitamin A deficiency, leading to impairments in physical and cognitive development. Reduction in cognitive abilities leads to decreased school participation and future economic productivity. Because of the large size of the adult Ascaris worm, small bowel obstruction, typically in the terminal ileum, is common in hosts (especially young children) with high worm burden and may necessitate surgical removal. Less commonly, volvulus, intussusception, perforation, and peritonitis can occur. Chronic Ascaris infection can also increase the risk of developing wheezing and bronchohyperreactivity, possibly because of the high levels of IgE and cross-reactivity with aeroantigens or because of parenchymal lung damage during acute larvae migration. This syndrome produces a Loeffler pneumonitis, which clinically resembles asthma.
Despite living in the small intestines, adult worms are motile. Under stress, adult worms will migrate into the appendix, stomach, oropharynx, or biliary and pancreatic ducts, causing mechanical obstruction, appendicitis, cholecystitis, cholangitis, pancreatitis, and biliary strictures. Surgical intervention through an endoscopic removal of common bile duct worms or open surgery may be required in circumstances of worsening intestinal obstruction, persistent biliary impaction, or hepatic disease with an abscess. Dead adult A scaris worms within the hepatobiliary system can become a nidus of pigmented hepatic stone formation within the common bile duct. Biliary migration of adult Ascaris worms is enhanced in patients with previous sphincterotomy or sphincter ablation, cholecystectomy, or bilioenteric anastomosis. Infected pregnant women are also at high risk of Ascaris migration into the hepatobiliary system secondary to relaxation of the sphincter of Oddi due to high progesterone levels.
Detection of Ascaris eggs in stool through use of the Kato-Katz thick smear method is the gold standard for diagnosis of Ascaris infection (see Fig. 286.2 ). Kato-Katz is a standardized microscopy technique that measures worm burden by quantitating the number of eggs per gram of stool in two separate stool samples. The technique is cheap and has few false-positive test results but is labor-intensive. Daily fluctuation in egg excretion and dependence on trained microscopists can cause highly variable results, particularly in areas of low disease burden, leading to a false-negative test result. To overcome the variability in areas with low worm burden, concentration techniques such as FLOTAC can increase the sensitivity by using larger volumes of stool. Use of concentration techniques in combination with Kato-Katz is critical for increasing the diagnostic accuracy in epidemiologic mapping and for monitoring mass preventive chemotherapy programs. However, the use of concentration techniques remains labor-intensive and increases the cost of the test by requiring additional equipment.
Highly sensitive, specific, and rapid diagnostic methods are required for eradication efforts. Polymerase chain reaction (PCR) methods using multiplex, multiparallel real-time technology improve accuracy in estimating Ascaris egg burden and increase sensitivity to detect infection in areas with low worm burden. Although more expensive in terms of upfront costs, PCR methods are less labor-intensive, use a single stool sample, and can be used to identify a higher distribution of polyparasitism compared with standard microscopy, making such methods ideal for monitoring both organism elimination and organism resurgence. False-negative and false-positive results can occur with PCR owing to variation in DNA extraction and target amplification.
Use of an oral benzimidazole, albendazole 400 mg daily or mebendazole 500 mg daily, is efficient to treat Ascaris infection and reduce morbidity in persons with high worm intensity. Single-dose oral albendazole, mebendazole, and pyrantel pamoate, used in mass preventive chemotherapy programs, have cure rates of 88%, 95%, and 88%, respectively. However, mass preventive chemotherapy programs targeting high-risk groups are not sufficient to lead to elimination because rapid reinfection is common. More frequent anthelmintic therapy applied to a broader age group within a community is required to overcome high reinfection rates. Improvements in water, sanitation, and hygiene (WASH), including proper disposal of human and animal feces, and increased access to potable water and health infrastructure will be essential to interrupt transmission and prevent reinfection.
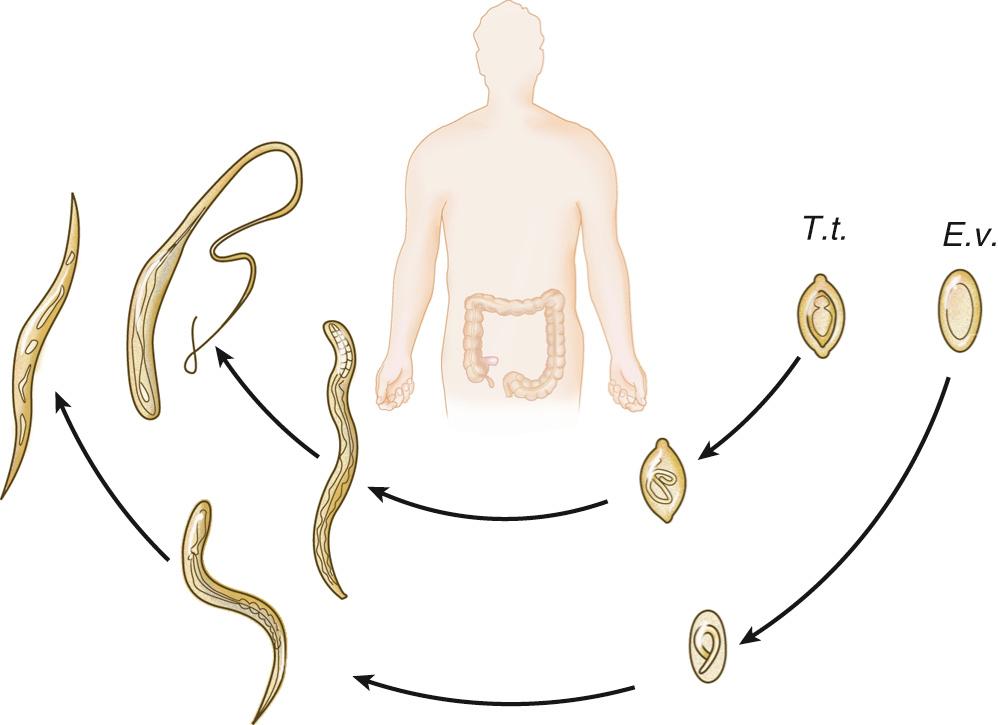
Also known as pinworm, E. vermicularis is endemic in Western countries, including the United States. Updated studies in the United States are lacking; the last prevalence study estimated 40 million infections in the 1980s. Worldwide, the prevalence can be as high as 30%. Pinworm infections are not limited to lower socioeconomic groups and can affect families and people living in close quarters.
The gravid female measures approximately 1 cm; the organism is limited to fecal-oral transmission, and the main gastrointestinal location is the cecum and appendix. The lifespan of a worm is less than 3 months; it spends 1 month as an adult and releases over 10,000 eggs (see Fig. 286.2 ). The females migrate to the perianal area at night, where they release their eggs and die ( Fig. 286.3 ). Eggs will embryonate and become infectious at 6 hours. These activities create a nidus of pruritus, leading to scratching and contamination, especially underneath the fingernails. The infectious eggs can then be transferred via the fecal-oral route to other individuals or through autoinfection to the host. Bedding, clothing, or surrounding surfaces can be contaminated with eggs. Eggs will become noninfectious after a few days unless ingested, continuing the life cycle. Sexual transmission can occur between partners engaging in oral-anal sex.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here