Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intestinal nematode infections are caused by a group of helminths, sometimes referred to as soil-transmitted nematodes, soil-transmitted helminths, or geohelminths. They are among the most common parasitic infections in humans and affect more than one-seventh of the world’s population. In most endemic populations, children are affected disproportionately by intestinal nematodes and often acquire infections of high intensity (i.e., large numbers of worms in the gastrointestinal tract) relative to their adult counterparts living under similar conditions. Child health and development are adversely affected by intestinal helminths throughout much of the developing world. Chronic intestinal nematode infections during childhood negatively impact school performance and attendance, as well as future economic productivity. Infection with intestinal nematodes also may impact susceptibility to or clinical progression of other infectious diseases as a result of the polarizing type-2 immune response induced by intestinal nematodes. In addition, these worms can cause anemia, which exacerbates the anemia caused by malaria or other etiologies.
It is estimated that intestinal nematodes cause the loss of over 1.9 million disability adjusted life years (DALYs), indicating an extraordinary impact on community health. Providing anthelminthic therapy to children has demonstrated improvement in long-term cognition and nutritional status leading to improved educational and economic outcomes in certain studies, but has shown negligible effects in others. This variation in study outcomes is likely due to differences in study populations and methods, and warrants continued evaluation.
Intestinal nematodes infect persons living in extreme poverty in all countries, regardless of overall income. It is estimated that nearly one-half of all helminth infections, including intestinal nematodes, occur among the poor living in wealthy countries, including the US. Several intestinal nematodes, thought to have been previously eradicated, have now re-emerged in areas of poverty in the US. , , Ascaris suum (porcine roundworm) , Enterobius vermicularis, hookworm, toxocariasis, and Strongyloides stercoralis are all endemic within the US, particularly in the South Gulf Coast and Appalachia regions. ,
Infection with intestinal nematodes generally occurs by 1 of 2 routes. For A. lumbricoides, T. trichiura, and E. vermicularis, infection results from ingestion of eggs. For Strongyloides (S. stercoralis and S. fuelleborni) and hookworms (A. duodenale, Ancylostoma ceylanicum, Ancylostoma caninum, and N. americanus) , infective third-stage larvae (L3), usually found in soil, penetrate host skin. Infection with Ancylostoma and the non-human primate threadworm S. fuelleborni subsp. felleborni and S. fuelleborni subsp. Kellyi can be acquired through ingestion of L3 and have even been linked to neonatal helminth infections associated with larval contaminated breast milk. Additionally, one report indicated potential transplacental acquisition of ascariasis in a neonate. The clinical findings associated with intestinal nematode infections are summarized in Box 276.1 . Recognized syndromes caused by perinatal infection with A. lumbricoides, A. duodenale, and Strongyloides are listed in Table 276.1 .
Löeffler pneumonia
Asthma
Intestinal obstruction
Extraintestinal migration
Malabsorption
Eosinophilia
Chronic colitis
Dysentery
Intussusception
Rectal prolapse
Anemia
Iron deficiency anemia
Hypoproteinemia
Chlorosis
Eosinophilia
Cognitive impairment
Eosinophilic enteritis
Aphthous ileitis
Enteritis
Eosinophilia
Eosinophilia
Epigastric pain
Cough
Autoinfection
Hyperinfection and disseminated disease
Pruritus ani
Vaginitis
Extraintestinal migration
| Syndrome | Nematode Species | Speculated Routes of Transmission |
|---|---|---|
| Neonatal ascariasis | Ascaris lumbricoides | Transplacental |
| Infantile ancylostomiasis | Ancylostoma duodenale | Transmammary, perinatal |
| Infantile strongyloidiasis (swollen belly syndrome) | Strongyloides fuelleborni and Strongyloides stercoralis | Transmammary, perinatal |
The diagnosis of Ascaris, hookworm, and Trichuris infection is established most frequently by identification of characteristic eggs in feces through light microscopy ( Fig. 276.1 ). Pinworm eggs are found deposited on the perianal skin and use of a tape test can identify eggs. In contrast, Strongyloides first-stage larvae, not eggs, are infrequently excreted in feces and can be difficult to diagnosis without several samples. Serologic testing for Strongyloides is more commonly used, especially in light infection or in asymptomatic people, and provides additional information on cure post-treatment. Molecular diagnostics are becoming more widely available and offer advantages, including increased sensitivity and specificity, compared to light microscopy. ,
Benzimidazole anthelminthics (i.e., albendazole and mebendazole) remain the standard therapy for Ascaris , hookworm, Trichuris, and pinworm, while Strongyloides is treated routinely with ivermectin. However, there is growing concern regarding the risk of developing wide-spread drug resistance as anthelminthic mass drug administration scales up. Use of drug combinations (e.g., albendazole plus ivermectin) in the setting of polyparasitism has been used to increase anthelminthic efficacy and also may aid in reduction in the development of drug resistance. Due to the massive impact of intestinal nematodes on childhood morbidity, mass drug administration (MDA, also known as preventive treatment or preventive chemotherapy) with benzimidazoles is recommended by the World Health Organization (WHO) for all children aged 12 months or older, nonpregnant adolescent girls, and non-pregnant women of reproductive age living in endemic regions. Preventive chemotherapy should also be given routinely to pregnant women after the first trimester in endemic regions where the prevalence of hookworm or Trichuris is >20% and the prevalence of anemia in pregnant women is >40%. Preventive chemotherapy targeting intestinal nematodes also has benefit for other neglected tropical diseases, including mansonelliasis, oesophagostomiasis, scabies, Plasmodium vivax , and potentially Plasmodium falciparum. Addition of newer anthelminthic drugs (e.g., tribendimidine, oxantel pamoate, or moxidectin) to MDA campaigns may further expand the health impact of preventive chemotherapy. Despite preventive chemotherapy, the rate of intestinal nematode reinfection remains high and there is growing concern of treatment failure due to potential drug resistance. Alternative therapies including pan-helminthic vaccines and new drug targets are needed in combination with improvements in water, sanitation, and hygiene (WASH) to reduce the morbidity and mortality associated with intestinal nematode infections. ,
Ascariasis is the most common helminth infection in humans, with over 400 million people infected worldwide. , Morbidity is most apparent in children who harbor the heaviest burden of infections. A. lumbricoides eggs are resistant to a wide array of environmental stresses allowing for ubiquitous contamination of environments with poor sanitation and waste management. , Ascariasis still occurs in the US as an imported infection, usually in recent immigrants from Latin America and Asia, and in internationally adopted children. , , However, ascariasis likely remains an autochthonous infection in the southeastern US, where the rate of infection in children has historically ranged from 2.3% to 49%. Additionally, transmission of Ascaris suum , the porcine roundworm, which is morphologically indistinguishable from human roundworm and genetically similar, also can infect humans, causing disease typically in children living near pig farms. ,
Chronic ascariasis in childhood can be associated with impaired physical growth and cognitive development, which in some cases can be linked to vitamin A and other micronutrient malabsorption as well as protein-calorie malnutrition. Young children also are more susceptible to life-threatening complications such as intestinal obstruction, perforation, and peritonitis. Globally, intestinal and biliary tract obstruction caused by Ascaris accounts for 10,000–100,000 deaths a year, mostly in children. , In low- and middle-income countries, the rate of complications secondary to ascariasis ranges from 11% to 67%, with intestinal or biliary tract obstruction the most common serious sequela. There have been case reports of transplacentally acquired neonatal ascariasis, but the prevalence of this mode of transmission remains unclear. ,
The life cycle of A. lumbricoides in humans begins with ingestion of embryonated eggs that have been deposited in moist soil. The acidic environment in the stomach followed by the bile salts and the alkaline pH of the small intestine aids in larvae hatching. Emerging third-stage larvae penetrate the bowel wall and ultimately invade the microcirculation of the lamina propria. Once in the circulatory system, larvae are carried to the liver through the portal veins and eventually are swept into the pulmonary vasculature, where they lodge in capillaries and rupture into the alveolar space. While in the lungs, third-stage larvae molt, shedding the outer chitin layer, into fourth-stage larvae. Fourth-stage larvae penetrate through the pulmonary epithelium into the bronchi, ascending the bronchi to the trachea; larvae are swallowed and again migrate to the small intestine. Ascaris larvae mature into sexually differentiated adults in the lumen of the small bowel, mate, and begin laying eggs. Adult female worms produce over 200,000 eggs per day and can live up to 2 years in the human host.
Illness associated with ascariasis can be attributed to the migration of both larvae and adult worms. As larvae migrate through the lung parenchyma, they cause both mechanical and immune-mediated damage. Pulmonary microhemorrhages from ruptured alveolar capillaries result in inflammation and fluid exudate. Neutrophils and eosinophils are recruited to the lung in response to proteins released by the migrating larvae. , Pulmonary infiltrates in association with cough, dyspnea, wheezing, and mild hemoptysis (Löeffler pneumonia) can occur and last up to 2 weeks. Children with ascariasis have increased risk of asthma and atopy and, as such, ascariasis may be a leading cause of asthma worldwide. The intensity of illness caused by migrating larvae is proportional to larval numbers, with lightly infected people having fewer pulmonary symptoms. In rare instances, adult worms from the intestines can migrate into the airways and cause airway obstructed respiratory distress.
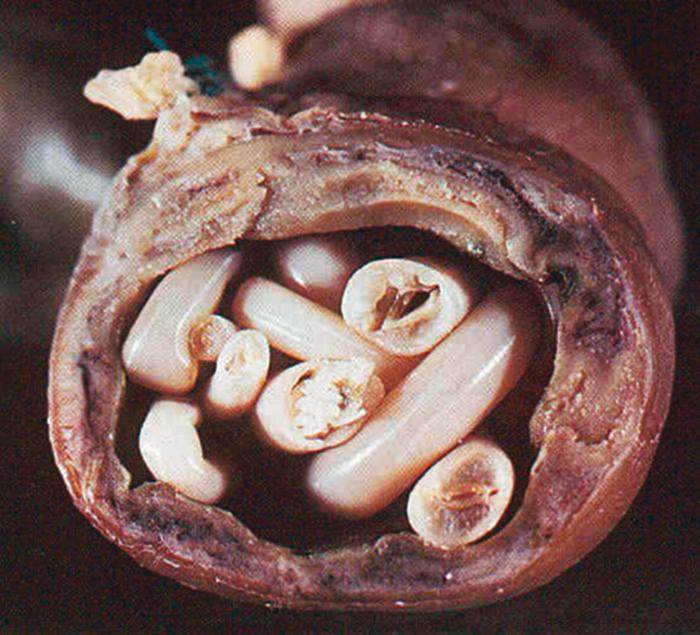
Symptoms associated with the presence of adult Ascaris worms in the small bowel include epigastric pain and diffuse abdominal discomfort. Because adult worms can grow to 35 cm in length, heavy infestations can result in acute intestinal obstruction and perforation, especially in the region of the ileum, which requires emergency medical or surgical intervention ( Fig. 276.2 ). , , Migration of adult worms into the biliary tree, and then to the liver or pancreas, can precipitate acute cholangitis, cholecystitis, hepatitis, pancreatitis, and hepatic abscess if the intrahepatic ducts are obstructed.
Chronic ascariasis has been associated with malnutrition, which is caused by malabsorption of dietary protein, fat, and vitamin A. The pathogenesis of malabsorption of nutrients is likely multifactorial. , , Investigators have postulated that adult worms successfully compete for nutrients by secreting a variety of enzymes and protease inhibitors into the proximal region of the small intestine. Whether the physical and mental growth restriction syndromes associated with chronic ascariasis in childhood have a purely nutritional basis remains unknown. Furthermore, Ascaris infection frequently occurs in the setting of infection with other intestinal nematodes, especially Trichuris and hookworm, thus making it difficult to ascribe long-term sequelae to an individual parasitic species.
The diagnosis of Ascaris infection is best established by examination of stool for characteristic ova using light microscopy ( Fig. 276.1 ). The Kato-Katz stool preparation method is low-cost and semi-quantitative which correlates with worm burden. Repeat testing and multiple slides may be needed due to daily fluctuations in egg release, and an experienced microscopist is required to increase sensitivity. The use of concentration techniques may aid in identification of Ascaris eggs, particularly in low burden disease. In areas with low worm burden, molecular techniques, such as quantitative polymerase chain reaction (qPCR), have been beneficial to determine worm burden. For identification and quantitation of ascariasis, qPCR has been shown to have increased precision compared to Kato-Katz. , However, the cost and the need for additional equipment and an energy source remain barriers to the use of qPCR in endemic regions.
When migration of Ascaris larvae through the lungs is associated with peripheral blood eosinophilia and pulmonary infiltrates on a chest radiograph, examination of sputum can reveal larvae, eosinophils, and Charcot-Leyden crystals. The differential diagnosis includes other causes of parasitic pneumonia, including strongyloidiasis, toxocariasis (visceral larva migrans), hookworm infection, and filariasis (tropical pulmonary eosinophilia).
In areas endemic for A. lumbricoides, any child manifesting acute abdominal symptoms consistent with intestinal obstruction or perforation should be evaluated for ascariasis. Occasionally, an adult worm is present in vomitus. Abdominal radiographs after barium administration can diagnosis A. lumbricoides as coiled radiolucent filling defect within the intestine. Similarly, computed tomography (CT) with oral contrast may demonstrate an elongated linear filling defect within the lumen of the intestines. Using ultrasonography, adult Ascaris worms appear as a “linear intraluminal mass” longitudinally or as a “target” size on cross section. Endoscopic retrograde cholangiopancreatography (ERCP) and ultrasonography can be helpful in identifying adult Ascaris worms in the hepatobiliary tree. , , ERCP can be used for removal of the adult worm from the biliary tract. , Occasionally, however, the diagnosis of intestinal ascariasis is made during exploratory laparotomy typically performed due to intestinal obstruction of unknown etiology. ,
Benzimidazoles, such as mebendazole (100 mg twice a day for 3 days or 500 mg once) and albendazole (400 mg in a single dose), are effective for treatment of intestinal ascariasis, with cure rates >90%. , , Because of their potential teratogenic and tumorigenic effects seen in animal models, but not demonstrated in human studies to date, benzimidazoles generally are not given to children aged <12 months or women during their first trimester of pregnancy. However, extensive experience with the use of these agents in children supports that a reduced dose of albendazole (200 mg) in a single dose given to children 12–24 months of age, or even children <12 months of age, is not problematic and should be used when necessary. Additionally, pregnant women after their first trimester of pregnancy can also be treated with benzimidazoles at standard dosing. For this reason, benzimidazoles increasingly are being distributed in low- and middle-income countries with heavy disease burdens for treatment and for preventive chemotherapy as part of MDA programs. Side effects are rare, with mild gastrointestinal symptoms, dizziness, headache, transaminitis, and leukopenia reported. , Benzmidazoles should be taken with a high fat meal. Reports of benzimidazole resistance have emerged for Ascaris lumbricoides secondary to a mutation in the β-tubulin isotype-1 gene, similar to reports in other nematodes. Alternative therapies include ivermectin (150–200 μg/kg once) which has been shown to be highly effective against ascariasis; however, safety data for the use of ivermectin in children weighing <15 kg and pregnant women limits its widespread use and it is not approved by the US Food and Drug Administration for ascariasis. Nitazoxanide is comparable to albendazole for treatment of Ascaris infections and is approved for use in children aged 12 months. The recommended dose is 100 mg (5 mL) twice a day for children 12–47 months of age, 200 mg (10 mL) twice a day for children 4–11 years of age, and 500 mg twice a day for older children and adults, completing a 3-day course; bioavailability is significantly increased when taken with food. Pyrantel pamoate (11 mg/kg up to maximum 1 g/day for 3 days) is an additional suitable alternative. In some heavily infected children, malnutrition, steatorrhea, and growth restriction are partially reversible with treatment; however, there remains conflicting data on the long-term impact of treatment on chronic morbidity.
In the case of intestinal obstruction, mineral oil or gastrografin (a solution of diatrizoate meglumine and diatrizoate sodium) given orally or by nasogastric tube also can cause relaxation of the obstructing bolus of worms and enhance expulsion; however, it is unclear if use of gastrografin reduces need for laparotomy. Standard supportive measures for treatment of small bowel obstruction also should be administered, including intravenous hydration, nasogastric suctioning, and monitoring of electrolyte status. Laparotomy to remove the adult worm bolus should be undertaken in patients who do not respond to therapy with anthelmintic drugs.
Prevention of ascariasis requires elimination of contact with soil contaminated by eggs. Although Ascaris eggs are killed by prolonged exposure to sunlight or temperatures >40°C, they can survive in extremely cold temperatures for many months and are resistant to many chemical disinfectants. Ascaris eggs survive in temperate climates and urban environments.
Enhanced diagnostics and effective anthelminthic treatment in combination with WASH programs is the most reliable means of combating widespread Ascaris transmission within a community. In endemic areas where the prevalence of infection with intestinal nematodes is >50%, preventive chemotherapy using benzimidazole agents should be administered to high risk populations (children aged >12 months, non-pregnant adolescent girls, and non-pregnant women of reproductive age) twice yearly as part of a targeted deworming program; prevalence of 20% to 49% requires annual treatment within the same high-risk groups. However, the effects of a single intervention are likely to be short-term given the inevitability of reinfection in endemic regions. Because ascariasis is an infection that is associated with poverty and poor sanitation, improved water, sanitation, and hygiene, together with sustained economic growth, are requirements for long-term parasite control and potential for elimination.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here