Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The GI tract processes 8 to 9 L of fluid daily that is derived from oral intake and endogenous exocrine secretions. Intestinal fluid absorption functions with 98% efficiency, allowing only 100 to 200 mL to be excreted each day. The intestine also extracts nutrients, vitamins, and minerals from ingested materials; excludes destructive antigens and microbes; and excretes waste ( Fig. 101.1 ). This multitasking is achieved by the uniqueness of the cellular and molecular architecture of the small and large intestine in combination with complex intricate regulatory mechanisms ( Fig. 101.2 ). Regulation is accomplished by crosstalk between endocrine and paracrine hormones, neurotransmitters, immunomodulators, and luminal factors, including the microbiome. Remarkably, this orchestration proceeds smoothly on a daily basis, but when the balance is perturbed, as occurs with an enteric infection, diarrhea ensues.
Over the past 5 decades, our understanding of intestinal ion transport processes has been revolutionized by elucidation of the molecular basis of cholera and CF, 2 devastating diseases affecting opposite ends of the physiologic spectrum—excessive versus insufficient fluid secretion, respectively. Development of increasingly sophisticated tools, beginning with molecular cloning and patch clamping methodologies to organoids, organ on a chip, cryo-electron microscopy, and CRISPR-CAS, has vastly advanced our knowledge of the physiology, regulation, and genetics underlying these diseases. This growing insight has had significant clinical impact, most notably in the development of oral rehydration therapy (ORT) for diarrheal diseases, a major health advance of the 20th century, and targeted drugs for specific CF-related mutations.
In this chapter, we review current understanding of the cellular and molecular underpinnings of ion and solute trafficking in different regions of the small and large intestine, and their regulation in health and disease states. The functional activities of intestinal transporters have long been recognized, but only recently have the nuances of the many underlying transport proteins and their regulation been delineated. This understanding is critical for appreciating normal intestinal function, the pathophysiology of intestinal absorptive abnormalities, and the development of therapeutic strategies for specific diseases.
Intestinal structure and function are optimally geared to absorb nutrients and transport fluids. In the small intestine, the circular folds of Kerckring (plicae circulares), villus-crypt architecture, and microvilli contribute to a 600-fold amplification of the absorptive surface. Using a cylinder as the model, the surface area of the small intestine is estimated to be about 3300 cm 2 ; the plicae circulares, villi, and microvilli amplify the surface area by factors of 3, 10, and 20, respectively, ultimately giving a surface area of about 2,000,000 cm 2 . In the large intestine, the spatial separation of crypts and surface cells allows efficient reabsorption of fluid. Although the architecture of the intestinal musculature influences bulk fluid flow and transit time via changes in motility (see Chapter 100, Chapter 99 and Chapter 100, Chapter 99 ), the work of fluid transport occurs in the epithelia.
Most epithelia are semipermeable barriers acting as the first line of defense between the mucosal (luminal) and serosal (blood-side) compartments and are capable of bulk transport of fluid from one compartment to the other. These epithelia, including those of the intestine, share common characteristics. One fundamental property of epithelia is cellular polarity, with molecularly distinct apical (luminal) membranes (AMs) and basolateral (serosal) membranes (BLMs) demarcated by intercellular tight junctions (TJs). TJ permeability varies from being relatively leaky in the small intestine to fairly tight in the large intestine, and these differences determine an individual epithelium’s effectiveness as a barrier. A loss of TJ integrity disrupts the barrier function and vectorial transport capabilities of the tissue.
All GI epithelial cells have 2 fundamental similarities: discrete apical and BLMs with distinct biochemical and biophysical properties, separated by TJs; and a basolateral Na + pump (ouabain-inhibitable Na + /K + -ATPase [adenosine triphosphatase]) that establishes a specific intracellular electrochemical environment with low intracellular Na + concentrations ([Na + ] i ) and a negative intracellular voltage.
This basic cell model is modified by insertion of specific transporters into either the apical and/or BLM and/or by TJ features that determine the distinctive qualities of specific epithelial segments. A complex interaction of protein-sorting signals, cytoskeletal elements, and intracellular trafficking processes determines whether a newly synthesized protein is targeted to either the AM or BLM. Proteins with a glycosyl phosphatidyl inositol anchor (e.g., alkaline phosphatase, CEA) are often associated with lipid rafts, and the glycosyl phosphatidyl inositol anchor serves to direct them toward the AM. Membrane proteins destined to be delivered to the BLM carry specific membrane-sorting amino acid sequences in their cytoplasmic tails. Other proteins can insert randomly into either an apical or basolateral domain, but they may be retained in the basolateral pole by specific components such as ankyrin.
Regulation of intracellular trafficking ensures accurate delivery and is critical for establishing epithelial polarization and vectorial transport. When TJs are disrupted, diffusion and intermingling of apical and basolateral proteins in the fluid phase of the membrane result in a loss of epithelial cell polarity. Critical to epithelial cell polarity is targeting of the Na + /K + -ATPase pump to the BLM. The Na + /K + -ATPase has 3 subunits, α, β, and γ, which are transmembrane proteins with a 1:1:1 stoichiometry. The α subunit binds to ATP and is responsible for cation transport, undergoing phosphorylation and dephosphorylation during enzyme turnover. The β subunit is essential for proper trafficking and insertion of the α subunit into the BLM, and the β and γ subunits modify the affinity of the α subunit for ATP, K + and Na + . The Na + pump is electrogenic, extruding 3 Na + ions in exchange for 2 K + ions, and thereby maintaining relatively low intracellular Na + and high intracellular K + concentrations compared with concentrations of these electrolytes outside the cell (see Fig. 101.2 ). There is also greater membrane permeability for K + over Na + , favoring diffusional cellular exit of K + over diffusional cellular entry of Na + . These features, in combination with the large number of intracellular proteins with fixed negative charges, lead to the characteristic negative intracellular potential difference compared with either the mucosal or serosal compartments. ∗ Low [Na + ] i and electronegativity establish a favorable electrochemical gradient for passive Na + entry into the cell. Functionally, the epithelial cell uses the energy of this Na + gradient to transport not only Na + ions but also a variety of nutrients, vitamins, and electrolytes.
∗ There are several potential differences across the epithelium: across the apical membrane into the cell, from the cell interior across the BLM, across the epithelium, across the mucosa, and across the entire GI tract. Conventionally, the potential differences across the epithelium, mucosa, and entire GI tract are considered the same.
These properties provide the basic mechanisms of ion and water transport that apply to all epithelia. In the intestine, differences in transport occur along its cephalocaudal length and along the surface-crypt axis within a particular intestinal segment. Tissue- and segment-specific nuances arise from structural-functional and regulatory differences of both intracellular and intercellular proteins.
All intestinal segments from the duodenum to the distal colon exhibit transepithelial fluid movement, supported by their individual array of transporters. For example, the glucose- and amino acid–coupled transporters in the jejunum are well-suited for absorption of large volumes of nutrients and water, whereas, the electrogenic Na + absorption in the distal colon accomplishes the necessary final fluid extraction to prepare feces. Although different transporters have been localized to specific GI segments, it is not always clear what governs this specific distribution. For example, anion exchange activity occurs throughout the intestine, but the transporters in the small intestine and in the colon (see discussion of “Bicarbonate Transport”) are distinct. Recent evidence, however, implies that nuances in how these transporters contribute to base transport may account for their distinct distribution.
There is also segmental heterogeneity along the crypt-villus axis. Stem cells near the base of the crypt differentiate and migrate upward to form villus enterocytes in the small intestine or surface colonocytes in the large intestine while undergoing important changes in their transport and barrier properties ( Figs. 101.3 and 101.4 ). In the small intestine, as enterocytes migrate away from the proliferative zone, the complexity of their TJs increases, their apical membrane microvillous architecture becomes more pronounced, their cytoskeleton and signaling molecules undergo change, and the expression of brush border membrane Na + nutrient-coupled transporters, Na + /H + exchangers, and hydrolases increases. In contrast, the levels of the Na + pumps remain relatively constant, and others (e.g., the signaling molecule adenylate cyclase and the cyclic adenosine monophosphate [cAMP]-stimulated Cl − channel CFTR) decrease in more mature villus cells.
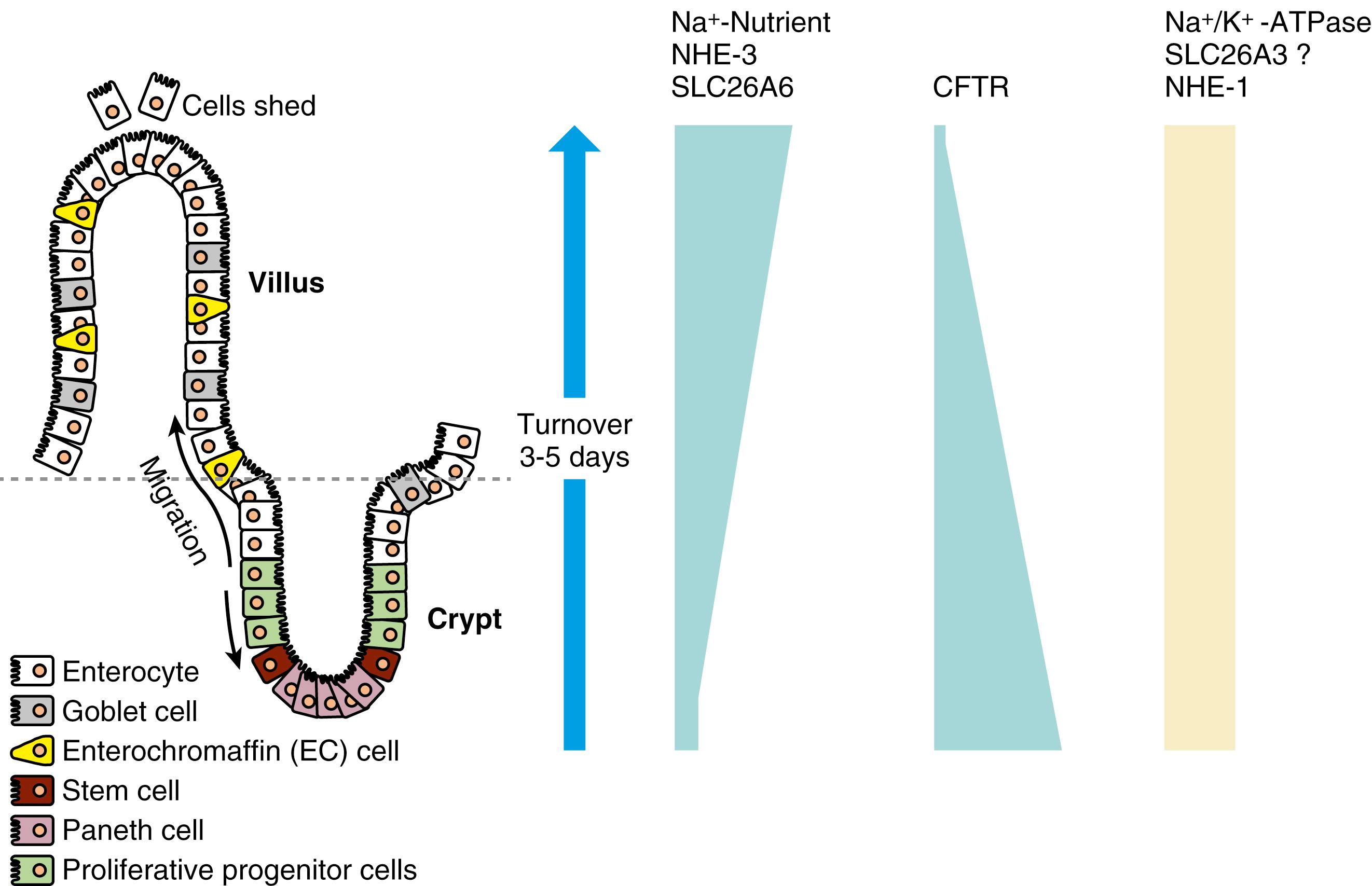
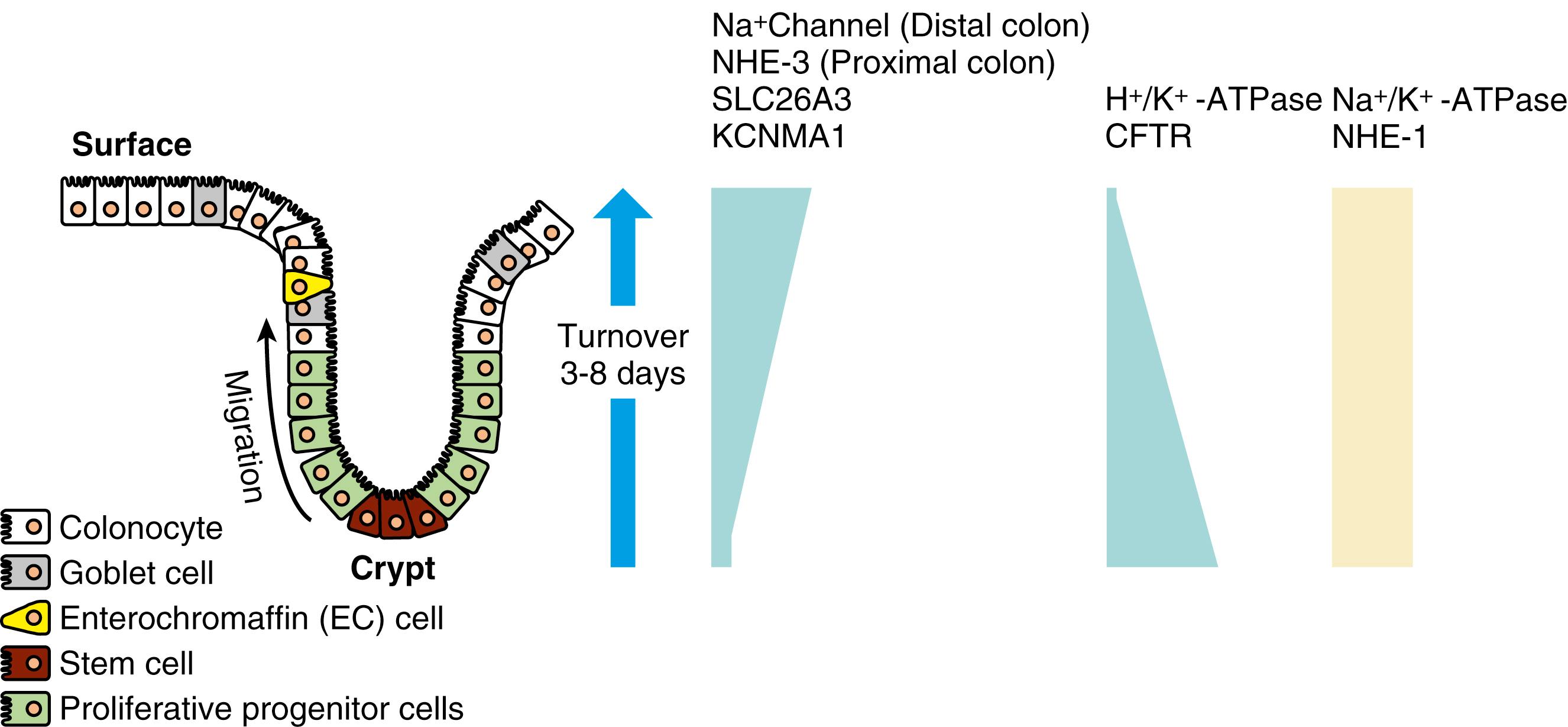
This spatial distribution of transporters (see Figs. 101.3 and 101.4 ) is consistent with a model in which secretory function resides primarily in the crypts and absorption occurs in villus or surface cells. Although the role of crypt cell Cl − secretion was challenged, this challenge has been refuted with well-substantiated evidence that crypts are the chief sites of Cl − secretion. Undoubtedly the cellular architecture of the columnar crypt and villus enterocytes are distinct and the segregation of absorptive and secretory functions explains why, in diseases that selectively damage villi (e.g., celiac disease), impairment of absorption is greater than impairment of secretion, and secretion predominates. The dichotomy between absorptive and secretory cells does exhibit plasticity, varying with altered physiologic and pathophysiologic states. For example, colonic crypts absorb Na + , and small intestinal villus cells secrete Cl − via CFTR. The localization of key transporters, their signaling mechanisms, and their crosstalk of interaction varies along the crypt-villus axis, and contribute to the fine-tuning of intestinal function.
Movement of ions and solutes across the epithelium is bidirectional and occurs transcellularly and paracellularly. Paracellular movement is largely passive in response to a variety of gradients, including concentration, electrical, osmotic, and hydrostatic; transcellular movement of ions and solutes occurs by active and passive transport mechanisms. Net transport is termed absorptive if the mucosal-to-serosal flux (J ms ) is greater than the serosal-to-mucosal (J sm ) flux, and it is termed secretory if J sm exceeds J ms . Changes in either or both can alter the direction of net movement; for example, the ileum, which normally exhibits an absorptive flux, responds to cholera toxin with a decrease in J ms and an increase in J sm for Cl − , resulting in massive fluid secretion. In contrast, increasing evidence points to a failure of Na + and Cl − absorption as the major ion transport aberrations associated with IBD.
Characteristics of the TJs (i.e., tight vs. leaky) vary along the length of the intestine and dictate the contribution of paracellular fluxes to overall transport. The effectiveness of a transepithelial gradient may be modified by a series of physical barriers, including an unstirred layer created by the glycocalyx above the apical membrane, the lipid composition of the apical and BLMs, the TJs, the geometry of the basolateral space between cells, and the basement membrane. Generally, movement of an uncharged particle is dictated solely by concentration gradients, whereas the transport of an ion is governed by the electrochemical gradient across the transported surface. Solvent drag , a nonspecific entraining of solutes along with the movement of water across paracellular pathways, is an absorptive mechanism that may be especially important in the small intestine, in Na + -coupled solute absorption, and in K + absorption.
The paracellular space and junctional complexes between cells define the barrier function of epithelia. Epithelia with a low transepithelial voltage and low resistance are considered leaky, and those with a high transepithelial voltage and high resistance are considered tight. The TJs in villi have higher resistance than those in crypts, and transepithelial resistance increases in a cephalocaudal direction (see Fig. 101.1 ).
Since the 1990s, the model of paracellular transport and TJs has rapidly evolved from a static rigid barrier to a finely regulated, dynamic complex structure (see Fig. 101.2 ). Movement through the space is exclusively passive, but it is influenced by its geometry, electrical conductivity, charge selectivity, and its ability to be regulated. A series of discrete structures, made of membrane and intracellular proteins, define the physical and biological properties of the paracellular space and the communication between adjoining cells; bicellular between 2 cells and tricellular between 3 cells. The bicellular TJ, or zonula occludens (ZO), is made up of a network of strands and grooves composed of 50 or more families of proteins. These include membrane proteins such as claudins, occludins, junctional adhesion molecules, and tricellulins. Claudins are a family of 26 membrane-spanning proteins (24 to 27 kd) that form pores and determine TJ charge selectivity by homotypic interactions of the extracellular domains of claudins of adjoining cells; absence of claudins mitigates transepithelial resistance. Tricellular TJ assembly requires the lipolysis-stimulated lipoprotein receptor and transmembrane tricellulin proteins; the latter is biochemically related to occludins. Most of these transmembrane proteins interact with membrane-associated or cytosolic scaffolding proteins such as the ZO proteins (ZO-1, ZO-2, ZO-3), multi-PDZ domain protein-1(Mupp-1), and cingulin. For example, the cytoplasmic tail of tricellulin associates with ZO-1.
Scaffolding proteins link membrane proteins to an array of protein kinases, phosphatases, monomeric guanosine triphosphatase proteins of the Ras superfamily (small G proteins) and cytoskeletal elements such as filamentous actin and myosin in the terminal web. This allows for a complex network to regulate paracellular permeability in health and disease. For example, enteropathogenic Escherichia coli (EPEC) act via protein kinase C ζ to disrupt TJs.
Other structures contributing to the paracellular pathway include, in order below the TJ: zonula adherens (ZA), desmosomes, and gap junctions. In epithelia, the ZA is primarily made up of transmembrane nectins (MW ≈ 90 kd) and E-cadherins (MW ≈ 110-130 kd). The former are Ca 2+ -independent adhesion molecules with immunoglobulin-type repeats in their extracellular domains and the latter are glycoproteins with extracellular motifs that engage in Ca 2+ -dependent homotypic interaction with cadherins of adjoining cells. Intracellularly, cadherins bind to a family of adhesion molecules, α, β, and p120 catenins, or afadin in the case of nectins, which in turn anchor to a dense actin-filament network. Dysfunction in cadherin-catenin interaction promotes cancer progression to invasion and metastasis. Desmosomes are structurally similar to ZA junctions and act as “spot-welds” providing mechanical integrity to the epithelium. They are comprised of desmoglein and desmocollin cadherins which associate with a dense plaque of intracellular anchor proteins, plakophilin, plakoglobin and desmoplakin that link to intermediate filaments instead of actin. Interestingly, EPEC uses distinct signaling pathways in its virulence armamentarium to alter ZO, ZA, and desmosomal functions, thereby altering paracellular permeability. Finally, gap junctions uniquely allow neighboring cells to exchange small molecules. Each cell has an assembly of 6 connexins, a 4-pass membrane–spanning protein, to form a hemichannel. When hemichannels of 2 adjoining cells align, they form a continuous pore connecting the interior of the 2 cells.
Our current understanding of the movement of ions, solutes, and fluid across epithelia is gleaned from a combination of in-vitro studies using reductionist models of cell lines or isolated epithelial sheets, and from complex methodologies such as genetically manipulated animal models and the in-vivo triple-lumen perfusion technique. Within the last decade, the ability to grow and differentiate stem cells into organoids that can also be studied as epithelial monolayers has transformed the field, both for validating key observations in relevant human tissues and providing tools for personalized medicine. All these models underscore that transepithelial ion (largely Na + ) movement from the mucosa to the serosa drives fluid absorption, whereas net ion (largely Cl − ) movement in the reverse direction drives fluid secretion. Different models help elucidate complex mechanisms, but ultimately have to be contextualized to human pathophysiology, with its inherent limitations. For example, some in-vitro studies report decreased Cl − secretion and increased Na + absorption in the jejunum of CF patients, implying fluid hyperabsorption. However, in-vivo studies show decreases in both Cl − secretion and passive Cl − absorption, which suggest that disease severity is reflected by decreased fluid absorption.
The reductionist models allow us to focus on transport processes at the cellular and paracellular levels. In the intact intestine, however, things are more complicated. The geometry of the intestinal wall and the unstirred layer influence the distance an individual molecule must traverse to reach the apical membrane. The extracellular glycosylated domains of apical membrane proteins make up the glycocalyx, which contributes to the thickness and permeability of the unstirred layer; this layer can be a diffusive barrier to the movement of large lipophilic molecules in a chiefly aqueous milieu. Physical parameters such as the mixing of luminal contents by peristalsis, villus motility, and the finer movement of the microvilli influence this rate.
Transcellular transport of ions and solutes can be passive or active. Because of the semipermeable nature of the lipid membrane, movement through the cell requires deployment of specialized membrane proteins such as channels, carriers, and pumps. The negative intracellular potential favors cation entry into and anion exit from the cell. This leads to the curious situation in which ions can move passively against their concentration gradient. For example, although the chemical concentration of Cl − in the cell is relatively low (≈35 mmol) compared with the outside concentration (≈110 mmol), the intracellular electronegativity creates a driving force for Cl − exit out of the cell.
The intestines are exposed to a constantly fluctuating external milieu that requires nuanced processes for moving water across the epithelium. The emerging picture is that multiple mechanisms contribute to intestinal water movement. Transepithelial water movement is inextricably linked to the movement of solutes; about 175 molecules of water can be transported per ion or molecule of solute. Two processes transport water across epithelial cell membranes: osmosis, which is passive and governed by even small differences in the chemical potential of water and hydrostatic pressure, and “active” processes, which are energized by and coupled to the movement of solutes with coupling ratios of solute/water molecules. Water movement across the epithelium can follow 4 routes: (1) diffusion through the lipid bilayer, or via proteins including (2) water channels, (3) uniporters, and (4) co-transporters.
Aquaporins (AQPs) are a family of water-channel proteins with 13 members in humans; AQP 0, 1, 2, 4, 5, 6, 8 are classified as classic water transporters; AQP 3, 7, 9, 10 as aquaglyceroporins because they also transport glycerol; and AQP 11 and 12 as “superaquaporins” with indeterminate functions. At least 6 have been localized to the GI tract and although considerable attention has been given to their regulation and role in the intestine, AQP knockout studies have not identified a specific functional intestinal water channel. For example, AQP4 is present in the BLM of colonic crypts; its absence there results in only a slight increase in fecal water content. The absence of a clear role for AQPs in the normal intestine is not surprising, considering the milieu. AQPs are advantageous in that they can increase the rate of water transport and save metabolic cost. In the intestine, however, where a hyperosmotic lumen-to-cell gradient during digestion may serve to favor water loss rather than absorption, they play a less prominent role than in the proximal renal tubule, where fluctuations in osmolarity are smaller. More recently, the aquaglyceroporin AQP3 present in the apical and BLM of colonic surface cells has been ascribed roles in diarrhea and constipation and in its capacity as a transporter of H 2 O 2 in the innate immune response to Citrobacter rodentium infection.
Water can also move via membrane transporters of ions and solutes. These include the apical Na + -glucose transporter (SGLT), the urea transporter, and the Na + /K + /Cl − co-transporter 1 (NKCC1). Water transport can be strictly osmotically driven, as occurs with AQPs and uniporters such as the urea channel; strictly coupled to each functional turnover of the protein, as seen with the K + /Cl − co-transporter (KCC; 1:500 molecules of water) on the BLM of absorptive epithelia and NKCC1 (1:590 molecules of water) on the BLM of secretory epithelia; or a combination of both, as occurs with the apical SGLT (1:220-400 molecules of water) and the Na + -independent uniporter glucose transporters, GLUT1 and GLUT2.
Ultimately, water movement across the intestine occurs under both isotonic conditions, when luminal and serosal osmolarity are 300 mOsm, and when the luminal osmolarity increases in the upper small intestine in response to a meal. Under isotonic conditions, water flux is maximal and decreases as luminal osmolarity increases; water flux ceases when luminal osmolarity is 250 to 300 mOsm greater than serosal osmolarity. Water movement through the paracellular pathway is governed by hydrostatic and osmotic gradients and tissue geometry. Cellular transport of water involves movement across the apical membrane, cytosol, and BLMs. In response to a meal, water is most likely absorbed by passive water permeability in combination with transport through SGLT1 and amino acid transporters on the apical membrane, and exits the BLM via GLUT2 and the KCC. In secretory intestinal cells, water enters coupled to the basolateral NKCC1, and although Cl − exits the cell through apical channels and Na + moves paracellularly, it is not known how water exits the apical membrane in the intestine.
Small hydrophobic and uncharged molecules move across the lipid bilayer of the cell by diffusion, the rate of transport determined by the concentration gradients and diffusion coefficients ( Fig. 101.5 ). Oxygen, carbon dioxide, fat-soluble vitamins, and unconjugated bile acids are examples of substances transported by diffusion. Because the majority of ions and solutes cannot cross the phospholipid membrane by diffusion, the cell uses an array of distinct integral membrane proteins, including channels, carriers, and pumps, to cross cell membranes (see Fig. 101.5 ).
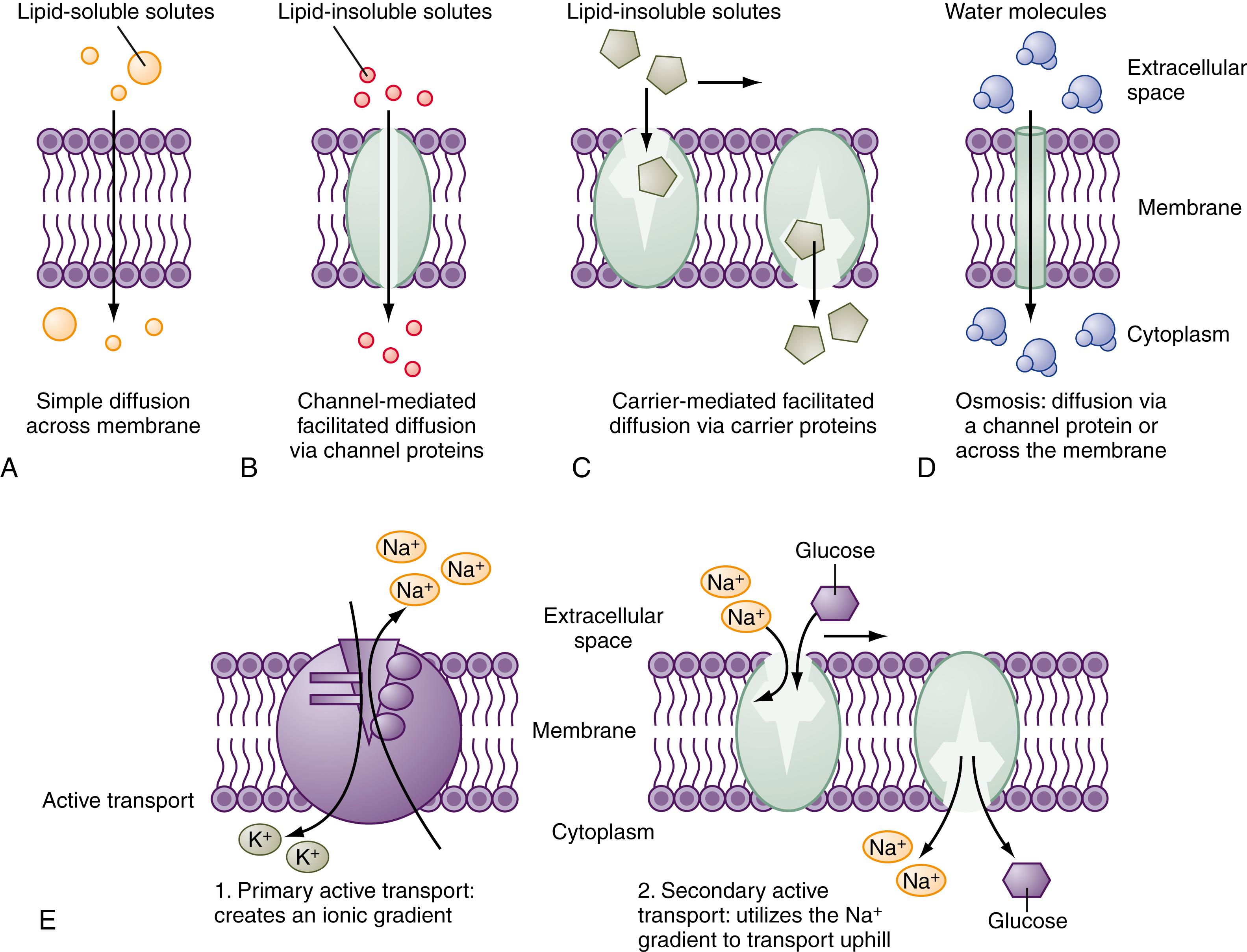
Channels are pores that allow swift (>10 6 ions/sec) and controlled (by rapid opening and closing) transit of ions across the membrane, driven by an electrochemical gradient. The advent of molecular cloning techniques, patch clamp methodology (which allows measurement of single-channel function), membrane protein crystallography, and, more recently, electron cryomicroscopy have greatly advanced our knowledge of how these proteins function. Channels tend to be ion selective. For example, Na + channels exclude K + despite its same charge and smaller size. Selectivity is determined by the hydration radius of the ion and the physiochemical nature of the pore. Overall transport of a particular ion is determined by the electrochemical gradient, density of channels, and gating (open-close time) of the channel; gating may be modulated by voltage or ion concentration or by intracellular regulation. Mutations of critical residues in the channel protein can have dire functional consequences; for example, in CF, specific mutations of the CFTR affect the ability to transport Cl − and HCO 3 − .
Carriers are another class of integral membrane proteins responsible for transport of ions and solutes, but at rates several orders of magnitude lower than channels. Carrier-mediated transport exhibits substrate specificity, saturation, and inhibitory kinetics. Carriers undergo a series of sequential conformational changes to facilitate transport of substrates across a membrane. When concentration or electrochemical gradients drive carrier-mediated transport, the process is downhill and is termed facilitated diffusion. For example, fructose enters the enterocyte via GLUT5, is rapidly isomerized to glucose, and the resulting downhill gradient for fructose allows for facilitated diffusion.
In contrast, other carriers harness the electrochemical energy established by the downhill movement of a second ion, usually Na + , to move a solute or another ion uphill. This process is termed secondary active transport because the specific gradient is indirectly created by a distinct energy-using process. For example, glucose uptake via apical membrane SGLT is driven by the Na + gradient generated by the basolateral Na + /K + -ATPase. Carriers exhibit substrate specificity, so SGLT transports d -glucose but not l -glucose. Equally important, carriers can transport single or multiple substrates and perform the transport in different directions. Uniporters , such as GLUT2 in the BLM, transport one type of substrate, hexoses. Symporters , such as the NKCC, move Na + /K + , and Cl − in the same direction, whereas anti-porters , such as the Na + /H + exchangers, move the 2 ions in opposite directions.
Pumps are the third class of integral membrane proteins and directly use energy, generally ATP hydrolysis, to move ions against an electrochemical gradient. This process is termed primary active transport. Although the Na + /K + -ATPase is the quintessential pump, the luminal gastric and colonic H + /K + -ATPases and the basolateral Ca 2+ -ATPases are also important in GI epithelial transport.
In the GI tract, the surface epithelial cells of the distal colon and rectum exhibit electrogenic Na + absorption against a fairly steep concentration gradient. The downhill electrochemical gradient created by the Na + pump drives Na + entry via an apical membrane Na + /specific ion channel ( Fig. 101.6 ) that belongs to the family of epithelial Na + channels (ENaCs). The ENaCs are multimeric proteins composed of α, β, and γ subunits, exhibit a high sensitivity to the diuretic amiloride, and are stimulated by mineralocorticoids and cAMP by increasing the synthesis and exocytosis of channels, respectively. Colonic ENaCs are inhibited by increases in intracellular Ca 2+ . Aldosterone or cAMP further increase cell membrane ENaC by blocking the degradation pathway for ENaC. Gain of function mutations in ENaC causes inappropriate increases in Na + and fluid retention as seen in Liddle syndrome, whereas loss of function mutations results in a reduction in Na + absorption and plays a role in inflammatory diarrhea.
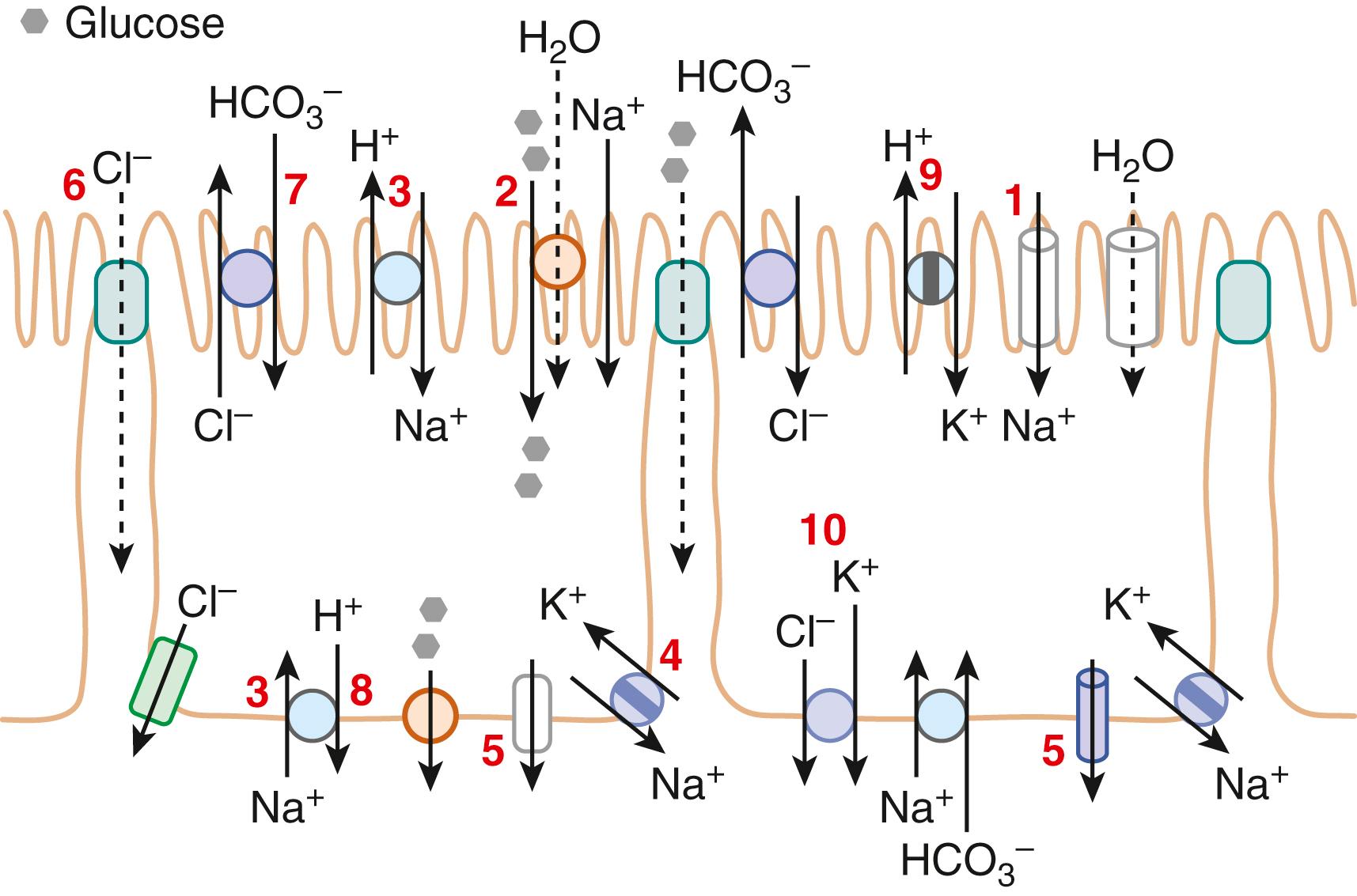
Nutrient transporters largely are found in the small intestine. Transport of many hydrophilic nutrients, including glucose, amino acids, and some vitamins occurs against their concentration gradients via secondary active transport at the apical membrane and facilitated diffusion across the BLM.
Glucose transport processes, elegantly elucidated by Wright and coworkers, provide a good example of nutrient transport. Transport across SGLT1 is electrogenic (2 Na + to 2 glucose), stereospecific ( d -isomer), and transports galactose but not fructose. Glucose exit across the BLM occurs via a separate family of facilitated diffusion carriers, the glucose transporters (GLUT2) (see Fig. 101.6 ). Fructose enters the cell via another member of this family (GLUT5) and exits via GLUT2.
Although it is clear that mucosal-to-serosal Na + and glucose movement stimulates water absorption, there are multiple underlying mechanisms. The classic explanation is that basolateral exit of glucose creates a hypertonic compartment in the paracellular space, thereby generating an osmotic gradient for fluid entry from the lumen. As discussed under “Water Movement,” SGLT can transport water (9000 molecules/sec) and could account for about 5 L of fluid reabsorption in the fed state. Transcellular transport via SGLT also triggers contraction of the actomyosin ring in the terminal web (described in Fig. 101.2 ), resulting in increased paracellular permeability to glucose and to water. Additionally, SGLT activation results in a protein kinase-dependent recruitment of GLUT2 to the apical membrane, which then serves as a high-capacity, low-affinity route for sugar entry during feeding. More recently it has been shown that SGLT activity increases the brush border recruitment and activity of the Na + /H + exchanger-3, NHE3, thereby increasing Na + and water absorption. The efficacy of using glucose-based solutions in ORT in the treatment of diarrheal diseases is based on the critical observation that SGLT activity is not affected by the secretagogue second messengers, cAMP or cyclic guanosine monophosphate (cGMP) (see “Sodium-Hydrogen Exchangers” and “Secretory Factors”).
For a description of similar advances made in our understanding of amino acid and vitamin transport, see Chapter 102, Chapter 103 and Chapter 102, Chapter 103 .
Exchange of extracellular Na + for intracellular H + is a process driven by the electrochemical gradient for Na + and by a pH gradient that results from a moderately acidic intracellular environment; this process occurs in almost every cell. In mammalian intestine, members of the Na + /H + exchange (NHE) gene family play an important role in electroneutral Na + absorption. This process may be down-regulated during eating and increases post-prandially after nutrient absorption.
Of the 10 mammalian isoforms of NHE that have been cloned, NHE1-4 and 6-9 exhibit species- and segment-specific distribution in the GI tract, whereas NHE5 and NHE10 are not expressed. The isoforms vary in their cellular localization with NHE1-5 and NHE8 primarily in the plasma membrane, and the remainder on intracellular membranes. NHE1 is a ubiquitous protein, expressed on epithelial BLMs, and functions as the housekeeper regulator of intracellular pH, cell volume, and growth. NHE2, NHE3, and NHE8 are apical membrane proteins restricted to epithelia and are the major conduits for electroneutral Na + absorption in the intestine and proximal colon (see Fig. 101.6 ). Further, NHE3 is expressed only in the villus or surface cells, and not in the crypts. NHE4 is located in the BLM, and was primarily ascribed a role in gastric parietal and chief cell function. Recent studies report a role for NHE4 also in the colon; NHE4 is involved in the modulation of intracellular pH in human colon and it is stimulated by aldosterone. 56, 57 The luminal-membrane NHE8 plays an important role during intestinal epithelial development and in goblet cell function in the colon. Epidermal growth factor (EGF) reduces basal transcription of NHE8 and might be critical in regulating NHE expression during intestinal maturation. The roles of intestinal NHE6, 7, and 9 remain to be determined. Genetic knock-out studies have highlighted the relevance and importance of various isoforms of NHE in intestinal Na + absorption. Thus, NHE3 −/− mice exhibited severe diarrhea and mild metabolic alkalosis, underscoring the importance of NHE3 in salt absorption. However, NHE2 −/− mice showed normal intestinal NHE activity, with enhanced NHE3 expression, but demonstrated gastric abnormalities. In NHE8 −/− mice, intestinal NHE activity was unaffected but the expression and activity of the anion exchanger, DRA (down-regulated in adenoma), was altered in the stomach and colon. Thus, multiple levels of regulation are involved in transporter expression and function.
NHE activity is differentially modulated by neural, paracrine, or endocrine stimuli through intricate scaffolding complexes that include the exchanger itself and a family of NHE regulatory factors (NHERFs) that act as a bridge between the exchanger and a variety of kinases, phosphatases, and other transporters. Different stimuli use differing scaffolding complexes to exert their effect. Glucocorticoids stimulate Na + absorption and up-regulate NHE3 and NHE8 65 but not NHE1, 2, or 4, consistent with their respective roles in vectorial transport and housekeeping. Glucocorticoids act via a serum- and glucocorticoid-inducible kinase, SGK1; SGK1 stimulates the activity of NHE3 by interacting directly with NHERF2.
Alternatively, increases in cAMP (as seen in cholera), cGMP (as seen in traveler’s diarrhea), or Ca 2+ (as seen in rotavirus-induced diarrhea) inhibit NHE3 activity. For cAMP-dependent inhibition, protein kinase A (PKA), which is recruited to the C-terminus of NHE3 by NHERF1, NHERF2, and the PKA anchoring protein, ezrin induces its inhibitory effect by phosphorylating NHE3 at Ser 554 and Ser 607 . For cGMP-dependent inhibition, agents such as E. coli heat-stable toxin A or guanylin activate brush border membrane guanylate cyclase C (GUCY2C) to increase cGMP which triggers the formation of a complex in the brush border membrane between cGMP-dependent protein kinase II (cGKII), NHERF2 and the cGKII anchor protein (GKAP) ( Fig. 101.7 ) leading to the phosphorylation of Ser 554 , Ser 607 , and Ser 663 . Calcium activates Ca 2+ /CAMKII and phosphorylates Ser 693 , Ser 694 , and Ser 810 to decrease NHE3 activity. All these intracellular messengers use NHERF/PK complexes to decrease NHE3 turnover, whereas cAMP and cGMP decrease NHE3 expression.
![Fig. 101.7, Second messengers: cAMP and cGMP. Five steps are involved in transduction of an external signal into a change in cellular function: (1) binding of either a stimulatory or an inhibitory agonist to an appropriate receptor of the transmembrane adenylate cyclase (tmAC) or membrane guanylate cyclase (GUCY2C) systems; (2) binding of the ligand to the receptor modulates cyclase activity, either within the same molecule in the case of GUCY2C or by activating the corresponding membrane-bound heterotrimeric guanine nucleotide regulatory proteins (heterotrimeric G proteins) in the case of tmACs; Cyclic nucleotides can also be generated by soluble AC (sAC) or GC (sGC) activated by Ca 2+ and NO, respectively; (3) an intracellular signal results from production of cAMP from ATP and cGMP from GTP; (4) an increase in [cAMP] i (intracellular cAMP concentration) activates protein kinases such as PKA, and an increase in [cGMP] i activates protein kinases such as PKG II, which is fixed to the membrane by myristoylation or soluble PKGI (not shown); involvement of kinase-anchoring proteins such as A kinase-anchoring proteins (AKAPs) and G kinase-anchoring proteins (GKAPs) has been demonstrated during the signaling; (5) protein kinase phosphorylation of specific target proteins results in a change in the activity of channels or transporters such as the CFTR chloride channel or the Na + /H + exchanger. (For easy explanation, steps 1 to 5 are drawn apart.) In the intact cell, the molecules described in steps 1 to 5 (receptors, cyclase, kinase, anchoring protein, and target transporter) may be in close proximity, allowing for spatial and localized regulation. In cAMP signaling, binding of stimulatory regulators (e.g., VIP, prostaglandins) to specific membrane receptors causes activation. Activated receptors couple via G s with tmACs to catalyze conversion of ATP to cAMP, which then activates specific cAMP kinases. An inherent GTPase returns G s to its nascent state; cholera toxin prevents this occurrence by covalently modifying G s , leaving enterocyte turnover as the only recourse for returning the tissue to its basal state. Other hormones, such as somatostatin, trigger the activation of inhibitory G proteins (G i ) to decrease cAMP. The tmAC cascade is localized to the BLM of epithelial cells. Soluble adenylate cyclase (sAC) is not dependent on heterotrimeric G proteins and can be activated by HCO 3 − and Ca 2+ . The sACs are present in the cytosolic, mitochondrial and nuclear compartments. In cGMP signaling, cGMP is generated by the activation of membrane or soluble guanylate cyclases (GCs). In contrast to the adenylate cyclases, membrane GCs are single-pass transmembrane proteins for which the extracellular domain serves as the receptor-binding domain and the intracellular domain catalyzes conversion of GTP to cGMP. Thus, the GCs are specific for their ligands, which include the endogenous atrial natriuretic peptides, guanylin, and uroguanylin, as well as enterotoxins such as the heat-stable enterotoxin of Escherichia coli . The intestinal cGMP protein kinase is tethered to the membrane via a myristoylated N-terminal region. The soluble GCs are the target of NO activation; they are minimally expressed in the small intestinal epithelium, but they are present in colonic epithelium, subepithelial elements, and smooth muscle, where they cause muscular relaxation. ATP , adenosine triphosphate; cAMP , cyclic adenosine monophosphate; cGMP , cyclic guanosine monophosphate; G i , inhibitory G protein; G s , stimulatory G protein; GTP , guanosine triphosphate; NO , nitric oxide; PKA , protein kinase A; PKG , protein kinase G; STa , heat-stable toxin; VIP , vasoactive intestinal peptide. Fig. 101.7, Second messengers: cAMP and cGMP. Five steps are involved in transduction of an external signal into a change in cellular function: (1) binding of either a stimulatory or an inhibitory agonist to an appropriate receptor of the transmembrane adenylate cyclase (tmAC) or membrane guanylate cyclase (GUCY2C) systems; (2) binding of the ligand to the receptor modulates cyclase activity, either within the same molecule in the case of GUCY2C or by activating the corresponding membrane-bound heterotrimeric guanine nucleotide regulatory proteins (heterotrimeric G proteins) in the case of tmACs; Cyclic nucleotides can also be generated by soluble AC (sAC) or GC (sGC) activated by Ca 2+ and NO, respectively; (3) an intracellular signal results from production of cAMP from ATP and cGMP from GTP; (4) an increase in [cAMP] i (intracellular cAMP concentration) activates protein kinases such as PKA, and an increase in [cGMP] i activates protein kinases such as PKG II, which is fixed to the membrane by myristoylation or soluble PKGI (not shown); involvement of kinase-anchoring proteins such as A kinase-anchoring proteins (AKAPs) and G kinase-anchoring proteins (GKAPs) has been demonstrated during the signaling; (5) protein kinase phosphorylation of specific target proteins results in a change in the activity of channels or transporters such as the CFTR chloride channel or the Na + /H + exchanger. (For easy explanation, steps 1 to 5 are drawn apart.) In the intact cell, the molecules described in steps 1 to 5 (receptors, cyclase, kinase, anchoring protein, and target transporter) may be in close proximity, allowing for spatial and localized regulation. In cAMP signaling, binding of stimulatory regulators (e.g., VIP, prostaglandins) to specific membrane receptors causes activation. Activated receptors couple via G s with tmACs to catalyze conversion of ATP to cAMP, which then activates specific cAMP kinases. An inherent GTPase returns G s to its nascent state; cholera toxin prevents this occurrence by covalently modifying G s , leaving enterocyte turnover as the only recourse for returning the tissue to its basal state. Other hormones, such as somatostatin, trigger the activation of inhibitory G proteins (G i ) to decrease cAMP. The tmAC cascade is localized to the BLM of epithelial cells. Soluble adenylate cyclase (sAC) is not dependent on heterotrimeric G proteins and can be activated by HCO 3 − and Ca 2+ . The sACs are present in the cytosolic, mitochondrial and nuclear compartments. In cGMP signaling, cGMP is generated by the activation of membrane or soluble guanylate cyclases (GCs). In contrast to the adenylate cyclases, membrane GCs are single-pass transmembrane proteins for which the extracellular domain serves as the receptor-binding domain and the intracellular domain catalyzes conversion of GTP to cGMP. Thus, the GCs are specific for their ligands, which include the endogenous atrial natriuretic peptides, guanylin, and uroguanylin, as well as enterotoxins such as the heat-stable enterotoxin of Escherichia coli . The intestinal cGMP protein kinase is tethered to the membrane via a myristoylated N-terminal region. The soluble GCs are the target of NO activation; they are minimally expressed in the small intestinal epithelium, but they are present in colonic epithelium, subepithelial elements, and smooth muscle, where they cause muscular relaxation. ATP , adenosine triphosphate; cAMP , cyclic adenosine monophosphate; cGMP , cyclic guanosine monophosphate; G i , inhibitory G protein; G s , stimulatory G protein; GTP , guanosine triphosphate; NO , nitric oxide; PKA , protein kinase A; PKG , protein kinase G; STa , heat-stable toxin; VIP , vasoactive intestinal peptide.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/IntestinalElectrolyteAbsorptionandSecretion/4_3s20B9780323609623001016.jpg)
The important role of scaffolding proteins in regulating NHE is highlighted by studies on NHERF2-null mice which demonstrate that NHERF2 is necessary to maintain basal NHE3 activity, its stimulation by lysophosphatidic acid (LPA), and its inhibition by cAMP, cGMP, and intracellular calcium ([Ca 2+ ] i ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here