Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
apical membrane vesicles
ascorbic acid
basolateral membrane
basolateral membrane vesicles
brush-border membrane
brush-border membrane vesicles
Ca/calmodulin
chromatin immunoprecipitation
cobalamin
conditional Knockout
dehydro- l -ascorbic acid
4,4′-diisothiocyano-2,2′-disulfonic acid stilbene
electromobility shift assay
enhanced green fluorescent protein
enteropathogenic Escherichia coli
enterotoxigenic Escherichia coli
flavin adenine dinucleotide
flavin mononucleotide
glucose transporter-2
glucose transporter-8
glutathione S -transferase
hereditary folate malabsorption syndrome
heterogeneous nuclear ribonucleic acid
inflammatory bowel disease
interferon-γ
microribonucleic acid
nicotinamide adenine dinucleotide
nicotinamide adenine dinucleotide phosphate
protein kinase-A
protein kinase-C
protein tyrosine kinase
proton-coupled folate transporter
PDZ domain containing 11
reduced folate carrier
riboflavin
riboflavin vitamin transporter-2
riboflavin vitamin transporter-3
riboflavin vitamin transporter-1
small interfering ribonucleic acid
sodium-dependent multivitamin transporter
sodium-dependent vitamin C transporter-1
sodium-dependent vitamin C transporter-2
tumor necrosis factor-α
thiamin pyrophosphate
thiamin pyrophosphate transporter
thiamin-responsive megaloblastic anemia
thiamin transporter-1
thiamin transporter-2
tight junction
transcobalamin
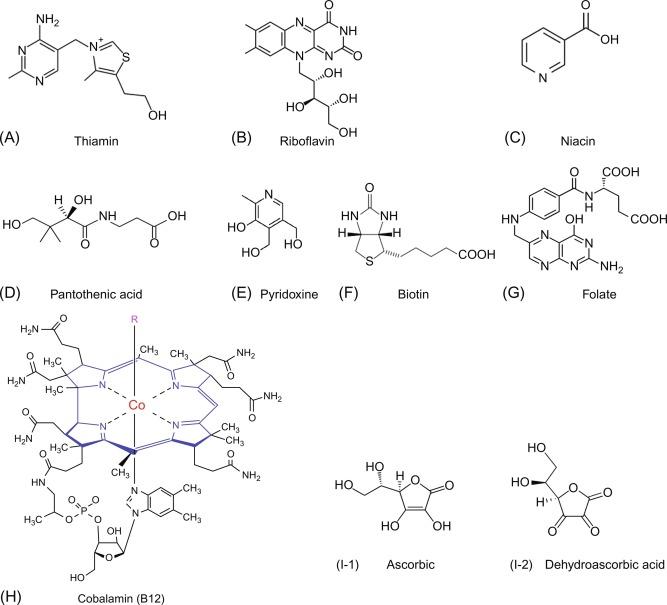
The water-soluble vitamins represent a group of structurally and functionally unrelated compounds ( Fig. 54.1 ) that share the common feature of being essential for normal cellular functions, growth and development. With the exception of some endogenous synthesis of vitamin B 3 (niacin), human cells cannot synthesize these micronutrients, and thus, must obtain them from exogenous sources via intestinal absorption. The intestine, therefore, plays a critical role in maintaining and regulating normal body homeostasis of these essential micronutrients. Interference with the normal intestinal absorption process of these vitamins leads to sub-optimal/deficiency states and development of metabolic derangments and clinical abnormalities. Impairment in intestinal absorption of water-solbule vitamins occurs in a variety of conditions including gentic defects (mutations) in the digestive or absorptive systems involved, intestinal diseases, chronic alcohol use, infection with certain enteropathogens, and drug-vitamin interaction.
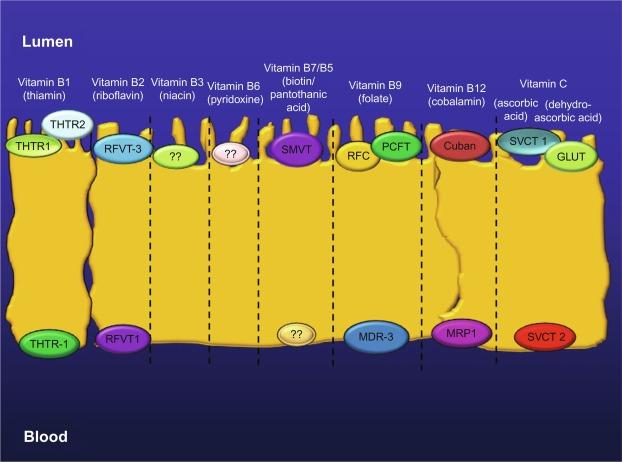
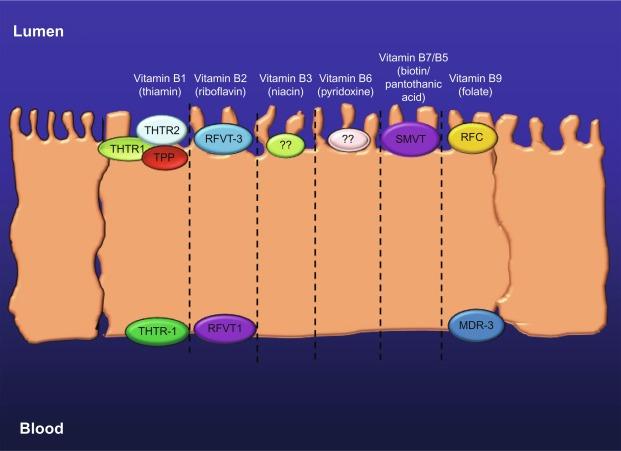
Significant progress has been made in recent years in our understanding of the cellular and molecular mechanisms involved in intestinal absorption of water-soluble vitamins and how these events are regulated. We now know that absorption of physiological concentrations of all these micronutrients is mediated via specific carrier-mediated mechanisms. In many cases the molecular identity of the systems involved ( Fig. 54.2 ), their cell biology, and their regulation have been delineated. We also know now that the large intestine possess specialized carrier-mediated systems for the absorption of the microbiota-generated water-soluble vitamins ( Fig. 54.3 ) and that this source of vitamins controbute toward overall host nutriton, and especially toward the cellular nutrition of the local colonocytes. Our aim in this chapter is to provide a comprehensive description of the current understanding of the physiology and cellular/molecular biology of intestinal absorption of water-soluble vitamins, and how external/environmental conditons and internal factors affect and interfer with these events.
Thiamin ( Fig. 54.1 ) was the first member of the family of water-soluble vitamins to be described, with reference to the effects of its deficiency (beriberi) being suggested some 4000 years ago in old Chinese medical literatures. Thiamin (mainly in its pyrophosphate forms) acts as an enzymatic cofactor for transketolase, pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and branched-chain ketoacid dehydrogenase, enzymes involved in many critical metabolic reactions that relate to energy metabolism. Because thiamin bridges the glycolytic and the pentose phosphate metabolic pathways (which generate chemical-reducing power in cells), it also plays an important role in reducing cellular oxidative stress. Thus, low intracellular levels of thiamin will lead to impairment in energy metabolism (acute energy failure) and to a propensity for oxidative stress. While most of thiamin’s biological activity is attributed to its pyrophosphate form (i.e., thiamin pyrophosphate, TPP, which is also the most abundant form of this vitamin in cells and accumulates mainly in mitochondria), recent studies have shown that other forms of the vitamin, namely, thiamin triphosphate, also possess biological activity in nerve cells where it regulates the function of membrane chloride channels and acts as a phosphate group donor to other membrane proteins (Ref. and references therein). Thiamin deficiency (and suboptimal levels) represents a significant nutritional problem worldwide and leads to several clinical abnormalities including neurological (neuropathy and/or Wernicke-Korsakoff syndrome) and cardiovascular (peripheral vasodilatation, biventricular myocardial failure, edema, and potentially acute fulminant cardiovascular collapse) disorders. On the other hand, optimization of thiamin levels appears to have the potential of preventing diabetic retinopathy and tissue damage caused by the hyperglycemia of diabetes and alleviating fatigue in patients with inflammatory bowel diseases (IBDs). Systemic thiamin deficiency and suboptimal levels occur in a high percentage of chronic alcoholics, diabetics, patients with celiac sprue and renal diseases, post bariatric surgery, patients with sepsis, and in subjects on long-term furosemide diuretic therapy. In addition, thiamin deficiency and suboptimal levels occur in the elderly population despite an average daily intake of the vitamin that exceeds their recommended requirement. Also recognized is the occurrence of thiamin deficiency disorders that occur despite normal plasma level of this vitamin. Examples of the latter are thiamin-responsive megaloblastic anemia (TRMA) and thiamin-responsive/Wernicke’s-like encephalopathy. TRMA is an autosomal recessive disorder characterized by megaloblastic anemia, sensorineural deafness, and non-type 1 diabetes mellitus, which is caused by mutations in the human thiamin transporter-1 (hTHTR-1; product of the SLC19A2 gene). Such mutations in hTHTR-1 gene led to an impairment in thiamin uptake (by the affected tissues) and the development of localized thiamin deficiency. Thiamin-responsive/Wernicke’s-like encephalopathy is characterized by seizures, ophthalmoplegia, nystagmus, and ataxia and is believed to be due to mutations in the human thiamin-transporter-2 (hTHTR-2; product of the SLC19A3 gene). In both of the latter disorders, oral administration of pharmacological doses of thiamin brings about significant improvements in many of the clinical symptoms of the affected individuals.
The human intestine encounters thiamin from two sources: a dietary source and from microbiota. Dietary thiamin exists mainly in the phosphorylated form, which must be hydrolyzed to free thiamin prior to absorption (the small intestine does not have a transport system for intact TPP, that is, it is unlike the large intestine that has such a system; see below), a process that is achieved by the action of the abundant intestinal phosphatases. Absorption of the liberated free thiamin (which exists in the monocationic form at gut luminal pH) then takes place mainly in the proximal part of the small intestine. The mechanism of thiamin absorption in the small intestine has been investigated using a variety of intestinal preparations from different species including human. Collectively, these studies have shown the involvement of a specific carrier-mediated mechanism that is inhibited by thiamin structural analogues, but not by unrelated organic cations. Studies using purified brush-border membrane vesicles (BBMV) isolated from human and animal small intestine have shown the involvement of a pH (but not Na + )-dependent, electroneutral, and amiloride-sensitive carrier-mediated mechanism for thiamin uptake. The mechanism by which thiamin exits out of enterocytes, that is, transport across the basolateral membrane (BLM), has also been studied using purified human and animal intestinal basolateral membrane vesicles (BLMV) and has been found to involve a specific, pH-dependent, electroneutral, carrier-mediated process. With regard to potential metabolism during absorption, studies have shown that some of the absorbed free thiamin is rephosphorylated (mainly to TPP) in intestinal epithelial cells; however, only free thiamin exits the intestinal absorptive cells into the serosal side. There is recent emerging evidence that indicates that the intracellularly generated TPP accumulates in mitochondria via a carrier-mediated process and this important process has major relevance to intestinal energy metabolism in health and disease.
As to the microflora-generated thiamin, this source provides considerable amounts of free and phosphorylated (TPP) forms of the vitamin. While gut microbiota of all human generate thiamin, recent studies that classified the human gut microbiota into three functionally different enterotypes (enterotypes 1, 2, and 3) have reported that enterotype 2 (which is enriched in Prevotella and Desulfovibrio ) is especially overrepresented in enzymes that are involved in TPP biosynthesis, implying a larger level of thiamin generation in the group that harbors this enterotype. As to absorbability of the microbiota-generated forms of thiamin in the large intestine, recent investigations have shown that the human colonocytes do indeed possess an efficient and specific carrier-mediated mechanism for uptake of free thiamin and a separate and specific carrier-mediated mechanism for uptake of intact TPP. The latter system was shown to have high affinity (apparent K m of 0.157 μM) and specificity for TPP (it does not transport free thiamin or thiamin monophosphate), is pH- and Na + -independent, and energy-dependent. It is also interesting to note that the gene that encodes the colonic TPP transporter (TPPT) (i.e., SLC44A4; see below) has recently been identified as an ulcerative colitis susceptibility gene. Based on the mentioned evidence, it is clear that the microbiota-generated thiamin is absorbable and contributes to overall thiamin nutrition, especially when it come to cellular nutrition and health of the local colonocytes.
Mammalian cells appear to utilize two members of the solute carrier SLC19A gene family (i.e., THTR-1 and THTR-2) to transport free thiamin and one to transport TPP. The SLC19A2 encodes a protein of 497 amino acids (THTR-1), while the SLC19A3 encodes a protein of 496 amino acids (THTR-2). Both of the human THTR-1 and -2 are predicted to have 12 putative transmembrane domains (TMDs) with their N- and C-terminal tails extending into cell interior. The hTHTR-1 and hTHTR-2 share 48% identity and 64% similarity with one another. They also share 40% and 39% identity at the amino acid level, respectively, with the human reduced folate carrier (hRFC). However, neither hTHTR-1 nor hTHTR-2 transport folate and the hRFCs do not transport free thiamin.
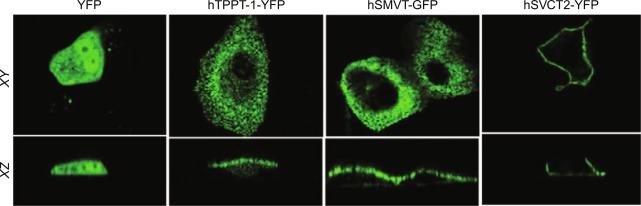
The hTHTR-1 appears to function in the micromolar range, while the hTHTR-2 appears to function in the nanomolar range. Both the hTHTR-1 and hTHTR-2 are expressed in the human small and large intestine, with expression of the former being markedly higher than that of the latter. The membrane domain of the polarized human enterocyte at which hTHTR-1 and hTHTR-2 proteins are expressed have also been investigated using immunological and confocal imaging approaches. Expression of the hTHTR-1 protein was shown to be at both the apical and BLM domains of the polarized enterocytes, with a slightly higher expression at the latter compared to the apical membrane domain. On the other hand, expression of the hTHTR-2 protein was shown to be restricted to only the apical membrane domain of the polarized absorptive epithelia.
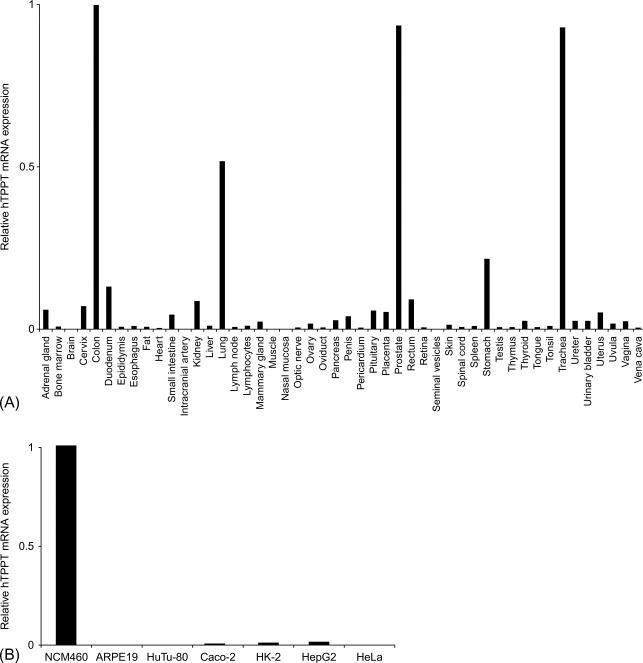
As to the molecular identity of the colonic TPPT, this has been recently delineated in studies by Nabokina et al., which showed that the uptake system is the product of the SLC44A4 gene. Expression of the human TPPT along the intestinal tract was found to be restricted to the colon ( Fig. 54.4 ). Also, cell surface biotinylation assay and live-cell confocal imaging studies have shown that the human TPPT is exclusively expressed at the apical membrane domain of polarized epithelial cells ( Fig. 54.5 ). The tissue-specific expression of the TPPT in the colon appears to be determined by epigenetics as well as microribonucleic acid (miRNA)-mediated mechanisms. The latter was suggested by findings that in the colon, histone H3 in the 5′-regulatory region of Slc44a4 is trimethylated at lysine 4 and acetylated at lysine 9, whereas the trimethylation at lysine 27 was negligible. In the jejunum, on the other hand, histone H3 was found to be hypertrimethylated at lysine 27 (repressor mark). Involvement of miRNA(s) in the tissue-specific expression of TPPT was suggested by findings showing the 3′-UTR of SLC44A4 to be the target of specific miRNAs/RNA-binding proteins in noncolonic, but not in colonic, epithelial cells.
Since the hTHTR-1 and -2 are both expressed in intestinal epithelial cells, it was important to understand their relative contribution toward total carrier-mediated intestinal thiamin uptake. This was done with the use of two approaches: (i) selective knockdown of hTHTR-1 and -2 with gene-specific small-interfering RNAs (siRNAs) and (ii) use of THTR-1 and -2-knockout mouse models. Results of the first approach showed that both the hTHTR-1 and -2 are involved in intestinal thiamin uptake and that together they account for total carrier-mediated thiamin uptake across the apical membrane of human intestinal epithelial cells. Results of the second approach showed a significant and specific reduction in thiamin uptake by the intestine in the THTR-2-knockout mice compared to uptake by the intestine of their sex-matched wild-type littermate. This was also associated with a significant reduction in blood thiamin levels in the knockout animals when compared to the control group. However, thiamin uptake by the intestine in THTR-1-knockout mice was not significantly different from that of their sex-matched wild-type littermates. Furthermore, the level of expression of THTR-1 was not altered in the intestine of THTR-2-knockout mice, while the level of expression of THTR-2 was markedly upregulated in the intestine (and kidney) of THTR-1-knockout mice. These findings show that THTR-2 is required for normal thiamin uptake in the intestine. The data also provided an explanation as to why patients with TRMA (who have a mutated and dysfunctional hTHTR-1) have normal plasma levels of thiamin. This is most likely due to an induction in the level of expression of hTHTR-2 in the intestine (and kidney), which leads to a higher intestinal uptake (and renal reuptake) of thiamin.
Insight into the mechanisms involved in intracellular trafficking and membrane targeting of the human thiamin transporters in epithelial cells have also been emerging from studies utilizing the live-cell confocal imaging approach. Using a series of truncated hTHTR-1 constructs fused with the enhanced green fluorescent protein (EGFP) (i.e., hTHTR1-EGFP), expression of the full-length fusion protein was shown to be at both the apical and the BLM domains of intestinal epithelial cells. Analysis of the localization pattern of truncated mutants of the hTHTR-1 has shown that while the C-terminus region of the polypeptide does not appear to have a role in membrane targeting, whereas an essential role for the N-terminal and the backbone was found. Also, truncation of the hTHTR-1 polypeptide within a region where several TMRA truncations are clustered results in intracellular retention of the mutant protein. As to intracellular trafficking, the hTHTR-1 protein was found to reside inside numerous trafficking vesicles whose movement is temperature dependent and requires an intact microtubule network for their mobility (Ref. ; real-time movies can be viewed at website: http://www.jcb.org/cgi/content/full/278/6/3976/DC1 ).
With regard to the hTHTR-2 protein, this carrier is expressed exclusively at the apical membrane domain of the polarized intestinal epithelial cells. Live-cell imaging investigations utilizing a series of hTHTR-2 truncations have shown that while the cytoplasmic C-terminal is not essential for membrane targeting of the protein, an essential role for the transmembrane backbone is evident. In addition, trafficking vesicles were found to be involved in the intracellular movement of hTHTR-2 to the cell surface, and that mobility of these vesicles depends on an intact microtubule network that was disrupted by overexpression of the dynactin subunit dynamitin (p50) (dynactin is involved in trafficking of vesicles via conventional cytoplasmic dynein, a minus end-directed motor protein).
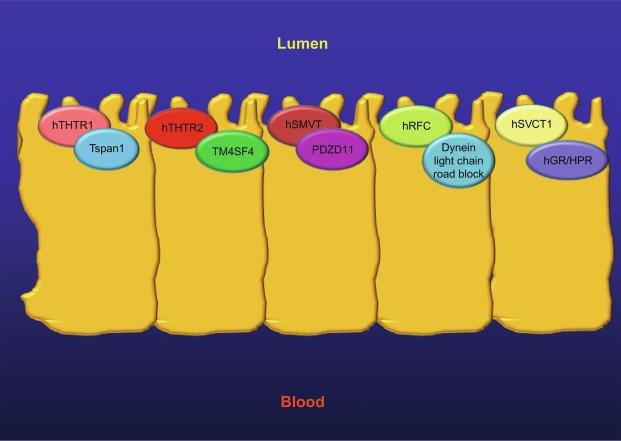
Other studies have identified accessory proteins that influence the functionality and cell biology of the hTHTR-1 and -2 in intestinal epithelial cells ( Fig. 54.6 ). Using a bacterial two-hybrid system to screen a human intestinal cDNA library with the complete coding sequence of hTHTR-1 as bait, studies have identified a member of the tetraspanin family of proteins, Tspan-1, as an interacting partner with hTHTR-1. Coimmunoprecipitation assay, glutathione S -transferase (GST)-pulldown assay, and live-cell confocal imaging have confirmed the existence of such an interaction between hTspan-1 and hTHTR-1 in human intestinal epithelial Caco-2 cells. This interaction appears to have biological and functional consequences as it decreased the rate of degradation of the hTHTR-1 protein, and thus, influenced its functionality. More recent investigations have utilized a yeast split-ubiquitin two-hybrid approach to identify the human transmembrane 4 superfamily 4 (hTM4SF4) as an interacting partner of hTHTR-2 in human intestinal epithelial cells ( Fig. 54.6 ). This interaction was again confirmed by GST-pull-down assay in vitro , by live-cell confocal imaging in intestinal epithelial cells, and shown to have functional consequences on the hTHTR-2 activity.
Knowledge about the structure-function activity of the hTHTR-1 and hTHTR-2 proteins has been emerging in recent years from clinical and experimental findings. With regard to the hTHTR-1, over 18 different clinical mutations have been identified in patients with TRMA. These mutations are either mis-sense or nonsense in nature with the latter type leading to an early truncation of the carrier protein. The effect of a number of these mutations (e.g., P51L and T158R, W358X, and Delta383fs) on functionality of the hTHTR-1 has been investigated experimentally using mutants generated by site-directed mutagenesis followed by expression in intestinal and other appropriate cell types. The results revealed a spectrum of mutant phenotypes with all exhibiting impaired thiamin uptake function due to either a change in hTHTR-1 level of expression/stability, mis-targeting, and/or altered transport activity. Other studies have examined the role of the only conserved anionic amino acid residue (located at position 138) in the predicted TMDs of hTHTR-1 in the transport of the cationic thiamin, as well as the role of the two putative N-glycosylation sites (predicted to be at positions 63 and 314). The result showed a critical role for the residue at position 138 in the function of hTHTR-1. On the other hand, neither of the putative glycosylation sites was found to play a role in the function or membrane targeting of the hTHTR-1 protein.
As to the hTHTR-2 protein, again data from clinical and experimental studies have shed light into important aspects of its structure-activity relationship. Two clinical mutations (K44E and E320Q) in hTHTR-2 have been described in patients with thiamin-responsive Wernicke’s-like encephalopathy. Experimental testing of these two mutants showed impaired functionality of hTHTR-2 in both cases. The K44E mutant caused thiamin transport dysfunction by impairing hTHTR-2 trafficking to the cell membrane. Another two mutations in the hTHTR-2 protein (G23V and T422A) have been reported in patients with biotin-responsive basal ganglia disease (although it is unclear at present as to why mutations in a specific thiamin transporter precipitates this condition despite the fact that hTHTR-2 does not transport biotin; Ref. ). Both of these mutations in hTHTR-2 were found to cause functional impairment, with no defect in the expression of this transporter at cell membrane. It is interesting to mention here that both of the affected residues (i.e., at positions 23 and 422) are conserved in the hTHTR-2 sequences of all species cloned so far, as well as in other members of the SLC19A gene family, that is, the hTHTR-1 and the (hRFC; product of the SLC19A1 gene), thus, supporting an essential role of these residues in transporter functionality. Hydropathy analyses suggested that these residues (G23V in TM1, T422A in TM11) lie within the TMDs of the hTHTR-2 protein. Crystallographic data from other major facilitator superfamily transporters showed that both TM1 and TM11 contribute to the central hydrophilic cavity of the transporters, underscoring the likelihood that mutation of residues within these regions could impair transporter function (reviewed in Ref. ). Other studies have examined the role of the three negatively charged conserved glutamic acid residues within the TMDs of hTHTR-1 and hTHTR-2 (i.e., E120, E320, and E346) as well as the role of the two putative N-glycosylation sites in the hTHTR-2 polypeptide (i.e., N45, N166) in the function and cell biology of the hTHTR-2 protein. Results of these investigations showed that mutating any of the conserved glutamic acid residues lead to a significant impairment in transport of the positively charged thiamin. Furthermore, while mutating E120 and E320 led to a decrease in the level of expression of hTHTR-2 at the apical membrane domain of intestinal epithelial cells, mutating E346 did not affect this parameter. Finally, the two putative glycosylation sites of the hTHTR-2 polypeptide (i.e., N45Q, N166Q) do not appear to affect the functionality or level of expression of the hTHTR-2 in intestinal epithelial cells.
With regard to the colonic TPPT, recent studies have shown that the protein is glycosylated at positions N69, N155, N197, N393, and N416. However, only glycosylation at N69, N155, and N393 appears to be important for function, most likely through an effect on protein conformation and/or interaction with the substrate.
The intestinal thiamin uptake process appears to be under the regulation of a variety of extracellular/environmental conditions and intracellular factors. Since a number of these conditions/factors exert their regulatory effect(s) at the transcription level, it is relevant to first describe the basal transcriptional regulation of the SLC19A2, SLC19A3 , and SLC44A4 genes.
The 5′-regulatory region (promoter) of the SLC19A2 gene was cloned and characterized in human intestinal epithelial Caco-2 cells using the Firefly luciferase reporter gene assay. The minimal promoter region required for basal activity of the SLC19A2 promoter was shown to be encoded in a region between − 356 and − 36 (considering the A of the initiation ATG sequence as position 1) and include multiple putative cis-regulatory elements. A role for a number of these cis-elements (namely, GKLF, NF-1, and SP-1) has also been established in studies involving mutational analysis, oligonucleotide competition assays, and electromobility shift/supershift assays. Functionality and physiological relevance of the full-length and minimal SLC19A2 promoters were confirmed in vivo by expressing constructs of these promoters fused to the Firefly luciferase reporter gene in transgenic mice. The pattern of expression of these promoter constructs in different tissues was found to be similar to the pattern of expression of the hTHTR-1 mRNA in different human tissues. Other investigations have determined the transcription initiation sites of the SLC19A2 gene in intestinal epithelial Caco-2 cells using 5′-rapid amplification of cDNA ends, with three such sites being identified at positions − 183, − 192, and − 220. Finally, studies have reported that the human SLC19A2 promoter is a target for activation by the p53 tumor suppressor transcription factor in murine erythroleukemia cells, but it is not clear if a similar activation also occurs in intestinal epithelial cells.
With regard to the SLC19A3 gene, its promoter has been cloned and characterized in human intestinal epithelial cells in vitro, and its functionality confirmed in transgenic mice in vivo. The transcription initiation site of the SLC19A3 gene was found to be at position − 88, and the minimal promoter region required for basal activity was shown to be encoded in a sequence between − 77 and + 59 and contains a number of putative cis-regulatory elements. A critical role for the stimulating protein-1 (SP1)/guanosine cytidine box (GC-box)-binding site (located at position − 48/−45 bp) was established and both SP1 and SP3 appear to interact with this site. The latter was confirmed in Drosophila SL2 cells (which lack endogenous SP factors), where both SP1 and SP3 were found to transactivate the SLC19A3 minimal promoter in a dose-dependent manner. Functionality of the full-length SLC19A3 promoter was also confirmed in vivo in transgenic mice carrying the human SLC19A3 promoter-luciferase reporter gene, thus, establishing the physiological relevance of the in vitro studies.
As for the SLC44A4 gene, the 5′-regulatory region of this gene has been cloned and characterized in human colonic epithelial NCM460 cells. The minimal region required for basal activity of the SLC44A4 promoter was mapped to a sequence between nucleotides − 178 and + 88. Mutational analysis performed on putative cis-regulatory elements established a role for ETS/ELF3 [E26 transformation-specific sequence (ETS) proteins], cAMP-responsive element (CRE), and SP1/GC-box sequence motifs in basal activity of the SLC44A4 promoter. Binding of ELF3 and CRE-binding protein-1 (CREB-1) transcription factors to the SLC44A4 minimal promoter was also shown by means of electromobility shift assay (EMSA). Contribution of CREB to SLC44A4 promoter activity was confirmed using NCM460 cells overexpressing CREB. A higher expression of ELF3 and CREB-1 in colonic (NCM460) compared with noncolonic cells was also observed, which may suggest possible contribution of these factors to colon-specific pattern of SLC44A4 expression.
The intestinal thiamin uptake process appears to be adaptively regulated by extracellular thiamin levels. Thiamin deficiency in humans leads to an increase in thiamin uptake when compared to control subjects. This effect appears to be mediated via changes in the V max and the apparent K m of the intestinal thiamin uptake process. The finding of adaptive regulation in intestinal thiamin uptake process in thiamin deficiency has been confirmed in studies with mice, and in studies utilizing cultured human intestinal epithelial cells. In mouse studies, dietary thiamin deficiency was found to lead to significant induction in intestinal carrier-mediated thiamin uptake; this was associated with a significant increase in the level of expression of the mTHTR-2, but not the mTHTR-1. In addition, activity of the luciferase reporter gene in transgenic mice carrying the human SLC19A3 promoter-luciferase construct was found to be significantly higher in the intestine of thiamin-deficient mice compared to pair-fed transgenic controls. In contrast, no changes in the activity of the luciferase reporter gene in transgenic mice carrying the human SLC19A2 promoter-luciferase construct were observed during thiamin deficiency. This provides evidence that the adaptive upregulation in intestinal thiamin uptake process in thiamin deficiency is mediated via an induction in the level of expression of THTR-2 and that the induction is, at least in part, mediated via transcriptional mechanism(s). To further confirm the involvement of transcriptional mechanism(s) in the adaptive regulation of intestinal thiamin uptake and to delineate the molecular mechanism(s) involved, human intestinal epithelial cells (Caco-2 cells) were exposed to different levels of extracellular thiamin while transfected with different 5′-truncated promoter-luciferase constructs. These investigations showed that the thiamin level-responsive region in the SLC19A3 promoter is located between − 77 and − 29 (using transcriptional start site as + 1). In addition, a key role for SP1/GC-box in mediating the effect of extracellular thiamin level on SLC19A3 promoter was established. Furthermore, extracellular levels of thiamin were found to affect the level of expression of the SP1 protein and its binding to the thiamin level-responsive region in the SLC19A3 promoter.
It is worth mentioning here that different cells appear to utilize different mechanisms to upregulate their thiamin uptake. Thus, thiamin uptake in the kidney is upregulated in thiamin deficiency via induction in the level of expression of both THTR-1 and THTR-2, while in the brain and heart, the upregulation appears to be mediated via induction in the expression of THTR-1 mainly.
As to the colonic TPP uptake process, this event also appears to be adaptively regulated by extracellular substrate (TPP) level and is mediated, at least in part, at the level of transcription of the SLC44A4 gene.
The intestinal thiamin uptake process is developmentally regulated during the early stages of life. A decrease in thiamin uptake by mouse intestinal BBMV was observed with maturation (suckling to weanling to adult). This decrease was associated with a decrease in the level of expression of both THTR-1 and THTR-2 ; and a decrease in the activity of the human SLC19A2 and SLC19A3 promoters in the intestine of transgenic mice that carry these promoters. These findings suggest that the developmental regulation of intestinal thiamin uptake is mediated, at least in part, via transcriptional mechanism(s).
The intestinal thiamin uptake process also appears to undergo differentiation-dependent regulation. This has been shown in studies utilizing the human intestinal epithelial Caco-2 cells (which differentiate spontaneously in culture upon reaching confluence to become enterocyte-like cells ) and native crypt/villus epithelial cells isolated from wild-type and transgenic mice carrying promoters of the human SLC19A2 and SLC19A3 genes. A significant upregulation in carrier-mediated thiamin uptake by Caco-2 cells was observed as cells differentiate in culture. This upregulation was associated with a significant increase in the level of expression of hTHTR-1 and hTHTR-2 protein and mRNA as well as in activity of the corresponding transfected SLC19A2 and SLC19A3 promoters. Deletion analysis identified the differentiation-responsive region to be at position − 356 to − 275 bp for the SLC19A2 promoter and at position − 77 to − 13 bp for the SLC19A3 promoter. In addition, a critical and specific role in the differentiation-mediated effects for an NF1-binding site (− 348 to − 345 bp) in the SLC19A2 promoter and a SP1/GC-box-binding site (− 48 to − 45 bp) in the SLC19A3 promoter were shown. The physiological relevance of these in vitro findings with Caco-2 cells was confirmed in wild-type and transgenic mice by demonstrating that thiamin uptake and mRNA levels of the mouse THTR-1 and THTR-2, as well as activity of human SLC19A2 and SLC19A3 promoters expressed in transgenic mice, were all significantly higher in intestinal villus compared to crypt epithelial cells. These findings suggest that the differentiation-dependent regulation of intestinal thiamin uptake is, at least in part, mediated via transcriptional mechanism(s).
Studies utilizing human intestinal epithelial Caco-2 cells and the human colonic epithelial NCM460 cells have reported that the intestinal thiamin uptake process is under the regulation of an intracellular Ca 2 + /calmodulin (CaM)-mediated pathway. This pathway appears to act through decreasing the V max (without affecting the apparent K m ) of the intestinal thiamin uptake process, suggesting that the effect is most probably mediated via changes in the activity (and/or number) but not affinity of the thiamin uptake carriers, respectively. The same mode of regulation was also observed for thiamin uptake by other cell types including renal epithelial cells, retinal pigment epithelial cells, and pancreatic beta cells, demonstrating extensive utilization of this regulatory pathway in different tissues.
As to the regulation of the colonic TPP uptake process, this event also appears to be under the regulation of an intracellular Ca + 2 /calmodulin-mediated regulatory pathway.
While chronic alcohol consumption is known to cause impairment in the intestinal thiamin absorption process, which leads to the development of thiamin deficiency in chronic alcoholics, the cellular and molecular mechanisms involved in this impairment have only been recently investigated. A study by Subramanya et al. showed that chronic alcohol feeding of rats leads to inhibition in carrier-mediated thiamin transport across both the brush-border membrane (BBM) and BLM domains of the polarized enterocytes and that the inhibition is evident as early as 2 weeks following the initiation of the alcohol feeding regime. This inhibition was associated with a significant reduction in the expression of THTR-1 (but not THTR-2) at the protein, mRNA, and heterogeneous nuclear RNA (hnRNA) levels (the latter suggests that the effect is, at least in part, mediated at the level of SLC19A2 transcription). Chronic alcohol feeding was also found to inhibit the carrier-mediated thiamin uptake process in the large intestine, suggesting that absorption of the bacterially synthesized thiamin is also a target for inhibition by chronic alcohol consumption. Similarly, chronic alcohol exposure of human intestinal epithelial HuTu-80 cells was found to lead to a significant inhibition in carrier-mediated thiamin uptake, and the effect appears to be, at least in part, mediated at the level of transcription.
Infection with the Gram-negative enteropathogenic Escherichia coli (EPEC), a food-borne pathogen, represents a significant risk to human health. While diarrhea is a major consequence of this infection, malnutrition also occurs especially in severe and prolonged cases, which may aggravate the health status of the infected hosts. A recent study has examined the effect of EPEC infection on thiamin uptake by human intestinal epithelial Caco-2 cells and showed that while wild-type EPEC causes a significant inhibition in thiamin uptake, neither the nonpathogenic E. coli nor killed EPEC, or the filtered supernatant, affect the uptake. The inhibition by EPEC of the intestinal uptake of this B-vitamin does not appear to be a generalized phenomenon affecting intestinal uptake of other water-soluble vitamins, as EPEC did not affect the intestinal uptake of vitamin B 2 (riboflavin) (Ghosal A, Ashokkumar B, and Said HM; unpublished observations). EPEC inhibition of thiamin uptake appears to be mediated via alteration in kinetic parameters of both the nanomolar (mediated by THTR-2) and the micromolar (mediated by THTR-1) saturable thiamin uptake processes. Cell surface expression of the hTHTR-1 and THTR-2 proteins (determined by biotinylation assay) was also significantly decreased in EPEC-treated cells compared to controls. Furthermore, EPEC infection affected the steady-state level of expression of hTHTR-1 and hTHTR-2 mRNA as well as activity of their respective promoters. These studies suggest that EPEC infection has a rapid onset via affecting the expression and activity of the thiamin transporters at the cell membrane, an effect that is then compounded and prolonged by the effects of EPEC on SLC19A2 and SLC19A3 transcription. EPEC structural components needed to cause the inhibition in thiamin uptake were also examined by infecting Caco-2 cells with EPEC mutants that harbor mutations in the escN gene (which encodes a putative ATPase for the EPEC type III secretion system, TTSS), or the espA, espB, or espD genes (which encode structural components of the TTSS) (Ref. and references therein). None of these mutants were found to affect thiamin uptake. On the other hand, mutations in the espF and espH genes (which encode effector proteins) lead to a partial inhibition in thiamin uptake.
Infection with the enterotoxigenic Escherichia coli (ETEC), another enteropathogen that affects a large segment of the global population, also appears to inhibit intestinal thiamin uptake. This was shown recently in studies with Caco-2 monolayers where infection with live ETEC (but not with boiled/killed ETEC or nonpathogenic E. coli ) or treatment with bacterial culture supernatant led to a significant inhibition in thiamin uptake. This inhibition appears to be caused by heat-labile and -secreted ETEC components and is mediated via activation of the epithelial adenylate cyclase system. The inhibition was associated with a significant reduction in expression of human thiamin transporter-1 and -2 (hTHTR1 and hTHTR2) at the protein and mRNA levels as well as in the activity of the SLC19A2 and SLC19A3 promoters. Finally, dual infection of Caco-2 cells with ETEC and EPEC was found to lead to compounded inhibition in intestinal thiamin uptake.
Thiamin deficiency is prevalent in patients with sepsis. This appears to be mediated in part via an effect of sepsis on intestinal thiamin uptake given recently reported observations of a significant inhibition in intestinal thiamin uptake in rat model of sepsis where the degree of inhibition was shown to correlate with the severity of sepsis. Also, the inhibition was associated with a significant decrease in the level of expression of THTR-1 and THTR-2 in the gut mucosa. Sepsis also caused a significant inhibition in level of expression of the mitochondrial TPPT and in level of mucosal ATP.
Riboflavin (RF; Fig. 54.1 ) is required for normal cellular metabolism, proliferation, and growth. In its biologically active forms [flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD)], the vitamin plays a key metabolic role in the transfer of electrons in biological oxidation-reduction reactions involving carbohydrate, lipid, amino acid, and certain water-soluble vitamins (pyridoxine and folate). Studies have also shown a role for riboflavin in protein folding in the endoplasmic reticulum and in the regulation of cellular energy expenditure. More recent investigations have demonstrated antioxidant and antiinflammatory properties for riboflavin, as well as a role for this vitamin in normal immune function. Riboflavin deficiency leads to a variety of clinical abnormalities, including degenerative changes of the nervous system, anemia, skin lesions, cataract, and growth retardation. It also leads to an increase in the susceptibility to cancer. Deficiency/suboptimal levels of riboflavin occur in chronic alcoholism, diabetes mellitus, IBD, Brown-Vialetto Van Laere, and Fazio Londe syndromes [the latter are rare neurological disorders caused by mutations in riboflavin transporters-2 and -3 (RFVT-2 and RFVT-3)]. In contrast to the deleterious effect of riboflavin deficiency, optimizing riboflavin status has been reported to reduce the risk of esophageal squamous cell carcinoma (Ref. and references therein).
The human intestine encounters two sources of RF: a dietary source and a microbiota source. Dietary riboflavin exists mainly in the form of FMN and FAD and is bound (noncovalently) to proteins. These coenzymes are first released from proteins via the combined action of gastric acid and hydrolases and then hydrolyzed further (prior to absorption) by intestinal phosphatases to free riboflavin. The mechanism of absorption of free riboflavin has been studied using a variety of human and animal intestinal preparations. Collectively, these studies have shown that transport of free riboflavin occurs mainly in the proximal part of the small intestine and involves an efficient and specific Na + -independent, carrier-mediated mechanism. This mechanism is inhibited by riboflavin structural analogues and by the Na + /H + exchange inhibitor amiloride ( K i for amiloride ≈ 0.48 mM). Other studies have shown that the intestinal riboflavin uptake process is sensitive to the effect of the tricyclic phenothiazine drug chlorpromazine, a compound that shares structural similarities with riboflavin. Once internalized, some of the riboflavin is phosphorylated and used by enterocytes, and the rest is transported out of the cell across the BLM via a specific carrier-mediated process.
With regard to the microbiota-generated riboflavin in the large intestine, the amount of the vitamin produced depends on the type of the ingested diet and is higher following ingestion of a vegetable-based diet compared to a meat-based diet. Also, a considerable amount of the microbiota produced riboflavin was found to exist in the large intestinal lumen in the form of free riboflavin, and thus, available for absorption. Other investigations have shown that the large intestine is capable of absorbing luminally introduced free riboflavin. Insight into the mechanism involved in colonic uptake of riboflavin came from studies using the human colonic epithelial NCM460 cells, which showed the involvement of an efficient and specific carrier-mediated mechanism that is similar to the one described in the small intestine. Subsequent studies in rats have confirmed the existence of a specialized carrier-mediated mechanism for riboflavin uptake in the colon. With the demonstration of existence of an efficient carrier-mediated system for riboflavin uptake in the large intestine, the belief that this source of riboflavin contributes to the overall host nutrition of the vitamin, and especially toward the cellular nutrition of the localized colonocytes, is further strengthened.
Insight into the molecular identity of the transport systems involved in intestinal riboflavin uptake has recently emerged with the identification of three putative human riboflavin transporters-1, -2, and -3 (RFVT-1, RFVT-2, and RFVT-3) that are encoded by three different but homologous genes: the SLC52A1 , SLC52A2 , and SLC52A3 genes, respectively. These transporters exhibit no similarity with the yeast or bacterial riboflavin transporters, nor do they show similarity with any other mammalian transporter. The hRFVT-1 and hRFVT-2 share > 87% sequence similarity at the protein level, while hRFVT-3 shares around 43% similarity with hRFVT-1 and hRFVT-2. The hRFVT-1, -2, and -3 are all expressed in the gut, with expression of the hRFVT-3 being significantly higher than that of hRFVT-1 and hRFVT-2 ; hRFVT-3 is also more efficient in transporting riboflavin than the other hRFVTs. Furthermore, live-cell confocal imaging of polarized intestinal (and renal) epithelial cells showed the hRFVT-3 to be predominantly expressed at the apical membrane domain, while expression of hRFVT-1 is mostly at the BLM domain of these cells; expression of hRFVT-2 was localized inside intracellular vesicles (with some expression at the BLM domain).
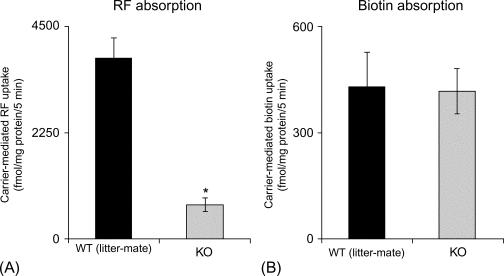
The relative contribution of the predominantly expressed riboflavin transporter in the intestine (i.e., RFVT-3) toward total carrier-mediated riboflavin uptake has also been recently addressed using an in vitro SLC52A3 gene-specific silencing siRNA approach and human intestinal epithelial Caco-2 cells with the results showing a major role for these transporters in intestinal riboflavin uptake. This was confirmed in vivo in studies utilizing an intestinal-specific (conditional) RFVT-3-knockout (cKO) mouse model developed by the Cre/Lox approach ( Fig. 54.7 ). In the latter study, all the RFVT-3 cKO mice developed systemic riboflavin deficiency indicating that RFVT-3 is involved in determining the overall riboflavin body homeostasis. Also observed was a significant increase in the level of expression of oxidative stress-responsive genes in the intestine of the cKO mice, which suggest a role for riboflavin in the maintenance of normal intestinal physiology.
As mentioned earlier, the RFVT-3 is expressed exclusively at the apical membrane domain of intestinal epithelial cells. What dictates cell surface expression of the protein appears to be a sequence in the C-terminal portion of the polypeptide that includes two conserved cysteine residues (one at position 463 and the other at position 467). Mutating these cysteine residues was shown to lead to severe impairment in riboflavin uptake due to retention of the protein in the endoplasmic reticulum. A potential disulfide bridge between C463 and C386 (also predicted by modeling analysis and mutating the C386 residue) was found to lead to intracellular retention of RFVT-3. Intracellular trafficking of RFVT-3 was shown to involve distinct vesicular structures whose mobility depends on existence of an intact microtubule network.
Early studies using Caco-2 cells exposed to specific sulfhydryl group reagents have reported a role for sulfhydryl groups in the function of the intestinal riboflavin uptake carriers. Since the sulfhydryl group reagents used in these studies were membrane impermeable, it was assumed that the affected sulfhydryl group(s) were located at the exofacial surface of the cell membrane. More recent investigations examined the effect of clinical mutations in SLC52A3 found in patients with the Brown-Vialetto-Van Laere syndrome (BVVLS; a rare neurological disease characterized by ponto-bulbar palsy, bilateral sensorineural deafness, and respiratory insufficiency) on cell biology of RFVT-3. Mutants P28T, E36K, E71K, and R132W were all found to be functionally impaired and that the impairment was due to retention of the mutated RFVT-3 within the endoplasmic reticulum. These findings suggest a role for the affected residues in normal cell biology of RFVT-3.
The intestinal riboflavin uptake process is under the regulation of extracellular/environmental conditions and intracellular factors. Since a number of these conditions exert their effect(s) at the transcription level, it is relevant to first describe the basal transcriptional regulation of the intestinally relevant SLC52A1 and SLC52A3 genes.
Recent studies have reported the cloning of the 5′-flanking region of the SLC52A1 gene and its characterization in human intestinal epithelial cells. These studies showed the gene to have one transcription start site (TSS) and has a core (minimal) promoter between − 234 and − 23. This minimal promoter lacks TATA elements, but is GC-rich and harbors several putative cis -regulatory (SP-1, KLFs, AP-2, and EGRF), of which Sp-1 appears to be the most relevant. Promoter supershift and chromatin immunoprecipitation (ChIP) analyses have confirmed the interaction between the Sp-1 cis-element and its Sp-1 nuclear factor; also an enhancement in promoter activity was observed following cotransfection of the minimal SLC52A1 promoter with Sp-1 into Drosophila SL-2 cells (which lacks endogenous Sp activity).
Identification of the minimal 5′-regulatory region of the SLC52A3 gene and determination of the regulatory element(s) involved in its activity in intestinal epithelial cells, together with confirmation of promoter activity in vivo in transgenic mice (to establish physiological relevance) have recently been accomplished. The minimal SLC52A3 promoter was found to be located between − 199 and + 8 bp (using the start of the TSS as position 1). An important role for the Sp1-binding site (at position − 74/−71 bp) in determining the activity of the SLC52A3 promoter was also identified. The latter has been established by means of mutational analysis, binding of Sp1 to the minimal SLC52A3 promoter (i.e., EMSA and supershift assays), and by ChIP analysis. The importance of Sp1 in driving the activity of the SLC52A3 minimal promoter was further confirmed in studies utilizing Drosophila SL-2 cells (which lack Sp activity) where a significant induction in the activity of the SLC52A3 minimal promoter upon cotransfection with the Sp3 nuclear factor was observed. Activity of the cloned SLC52A3 promoter was further confirmed in vivo in transgenic mice carrying the promoter construct fused to luciferase.
The intestinal riboflavin uptake process is adaptively regulated by the prevailing extracellular substrate level. This has been shown in vivo in rats supplemented with different levels of riboflavin and in vitro in studies utilizing cultured intestinal epithelial cells. In the rat study, induction of riboflavin deficiency (by dietary means) led to a significant and specific upregulation in intestinal riboflavin uptake, while oversupplementation of the animals with riboflavin led to a significant and specific downregulation in intestinal riboflavin uptake. In the in vitro studies, maintaining human intestinal epithelial cells (Caco-2 and NCM460) in a RF-deficient medium also led to a significant upregulation in riboflavin uptake, while maintaining these cells in the presence of high pharmacological concentrations of the vitamin led to downregulation in riboflavin uptake. The latter adaptive changes were mediated via changes in the V max (but not the apparent K m ) of the riboflavin uptake process, suggesting that the changes are mediated via an increase in the number (and/or activity) but not the affinity of the riboflavin carriers. Actinomycin D treatment of cells led to significant inhibition in the induction of riboflavin uptake under riboflavin deficiency, thus suggesting possible involvement of a transcriptional mechanism(s) in the adaptive response. The latter suggestion was confirmed in recent investigations showing the induction in riboflavin uptake in riboflavin deficiency to be associated with an increase in expression of the hRFVT-3 (and hRFVT-2; but not hRFVT-1). Focusing predominantly on the hRFVT-3, studies showed an induction in the level of expression of the hRFVT-3 protein, mRNA, and hnRNA, as well as in activity of its gene ( SLC52A3 ) promoter in deficiency. The latter studies also showed an increase in the level of expression of the Sp1 nuclear factor (which as mentioned above is important for activity of the SLC52A3 promoter) in riboflavin deficiency, and that mutating the Sp1/GC site in the SLC52A3 promoter led to a drastic decrease in the level of induction in SLC52A3 promoter activity in riboflavin deficiency. Furthermore, the study reported involvement of specific epigenetic alterations affecting the SLC52A3 promoter in riboflavin deficiency, and an increase in hRFVT-3 protein expression at the cell surface under the deficiency condition.
Whether the intestinal riboflavin uptake process is developmentally regulated has also been addressed. Riboflavin transport in rat intestine declines with maturation; this decline is mediated via a decrease in the V max and an increase in the apparent K m of the carrier-mediated riboflavin uptake process (i.e., as the animal moves from suckling to weanling to adulthood). The latter findings suggest that developmental maturation of the intestine is associated with a decrease in the number (and/or activity) of the riboflavin uptake carriers and a decrease in their affinity. The molecular mechanism(s) involved in the developmental regulation of intestinal riboflavin uptake, however, is not currently known and in need of further investigations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here