Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
One of the most preventable causes of death in abdominal and pelvic trauma is arterial hemorrhage that goes untreated or unrecognized. Currently, interventional radiology has undergone many advances, particularly in noninvasive imaging and conventional angiography, resulting in the ability to both recognize and treat critical, life-threatening arterial hemorrhage quickly. Interventional radiology also extends beyond angiography as computed tomography (CT)-guided percutaneous interventions and other imaging-guided techniques also play a significant role in the acute management of the trauma patient.
The cornerstone of arterial hemostasis is early intervention, whether via a direct approach to injured blood vessel, endovascular techniques, thoracotomy, open laparotomy, or a combination of interventions. Early intervention requires a highly sensitive and specific diagnostic study that can be both performed and interpreted quickly. With current technological advancements in CT, CT angiography (CTA) has become the foundation for identifying traumatic vascular injuries. Patients with blunt trauma who are hemodynamically stable typically forgo laparotomy and are assessed with CTA for nonoperative management. Interventional radiology has become an integral part of the management of trauma patients, as vascular embolization has become integrated in the management of both the nonoperative and operative patient. Further advances in endovascular techniques have allowed tremendous strides in the management of the unstable patient, and in the appropriate clinical setting, many leading trauma centers have utilized arterial embolization as a component of primary resuscitation, especially in pelvic trauma.
In short, interventional radiology plays a major role in the diagnosis, treatment, and management of the trauma patient. Ultimately, treatment of the trauma patient requires a multidisciplinary approach integrating the trauma surgeon and interventional radiologist.
CTA is typically reserved for those trauma patients who are hemodynamically stable. Hemodynamically unstable patients undergo resuscitation, and once vital signs are stabilized, CTA is performed. Yet, development in CT technology has pushed the envelope, so to speak, and CTA is being performed on less stable patients. This is largely due to advancements in multidetector CT (MDCT) technology, which has decreased time of acquisition and has allowed faster localization of active hemorrhage. MDCT not only provides improved temporal and spatial resolution but can acquire total body images in less than 1.5 seconds. MDCT acquires isovolumetric data, enabling the interpreter to reconstruct images in an infinite number of planes. Multiplanar reconstruction and maximum intensity projections aid in confirming suspected vascular lesions and uncover vascular injuries that may be obscured by adjacent hyperattenuating bone or foreign bodies.
In the 1980s, it was well established that between 50% and 70% of all liver and splenic injuries cease bleeding at the time of operation and can be treated conservatively as long as patient hemodynamic status is not compromised. However, vascular injury has become a primary focus in CTA evaluation, as its presence is associated with failure of nonoperative management. Solid organ injuries are also evaluated with CTA and historically have been defined and graded through the American Association for the Surgery of Trauma scale. Yet, these grading scales are based on anatomic findings and have not been shown to accurately select those patients who will fail nonoperative treatment or decompensate from delayed hemorrhage. In fact, Durham et al. compared CT scan results with operative findings and concluded that CT may actually underestimate the degree of liver injury. Subsequently, there have been a number of attempts to define clinically relevant CT imaging findings. Though currently there is no universally accepted standardization, the goal of this section is to define those CT findings considered high risk and that may benefit from early intervention.
Contrast extravasation is one of the most reliable CTA findings of vascular injury and has a 99% specificity and 80% to 97% sensitivity for identifying patients who will require embolization. In 1993, Shanmuganathan found that active bleeding can be differentiated from clotted blood on CT. Continuous bleeding is hyperattenuated on CT, averaging 130 Hounsfield units (HU), and clotted blood measures between 40 and 70 HU. Therefore, attenuation greater than 100 HU is suspicious for active hemorrhage. With contrast agent administration, a contrast blush can be seen, which often fades into a parenchymal hematoma or peritoneal fluid. The attenuation of extravasated contrast material will often be within 10 HU of the adjacent feeding arterial source. Active extravasation typically has ill-defined margins with either a linear or focal region of hyperattenuation. On delayed CTA imaging, a hematoma that has expanded in size or changed attenuation values suggests active hemorrhage. Overall, the key for the diagnosis of active extravasation is a hyperattenuated entity, which changes on delayed imaging.
It is critical to distinguish between active hemorrhage from intraparenchymal hematoma and laceration. If vascular injury is suspected despite no evidence of active extravasation, serial CTA may help monitor patient progression. Delayed bleeding may be seen as a hematoma that has increased in size, rupture of a central solid-organ hematoma, or rupture of a pseudoaneurysm in contact with fluid collection such as biloma or hematoma. Sudden change in clinical status or increase in pain should lead to suspicion of delayed bleeding. Moreover, hepatic lacerations involving more than three liver segments and extension of laceration into the hilum are associated with vascular injury and should prompt early intervention. Care should be taken in lacerations with hilar extent, as these are more likely unstable lesions involving the portal vein or inferior vena cava (IVC) and are better managed surgically.
The location of intraparenchymal hematoma is equally important. Certain locations potentially communicate with larger compartments and are at higher risk of decompression due to the lack of supporting structure for tamponade. Hepatic hematomas involving segment VII may extend to the bare area of the liver, causing decompression into the retroperitoneum. A central hepatic hematoma communicates with the perivascular spaces and may decompress into the hilum. Finally, hepatic or splenic parenchymal hematoma may extend into the capsule; if the capsule is compromised, hematoma can expand into the peritoneal cavity. In fact, the more quadrants containing free fluid, the more specific CTA is for vascular injury. Patients with free fluid extending into the paracolic gutters and pelvic cul-de-sac more frequently fail nonoperative management and require embolization, but free fluid contained in Morrison’s pouch and perisplenic regions are more likely to pass without embolization. Though hemoperitoneum is not a specific CT finding alone, free fluid in more than three quadrants with contrast extravasation or sentinel clot increases the specificity for vascular injury, requiring early intervention. If hemoperitoneum is found in more than one quadrant, comparison of the fluid attenuation should be made. Blood closest to the injury site has more time to retract and forms higher density clotted blood; the so-called “sentinel clot sign.” This is important for the interventionalist in patients with multiple solid organ injuries, as it can facilitate deciding which solid organ should be selected first for angiography.
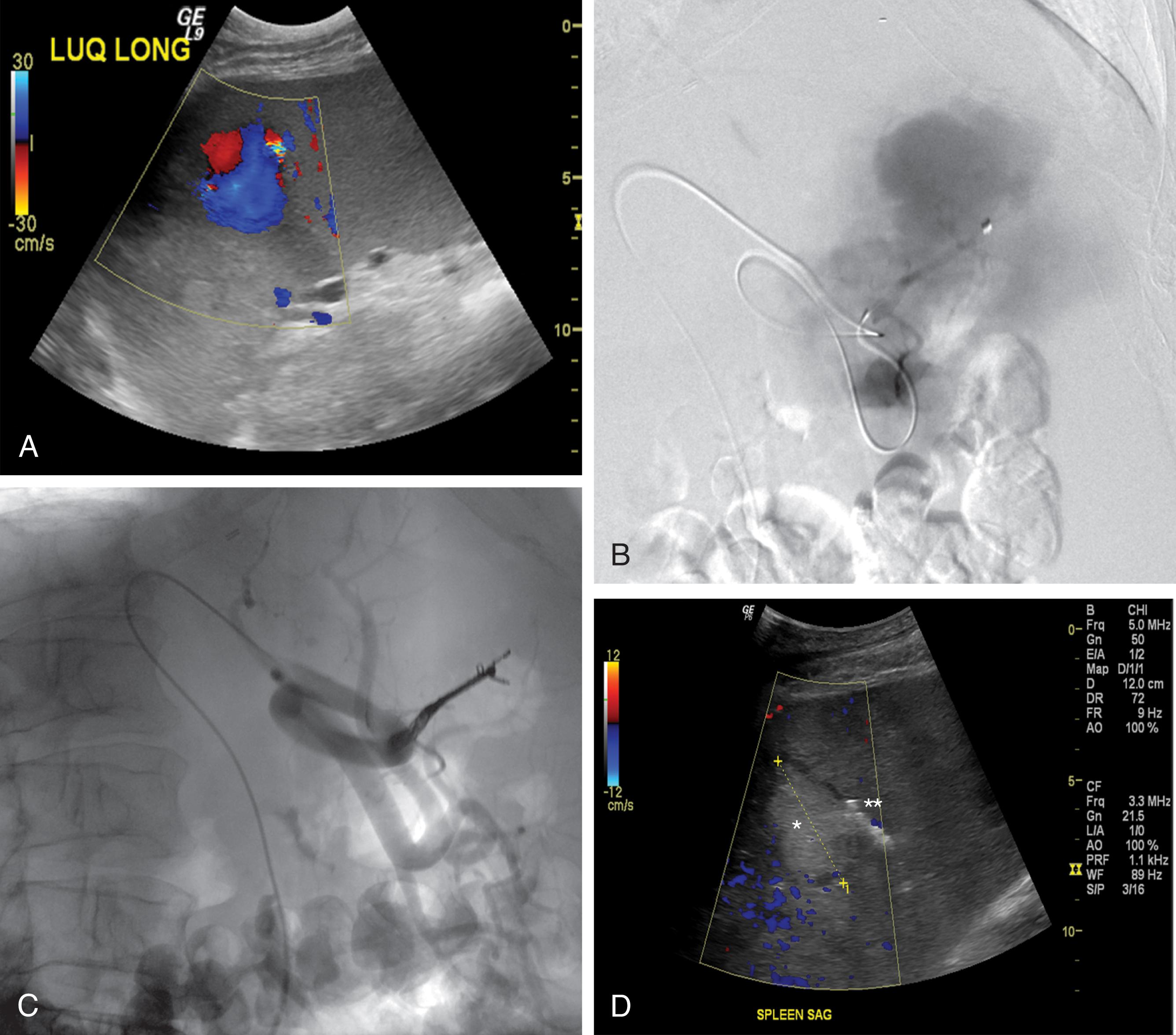
Other signs of vascular injury seen on CT include contrast-contained vascular lesions and intimal injuries. Pseudoaneurysm and arteriovenous fistula are classified as contained arterial lesions. Pseudoaneurysms exhibit different characteristics on CT when compared with active extravasation. They commonly exhibit attenuation that is similar to the adjacent feeding artery yet demonstrate well-defined margins in a round configuration. Pseudoaneurysm is also less apparent on delayed imaging, and if an adjacent hematoma is present, it will not change in size or attenuation. Depending upon the location, pseudoaneurysms may be readily characterized on Doppler ultrasound ( Fig. 1 ). Contained arteriovenous fistula is difficult to diagnose on CT, as CTA requires long intravenous injection times, which nonselectively opacify the vasculature. Finally, traumatic visceral intimal injuries may be detected on CTA and are most commonly found in the renal arteries. Blunt renal artery injuries are considered high-risk lesions owing to the risk of thrombosis and renal dysfunction.
Diagnostic CTA does have its limitations. It is important to understand that current CTA imaging is only a snapshot in time, and it may not detect all vascular lesions that are present. Furthermore, CTA is unable to accurately predict delayed hemorrhage. As stated earlier, CTA potentially underestimates solid organ injuries, and therefore clinical presentation is most critical. Ultimately, however, CTA is a powerful tool that reliably aids in management decisions of the trauma patient.
Angiography confirms 80% to 90% of CTA findings suspicious for arterial injury yet presents a continuous diagnostic challenge. Active hemorrhage and vascular injury may be subtle, and the appearances of these lesions differ on angiography from CTA. Contrast extravasation is seen as a persistent blush of contrast on angiography that appears earlier than the venous phase and fails to wash out in the delay phase. Pseudoaneurysm is diagnosed as a contained saccular outpouching, which has equal density to the adjacent vessel and no evidence of extravasation. Abrupt arterial truncation indicates transection and occlusion of the vessel.
When a discrepancy between CTA and angiography manifests, it is important to determine whether it is due to the cessation of arterial bleeding or if vasospasm, the physiologic response to hemodynamic instability, is masking the vascular injury. If active hemorrhage is highly suspected and diagnostic angiography does not locate the lesion initially, provocative angiography can be performed to reveal contrast extravasation in the setting of a hemodynamically stable patient. The most frequently used medications are nitroglycerin, heparin, or tissue plasminogen activator. Nitroglycerin is the safest in a trauma setting, due to its short half-life of 3 minutes. Its effect allows the interventionalist to identify potential life-threatening hemorrhage yet is brief enough as to typically not cause further hemodynamic demise. If a discrepancy persists after provocative angiography or if there is a high clinical suspicion of hemorrhage in the absence of CTA, prophylactic embolization may be performed. However, it is important to consider spontaneous cessation of arterial hemorrhage or that hemorrhage may be from a venous source.
Arterial access is extremely important prior to performing diagnostic and therapeutic angiography. The most common arterial access site in the trauma setting is the right common femoral artery, due to its superficial location. If the right common femoral artery cannot be accessed, it may be obtained from the contralateral side or via a brachial or radial approach. The upper extremity approach may prove particularly useful in patients requiring pelvic binders, as these can be left in place, unlike in the femoral approach. Brachial and radial access have an established risk of stroke (∼0.5%) due to the necessity of crossing the vertebral and carotid arteries. Additionally, there is a slight increased risk of hematoma formation and radial artery thrombosis, which has potentially adverse consequences in the upper extremity. Compressive hematoma risks medial brachial compartment syndrome and peripheral nerve injury. Finally, the left brachial or radial artery is preferred to the right, as there is only one cerebral vessel crossed with this approach.
Angiographic evaluation will largely depend on preassessment CTA. If CTA has been performed, selective angiography can be performed first to address the known or suspected vascular injuries diagnosed on CTA. After selective angiography, nonselective angiography should always be performed to identify undiagnosed vascular lesions and assure there are no additional sites of active hemorrhage. In unstable patients with pelvic trauma, CTA many times will not be available, and nonselective angiography should be implemented first to provide a roadmap for more selective arteriography.
Nonselective aortography not only offers a road map to the visceral vasculature, it may also demonstrate potential anatomic collateral pathways important for embolization. These collateral pathways, though rare, may provide sustained blood flow to active hemorrhage and thus are important to identify. Additionally, these collateral pathways may be important in the surgical management of the trauma patient in cases that may subsequently end up in the operating room. In abdominal vasculature, the arc of Buhler is an embryologic communication of the celiac axis and the superior mesenteric artery (SMA), which persists via the dorsal pancreatic artery. The arc of Barkow represents a collateral pathway between the celiac and SMA via the omental arteries. The right and left gastroepiploic arteries, which originate from the gastroduodenal and splenic arteries, respectively, give collateral flow to the greater omentum. Additionally, the posterior epiploic arteries of the transverse pancreatic and the middle colic arteries (from the SMA) supply the greater omentum and connect with the gastroepiploic arteries, thus forming the arc of Barkow. Though less important in the trauma setting, the arc of Riolan represents communication of the SMA with the inferior mesenteric artery via a central connection from the proximal middle colic artery and proximal left colic artery.
When considering vascular injury, a vast array of tools is available, and a good foundation in angiographic techniques is required. The main agents discussed in this chapter are stent grafts, occlusion balloons, and embolic agents (both liquid and solid). Stent graft may be used to stop arterial bleeding as well as presage native arterial flow to the distal organs and tissues. The successful utilization of a stent graft requires a suitable artery, and therefore cannot be used for every vascular lesion. Vessels ideally should be 5 mm or greater and should follow a relative straight course; tortuous vessels are not amenable to stent grafts due to migratory risk, though newer stent technology is currently underway that may allow for more flexible stent grafts in the future. Furthermore, bifurcating vessels are not candidates for stent grafts for similar reasons and risk of branch occlusion; they should also be avoided in young patients due to in-stent stenosis from neointimal hyperplasia. Arteries that are ideal for stent grafts include the external iliac and superficial femoral arteries. Occlusion balloons are typically used as temporizing measures as a bridge to surgery or as definitive endovascular treatment in rapid exsanguination. Additionally, aortic occlusion balloons have been utilized in patients requiring temporary stabilization before pelvic angiography.
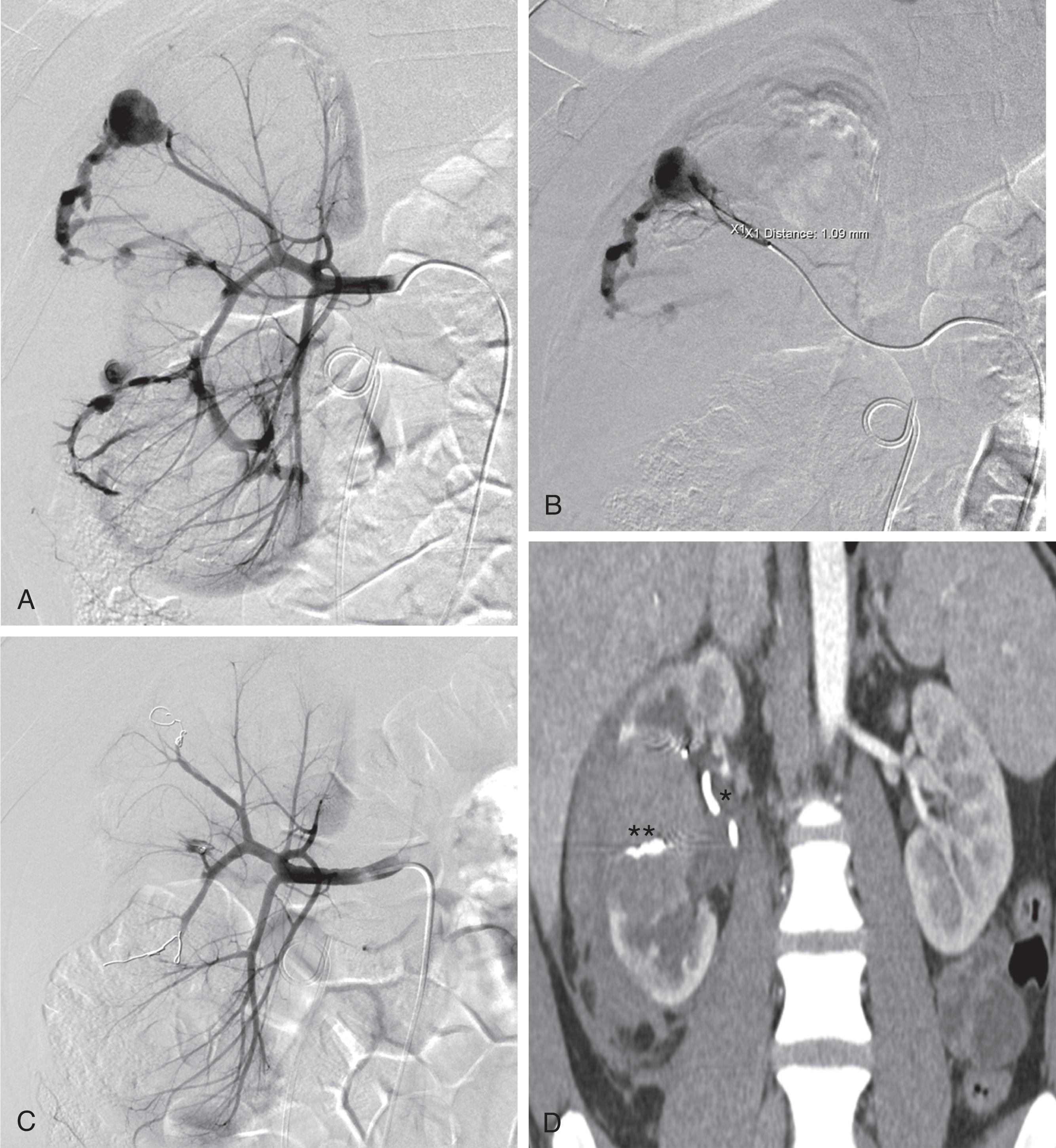
Embolization can be performed with endovascular coils, detachable vascular plugs, Gelfoam pledgets, particles, liquid embolics, or a combination of these. Endovascular coils traditionally functioned as embolic agents through inducing thrombosis, not mechanical occlusion, in which the thrombogenic effect is augmented by Dacron filaments incorporated in the coil. Coil technology has advanced greatly of the last decade with the introduction of detachable coils, in which the endovascular coils have a controlled detachment mechanism. While the detachment mechanisms vary among different manufacturers, all of these have allowed for more precise placement of coils for hemostasis and minimize the chance of nontarget embolization. A few newer coil technologies have introduced larger-diameter coils without Dacron filaments, which boast the ability to achieve hemostasis through complete mechanical occlusion of the vessel, unlike their smaller Dacron filament coil counterparts. Detachable coils allow for both accurate precise embolization, which is key for treating underlying vascular injuries while preserving normal tissue perfusion, especially in the setting of injury to end vessel organs, such as in renal trauma ( Fig. 2 ). Endovascular coils as a whole are very effective in proximal or distal embolization.
Endovascular plugs may also be used in cases of arteriovenous fistula or bleeding refractory to other embolic agents and are utilized predominantly in proximal embolization techniques. The major disadvantage of vascular plug embolization remains that once proximal embolization is performed, distal access to the vessel is blocked, and therefore repeat endovascular intervention is not possible. Another disadvantage for endovascular plugs in the past has been due to the large caliber of device, vascular plugs have required larger access sheaths or guide catheters for deployment (often 5-French or 6-French). In the last decade, however, further advances in endovascular plugs have extended the armamentarium of embolization as manufacturers have developed smaller vascular plugs that are able to be used in more distal embolization techniques.
Gelfoam is another embolic agent; it is water soluble and can be prepared rather quickly. It is a temporary embolic agent that is completely absorbed by the body. The arterial vessel typically recanalizes within 2 to 3 weeks. It is effectively used in regions of multiple pseudoaneurysms or active extravasation. It is the cornerstone for embolic agents in blunt pelvic trauma to seal active hemorrhage quickly.
Embolic particles are seldomly used in the trauma setting. Particles are available in many different sizes; however, those used in trauma range from 500 to 700 μm and 700 to 900 μm. Though not as frequently used, liquid agents are also very effective embolic materials. N -butyl cyanoacrylate, or NBCA, is commonly referred to as liquid adhesive or glue and polymerizes immediately on contact with ionized fluid such as blood. The glue forms as a result of an exothermic reaction, which destroys the vessel wall, and therefore it is reserved for smaller vessels accessed with a microcatheter. Particles and liquid embolics are permanent distal embolizing agents, and there is a potential risk of tissue ischemia, especially if there is occlusion of the capillary bed. In general, liquid and particulate embolic agents should be avoided in the spleen and inferior gluteal artery to reduce the incidence of abscess and sciatic nerve injury, respectively.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here