Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An ideal imaging guidance system for cardiovascular, catheter-based interventional procedures would offer real-time, high-resolution, three-dimensional (3D) imaging of important anatomic tissues and chambers, irrespective of respiratory, cardiac, or patient motion alongside excellent visualization of catheters, guidewires, and other interventional devices. Such tools would quickly enable novel, minimally invasive alternatives to open surgical procedures.
X-ray fluoroscopy guides most contemporary catheter-based procedures. However, fluoroscopy has important limitations ( Table 47.1 ). Iodinated radiocontrast, which provides the ability to outline chamber and vascular lumens, is injected only periodically. Tissue detail is minimal. Additionally, fluoroscopy provides only two-dimensional (2D) “projection” imaging, with limited depth perception. Iodinated radiocontrast is nephrotoxic in susceptible individuals. Ionizing radiation increases lifetime cancer risk, particularly in children. Finally, operators and personnel risk disabling orthopedic injuries from the weight of protective lead apparel.
| Imaging Modality | Advantages | Disadvantages |
|---|---|---|
| iCMR |
|
|
| X-ray fluoroscopy |
|
|
| Ultrasound |
|
|
Ultrasound is often used in combination with x-ray fluoroscopy procedures. For example, transesophageal echocardiography (TEE) provides adjunctive imaging during atrial septal defect closure or transcatheter aortic valve replacement, by assisting with device sizing, positioning, and postdeployment assessment. However, transthoracic echocardiography (TTE) offers limited acoustic windows, and suffers “shadowing” artifacts caused by devices.
Cardiovascular magnetic resonance (CMR) more closely approaches the idealized imaging guidance system described earlier. CMR offers superior tissue imaging, in any arbitrary orientation, without ionizing radiation or nephrotoxic contrast. Rapid imaging techniques and modified CMR visible devices now permit real-time CMR guided catheter applications. The ability to visualize both tissue and interventional devices may enable catheter-based procedures in the future that currently require open surgical exposure. In the meantime, CMR right heart catheterization provides superior hemodynamic characterization combining high fidelity flow, pressure, and function measurements.
Early real-time magnetic resonance imaging (MRI) systems employed low field strength magnets (0.2–0.5 T), open or closed bore configurations, and sometimes embedded x-ray fluoroscopy systems. Major limitations included relatively low signal-to-noise ratio (SNR), inhomogeneous magnetic field interfering with rapid imaging, and limited patient access when employing long, closed bores. Newer, short-bore, 1.5 T systems with high performance gradients provide excellent field homogeneity and higher SNR. Newer scanners also include large arrayed radiofrequency (RF) receivers (≥32), intended for parallel imaging techniques to improve imaging speed. These additional receiver channels can also be used to attach “active” catheter devices to improve their visibility. By contrast, higher field 3 T systems disproportionately increase heating but do not notably enhance image quality using workhorse (balanced steady state free precession [bSSFP]) pulse sequences, and are therefore difficult to justify for interventional CMR (iCMR) applications.
In addition, commercial vendors now provide interactive scan user interfaces that increasingly resemble echocardiography. These interfaces drive sophisticated image reconstruction hardware and software that permit images to be acquired with ease and displayed to operators with minimal delay in multiple scan planes.
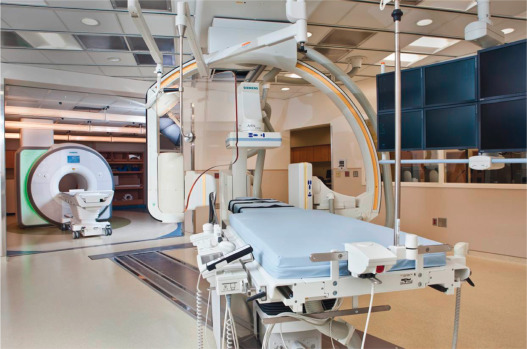
Combined x-ray and CMR catheterization laboratories, so-called XMR labs, now enable rapid and convenient CMR catheterization procedures, especially in pediatrics and heart failure ( Fig. 47.1 ). In these laboratories, conventional x-ray fluoroscopy guidance is immediately available to perform interventions using CMR fusion imaging or for situations requiring emergency bailout. XMR laboratories are now commercially available. For example, the National Heart Lung and Blood Institute (NHLBI) laboratory at Children's National Medical Center in Washington, DC, combines a biplane x-ray fluoroscopy system with a 1.5 T cardiac scanner ( Fig. 47.1 ). These systems are separated by RF-shielded doors and can be used independently or together for combined procedures. An automated transport table moves patients seamlessly between the two modalities. Alternative designs include single-room layouts, in which both x-ray and CMR systems are located inside the RF shield. Wave guides and penetration panels are strategically positioned in the room so that communication and patient monitoring hardware can be installed without disrupting the RF shield.
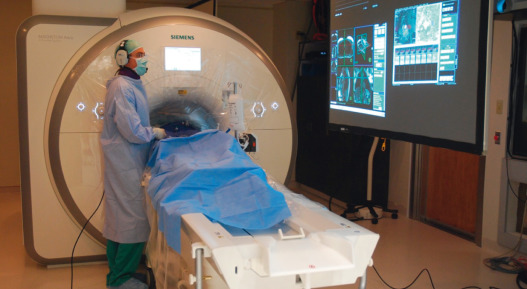
Rapid CMR requires rapid gradient switching that causes substantial acoustic noise. This noise prevents verbal communications between operators, the patient, and staff. Wireless noise-cancelling headsets are used at NHLBI ( Fig. 47.2 ). This versatile system allows staff and patients to speak simultaneously on open microphones and allows separate and parallel conversations with patients and among staff.
Gradients, RF interference, and magnetohydrodynamic effects severely distort the electrocardiogram (ECG). Electronic filters enable heart rate monitoring; however, ECG waveform morphology is not readily interpretable for ST-segment monitoring of myocardial ischemia or injury. Multichannel invasive pressure waveform monitoring, temperature monitoring, and oxygen saturation monitoring can use commercial systems, without modification from x-ray catheterization laboratories. Several manufacturers are developing dedicated hardware that will interface with existing catheterization laboratory high-fidelity hemodynamic recording systems. In the meantime, open source kits for local assembly of suitable hemodynamics interfaces are available ( http://nhlbi-mr.github.io/PRiME ).
Interventional catheterization suites require continuous operator monitoring of multiple information streams, including hemodynamics, imaging control, and interventional images. This is similarly true during iCMR procedures, especially using active devices. MRI-conditional liquid crystal display (LCD) video displays are available from most scanner manufacturers. We prefer two banks of less expensive LCD video projectors that project onto a fabric screen, which depict hemodynamics, scanner interface, and 3D-rendered images separately, one at each end of the CMR system ( Fig. 47.2 ). The projectors are housed in RF-shielded boxes and video signals are passed into the room via fiber optic cables through the waveguide.
The iCMR scanner user interface requires several key features not typical for diagnostic CMR. Commercial or investigational graphic user interface based scanning host computer systems are available from most systems vendors (Interactive Front End, Siemens ; MR Echo, General Electric; eXTernal Control, Philips Healthcare) and as standalone commercial (RT Hawk, Heart Vista) or open-source solutions (Vurtigo, Sunnybrook Health Sciences Center, http://www.vurtigo.ca ).
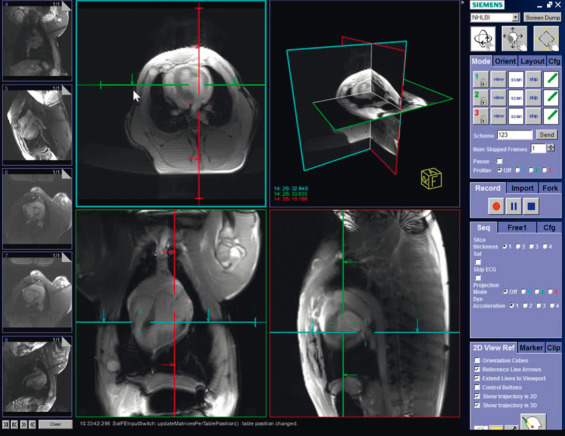
One of the most useful features is the colorized display of individual receiver channels that are attached to “active” catheter receiver coil devices. This makes catheter devices readily apparent and has proven useful in vivo. Another is interactive “projection-mode” imaging wherein slice select is disabled and catheter devices are displayed as the operator is used to seeing them under conventional x-ray fluoroscopy. For tracking balloon catheters filled with dilute gadolinium (Gd) contrast during CMR catheterization, we find it helpful to interactively adjust slice thickness and saturation magnetization preparation, to help find the catheter tip should it move outside of the selected plane, and to interactively adjust temporal resolution for catheterization steps requiring fine motor control. Operationally, we find it useful to display multiple slices during interventional procedures, both separately and combined in a 3D-rendered image ( Fig. 47.3 ). Other laboratories are experimenting with voice-control, automatic adjustment of slice location to correspond to catheter position, and automatic adaptation of imaging parameters to speed of catheter manipulation.
Patient care staff must undergo formal comprehensive training in CMR safety. For example, the hospital emergency medical response team is unlikely to be familiar with CMR operations and may unwittingly jeopardize themselves, their colleagues, or the patient by rushing into the room with a steel oxygen tank. Rapid patient evacuation from the CMR catheterization laboratory needs to be practiced repeatedly in mock drills. Ferromagnetic objects that cannot be removed from the room must be attached to the wall during CMR guided procedures. It is good practice for all devices on wheels in the x-ray section of the iCMR suite (e.g., anesthetic machine, intraaortic balloon pump, or defibrillator) to be tethered to the wall or floor. Oxygen, inhalational anesthetic, and vacuum for suction can be fed through RF-protective wall ports, eliminating in-room cylinders. At NHLBI, we drill emergency evacuation from the CMR room on a quarterly basis. Swift evacuation relies on clear delineation of roles for all staff. In an emergency, we can start chest compressions within 10 seconds and move the patient back into the x-ray room for defibrillation in under 1 minute. In the near future, CMR-conditional external defibrillators should be commercially available so that arrhythmias can be managed without evacuating the patient from the scanner.
RF energy from CMR transmit coils will concentrate on long conductive devices causing local heating, potentially leading to burns. Box 47.1 lists factors associated with heating, especially long conductive devices and cables. This complex problem is described later under “active” catheter devices. Interventionists must also take care that connections to intravascular coils as well as surface coils do not inadvertently form loops, which can cause patient burns.
Length of conductive elements
Geometric shape
Orientation in the magnet
Distance from the radiofrequency transmitter
Physical proximity to tissues
Insulation
Body mass and surface area
Convective cooling of intravascular devices by local blood flow
General body temperature
Implanted conductive devices
Tissue thermosensitivity
Field strength
Pulse sequence
Flip angle
Scanning duty cycle
Position relative to bore isocenter (closer is better)
Rapid CMR is required for invasive procedures, for catheter visualization, and for imaging of anatomic structures. Efficient image data sampling methods, parallel imaging, and coherent steady state techniques have enabled real-time CMR without significant degradation in image quality in the field of interest. Frame rates as high as 10 to 15 images per second are now possible using multichannel (≥32) coils that use parallel imaging techniques to achieve acceleration factors of three or more. In addition, interactive, real-time color flow imaging may supplement anatomic detail with critical physiologic detail, such as leaks or gradients, during therapeutic procedures and interventions. Slice orientation can automatically follow the tip of catheter devices as they move, so-called adaptive imaging, so that catheter features are kept in view during manipulation. These methods also can be applied automatically to alter scanning parameters such as field-of-view and temporal resolution.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here