Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Many diseases involve predominantly or exclusively the pulmonary interstitium. The differential diagnosis of the various entities on the chest radiograph and computed tomography (CT) is based on the pattern and distribution of abnormalities and on the presence of associated findings, such as lymph node enlargement or pleural effusion. Interstitial lung disease results in six distinct radiologic patterns of abnormality: septal, reticular, cystic, nodular, and ground-glass opacities and consolidation. Although each of these patterns can be seen on high-resolution CT and correlated with specific histopathologic findings, the appearances on the chest radiograph are frequently nonspecific and sometimes misleading. A reticular pattern on the radiograph may result from summation of smooth or irregular linear opacities, cystic spaces, or both. In approximately 10% of patients with interstitial lung disease, the chest radiograph is normal. Numerous studies have shown that CT, particularly high-resolution CT, is superior to chest radiography in the detection of parenchymal abnormalities and is more accurate in the differential diagnosis. High-resolution CT is performed almost routinely in the evaluation of patients with suspected or proven interstitial lung disease.
Because of its low cost and low radiation dose, the chest radiograph remains the initial imaging modality of choice in the evaluation of patients with suspected interstitial lung disease and in the follow-up of these patients. Serial chest radiographs often provide a clue to the correct diagnosis by showing the course of the disease process. In this chapter we include the radiographic and the high-resolution CT patterns of abnormality, recognizing that in a high percentage of cases accurate assessment of presence, pattern, and extent of interstitial disease can be made only on high-resolution CT. Consolidation may occur in interstitial lung disease but more commonly reflects the presence of airspace disease. Consolidation is discussed separately in Chapter 2 .
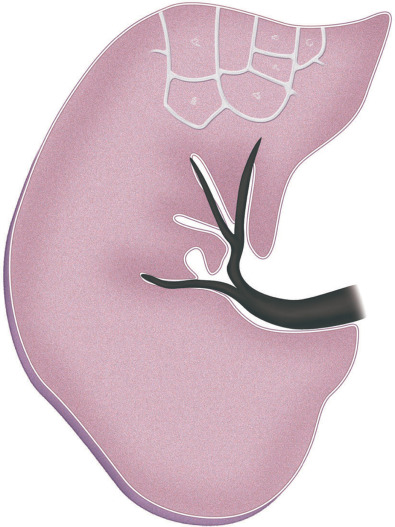
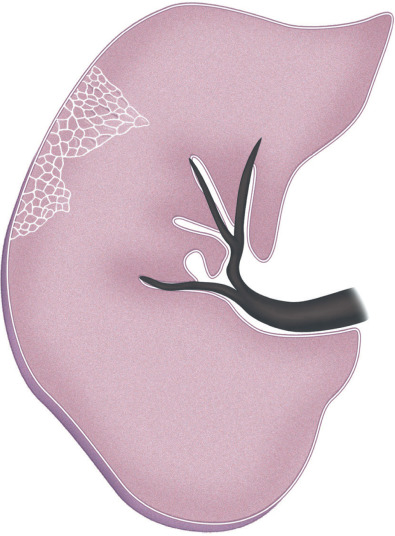
A septal pattern results from thickening of the interlobular septa (i.e., the tissue that separates the secondary pulmonary lobules) ( Fig. 5.1 ). Normally, no septal lines can be identified on the radiograph, and only a few can be seen on high-resolution CT, mostly in the anterior and lower aspects of the lower lobes. When thickened, interlobular septa (septal lines) are seen on the radiograph as short (1–2 cm) lines perpendicular to and continuous with the pleura (Kerley B lines) or as longer (2–6 cm) lines oriented toward the hila (Kerley A lines) ( Fig. 5.2 ). On high-resolution CT scan septal lines can be seen as short lines that extend to the pleura in the lung periphery and as polygonal arcades outlining one or more pulmonary lobules in more central lung regions (see Fig. 5.2 ). Thickening of the interlobular septa may be caused by edema, cellular infiltration, or fibrosis. Septal thickening can be smooth, nodular, or irregular in contour.
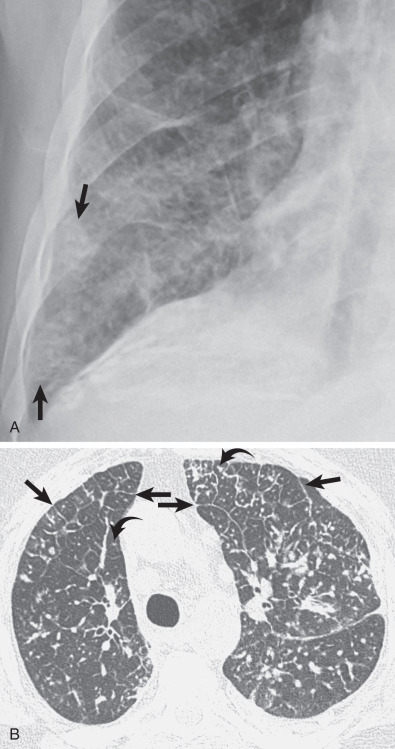
The most common cause of a septal pattern as the predominant or only pattern is hydrostatic pulmonary edema ( Figs. 5.3 and 5.4 ). Less common causes of smooth septal thickening include lymphangitic spread of carcinoma (see Fig. 5.2 ), lymphoma ( Fig. 5.5 ), leukemia, Churg-Strauss syndrome, acute lung rejection ( Fig. 5.6 ), congenital lymphangiectasia, and Niemann-Pick syndrome ( Fig. 5.7 ). These various conditions tend to result in extensive septal thickening that is usually bilateral and often symmetric. Localized smooth septal thickening is frequently seen adjacent to pleural inflammation ( Fig. 5.8 ), particularly in patients with empyema or after pleurodesis, lymphoid interstitial pneumonia, and idiopathic bronchiectasis. The interlobular septal thickening in patients with bronchiectasis is presumably due to impaired lymphatic drainage, and the extent of septal lines correlates with the extent and severity of bronchiectasis. Nodular septal thickening occurs most commonly in patients with lymphangitic carcinomatosis ( Figs. 5.9 and 5.10 ), sarcoidosis ( Fig. 5.11 ), Kaposi sarcoma, leukemia, and amyloidosis.
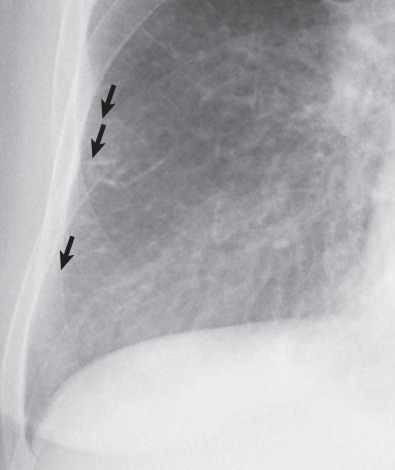
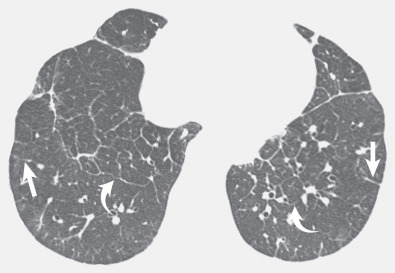
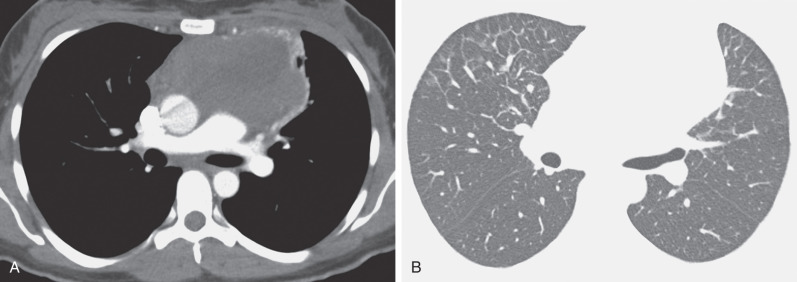
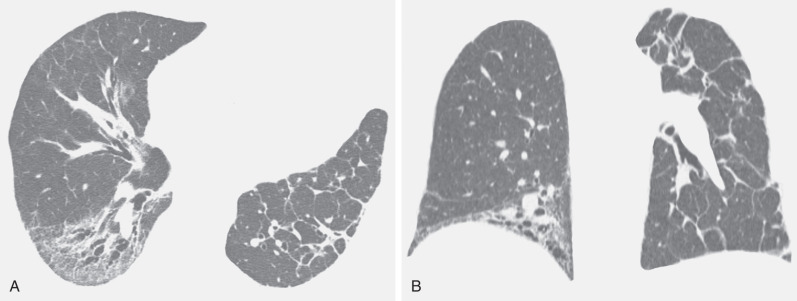
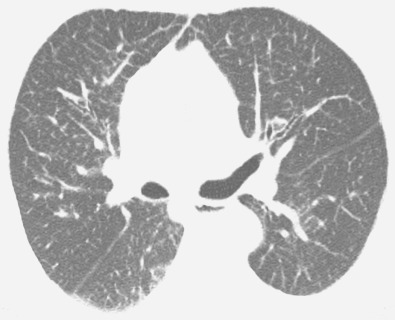
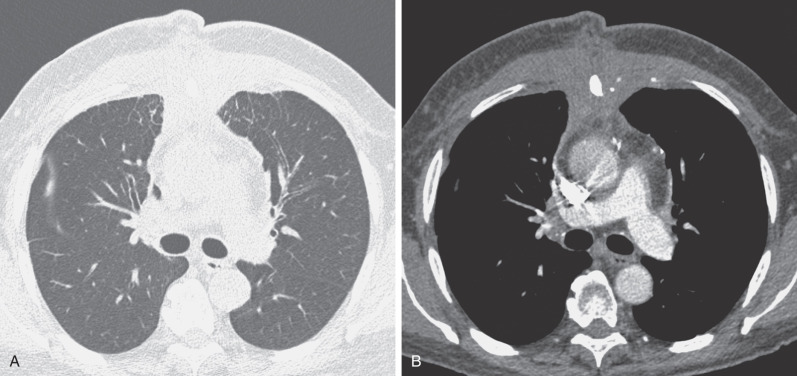
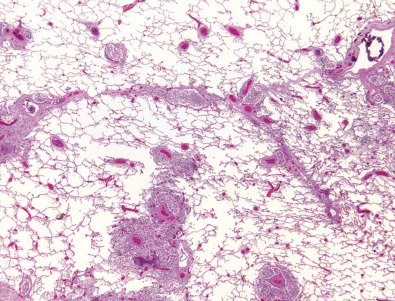
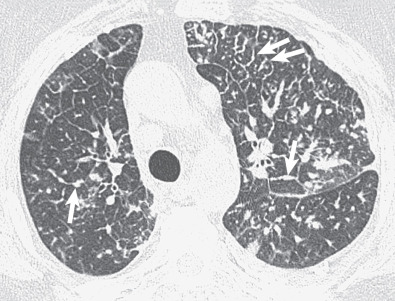
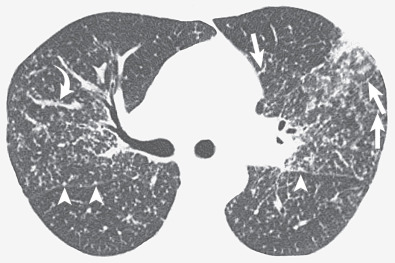
Patients with lymphangitic spread of tumor may have smooth or nodular septal thickening (see Figs. 5.2 and 5.10 ). In most cases septal thickening is the main abnormality seen in these patients. Other common findings include unilateral or asymmetric hilar lymph node enlargement and pleural effusion. Although septal thickening may be seen in sarcoidosis, it is seldom the main finding. The nodules in sarcoidosis are typically most numerous along the bronchoarterial and pleural interstitium and along the interlobar fissures (see Fig. 5.11 ).
Irregular septal thickening is seen most commonly in patients with interstitial fibrosis, particularly idiopathic pulmonary fibrosis, asbestosis, and sarcoidosis ( Fig. 5.12 ). It is usually associated with other findings of fibrosis, such as reticulation, traction bronchiectasis, and traction bronchiolectasis, and is seldom the predominant pattern. The main abnormalities in idiopathic pulmonary fibrosis typically consist of predominantly peripheral and basal reticulation and, commonly, honeycombing.
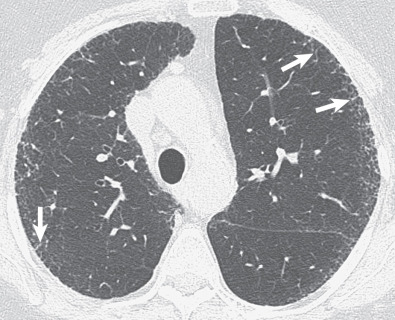
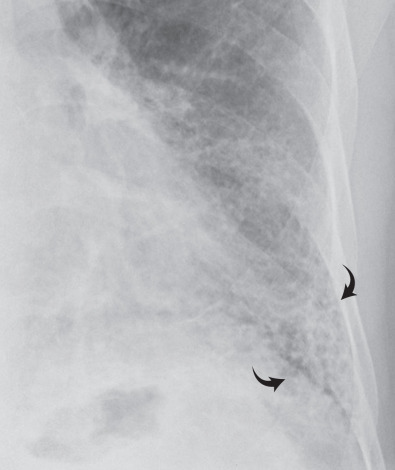
A reticular pattern is characterized by innumerable interlacing line shadows that suggest a mesh ( Fig. 5.13 ). On the chest radiograph the pattern may be the result of summation of smooth or irregular linear opacities, cystic spaces, or both ( Fig. 5.14 ). Although distinction between these abnormalities often is difficult on the radiograph, it can be made readily on high-resolution CT. The reticular pattern on high-resolution CT results from small irregular intralobular linear opacities separated by only a few millimeters ( Figs. 5.15 and 5.16 ). Intralobular linear opacities reflect thickening of the interstitium within the secondary pulmonary lobule and are most commonly caused by fibrosis. Fibrosis also typically results in distortion of the parenchymal architecture, traction bronchiectasis, traction bronchiolectasis, and honeycombing ( Fig. 5.17 ; see also Fig. 5.16 ). Common causes of a reticular pattern include usual interstitial pneumonia, nonspecific interstitial pneumonia, fibrosis associated with collagen vascular disease, chronic hypersensitivity pneumonitis, sarcoidosis, and asbestosis.
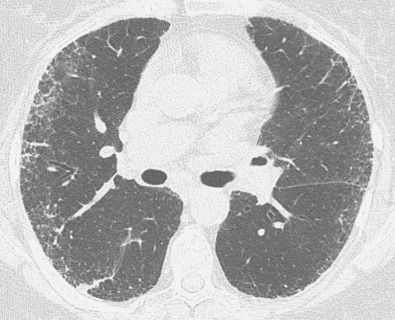
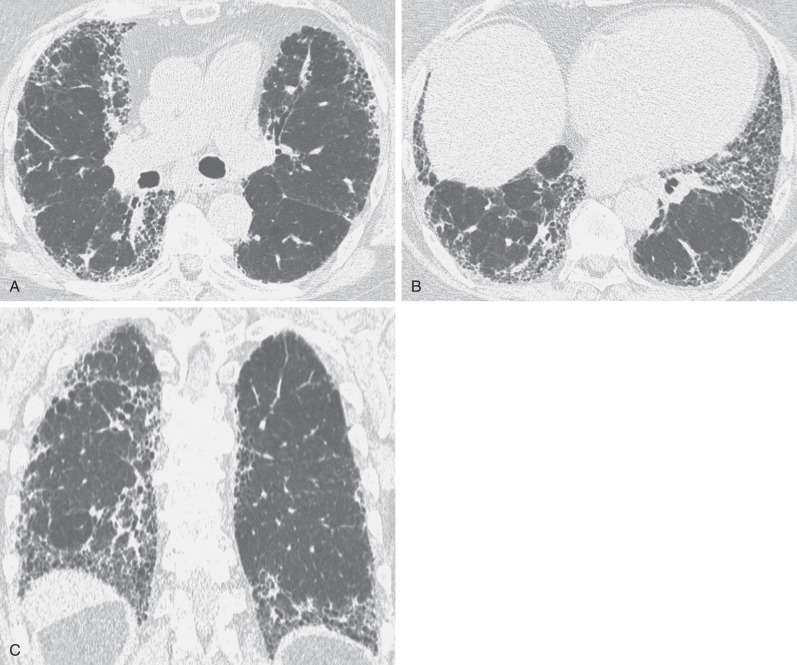
Idiopathic pulmonary fibrosis is a chronic interstitial fibrosis limited to the lung and associated with the imaging and histologic pattern of usual interstitial pneumonia. The radiographic findings consist of a symmetric, bilateral reticular pattern that may be diffuse but tend to involve predominantly the lower lung zones (see Fig. 5.14 ). In 60% of patients a predominant peripheral distribution is apparent on the radiograph. As the disease progresses, the reticular pattern becomes coarser and is associated with progressive loss of volume.
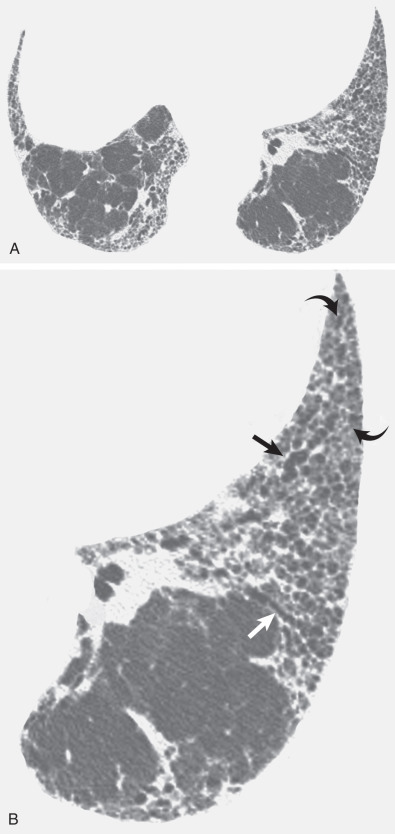
The characteristic high-resolution CT findings of idiopathic pulmonary fibrosis consist of a reticular pattern in a patchy distribution and involving all lobes but being most severe in the subpleural lung regions and in the lung bases (see Figs. 5.16 and 5.17 ). The reticular pattern is usually associated with irregular pleural, vascular, and bronchial interfaces; evidence of architectural distortion; and dilation of bronchi and bronchioles (traction bronchiectasis and bronchiolectasis) (see Figs. 5.16 and 5.17 ). Subpleural air-containing cysts measuring 2 to 20 mm in diameter (honeycombing) lining up in rows or stacks are present in 80% to 90% of patients at diagnosis.
A reticular pattern also frequently is seen in patients with nonspecific interstitial pneumonia. Nonspecific interstitial pneumonia is a chronic interstitial lung disease characterized histologically by a combination of interstitial fibrosis and inflammation that resembles idiopathic pulmonary fibrosis clinically but has a considerably better prognosis. Nonspecific interstitial pneumonia is rarely idiopathic and is more commonly secondary to connective tissue disease (particularly scleroderma), drug reaction, or hypersensitivity pneumonitis. The radiographic and high-resolution CT findings most commonly consist of ground-glass opacities and a fine reticular pattern ( Fig. 5.18 ). Evidence of architectural distortion with traction bronchiectasis and bronchiolectasis is commonly seen on high-resolution CT, but honeycombing is uncommon at presentation. The abnormalities may be diffuse, but they involve mainly the lower lung zones in 60% to 90% of cases and predominantly the lung periphery in 50% to 70%. Although the fibrosis in nonspecific interstitial pneumonia is often predominantly peripheral and basal, in approximately 50% of patients there is relative sparing of the immediate subpleural lung in the dorsal regions of the lower lobes ( Fig. 5.19 ). The presence of predominantly ground-glass opacities, relatively mild reticulation, absence of honeycombing, and relative sparing of the subpleural regions allow distinction of nonspecific interstitial pneumonia from usual interstitial pneumonia in most cases. It may be impossible, however, to distinguish fibrotic nonspecific interstitial pneumonia with a predominantly reticular pattern from usual interstitial pneumonia.
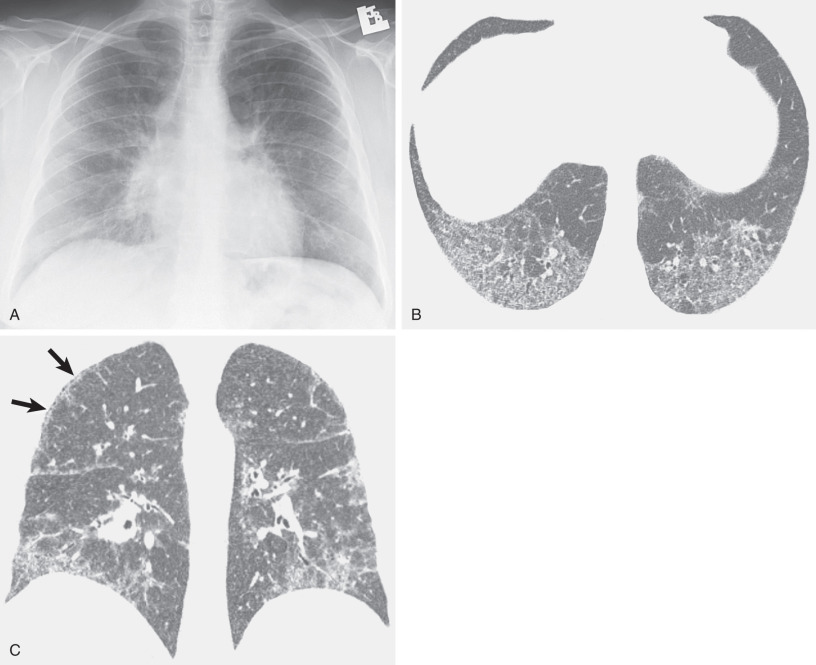
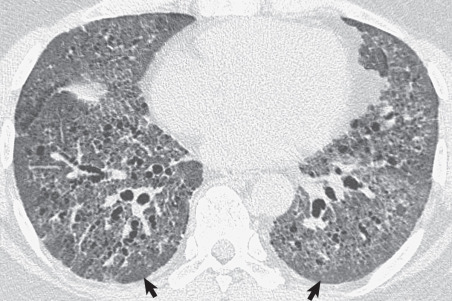
The most common collagen vascular diseases associated with a reticular pattern on the chest radiograph and high-resolution CT are scleroderma and rheumatoid arthritis. The histologic, radiographic, and high-resolution CT manifestations of scleroderma are usually those of nonspecific interstitial pneumonia ; rheumatoid arthritis tends to result in findings of usual interstitial pneumonia and, less commonly, nonspecific interstitial pneumonia.
In chronic hypersensitivity pneumonitis, the fibrosis is situated predominantly in the middle lung zones or shows no zonal predominance ( Fig. 5.20 ). Lung apices and bases are relatively spared. The distribution of fibrosis on high-resolution CT is commonly random but may be predominantly subpleural or peribronchovascular. In most cases there are superimposed findings of subacute disease, typically poorly defined centrilobular nodules, extensive bilateral ground-glass opacities, and lobular areas with decreased attenuation and vascularity (i.e., mosaic attenuation) with air trapping on expiratory images (see Fig. 5.20 ). The predominantly middle lung zone distribution of fibrosis and the presence of centrilobular nodules and lobular areas of air trapping allow recognition of hypersensitivity pneumonitis in most cases. In some patients the findings may mimic those of usual interstitial pneumonia or nonspecific interstitial pneumonia.
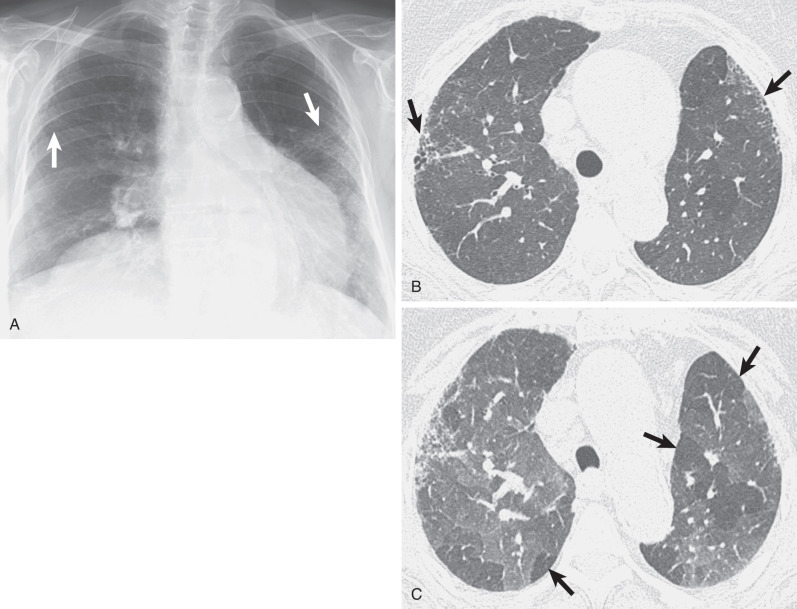
A reticular pattern is seen in 15% to 20% of patients who have sarcoidosis and radiographically evident parenchymal abnormalities. The fibrosis in sarcoidosis typically involves mainly the upper and middle lung zones. It is typically associated with superior retraction of the hila, traction bronchiectasis of the central bronchi, and compensatory overinflation of the lower lobes ( Fig. 5.21 ).
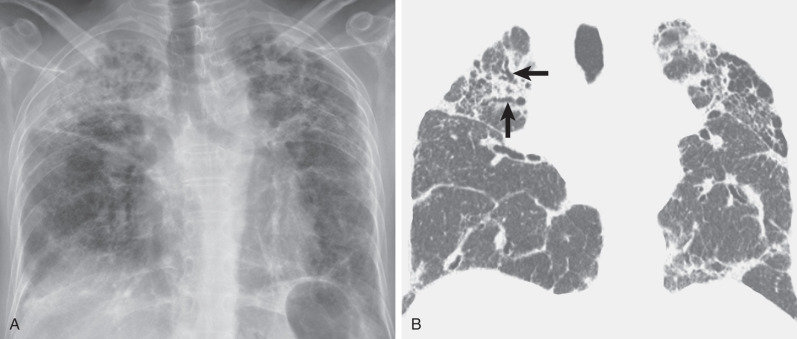
The reticular pattern in asbestosis is usually mild and involves predominantly or exclusively the lower lung zones. High-resolution CT typically shows subpleural reticulation, subpleural curvilinear opacities, and small rounded or branching subpleural opacities involving mainly the dorsal regions of the lower lung zones. Most patients have associated pleural plaques or diffuse pleural thickening.
Cystic airspaces are round, air-containing parenchymal spaces with a well-defined wall. The term is used to describe multiple diffusely enlarged airspaces (diffuse cystic lung disease) seen in Langerhans cell histiocytosis, lymphangioleiomyomatosis, and lymphoid interstitial pneumonia (although rarely, Birt-Hogg-Dubé syndrome, amyloidosis, and light-chain deposition disease can also manifest with this imaging appearance); in end-stage fibrosis (honeycombing) as may be seen in idiopathic pulmonary fibrosis; and, less commonly, in chronic hypersensitivity pneumonitis, nonspecific interstitial pneumonia, asbestosis, and sarcoidosis. Among the conditions that cause diffuse cystic lung disease, Langerhans cell histiocytosis and lymphangioleiomyomatosis are the most commonly encountered diagnostic considerations.
Pulmonary Langerhans cell histiocytosis is a disease characterized histologically by infiltration of the lung by Langerhans cells that typically manifests in young adults and is seen almost exclusively in smokers. The parenchymal abnormalities are typically bilateral, symmetric, and diffuse throughout the upper and middle lung zones, with sparing of the costophrenic angles. The chest radiograph tends to show a reticular or reticulonodular pattern ( Fig. 5.22 ). The characteristic findings on high-resolution CT consist of cysts (present in ≈80% of patients) and nodules (present in 60%–80%). The nodules predominate in the early phases, and the cysts predominate in the late stages of the disease. The nodules range from 1 to 10 mm in diameter, and the cysts range from a few millimeters to several centimeters in diameter and may be round or oval or have bizarre shapes with bilobed, cloverleaf, and branching configuration. Regardless of the stage of the disease, the abnormalities are diffuse throughout the middle and upper lung zones, with relative sparing of the lung bases (see Fig. 5.22 ).
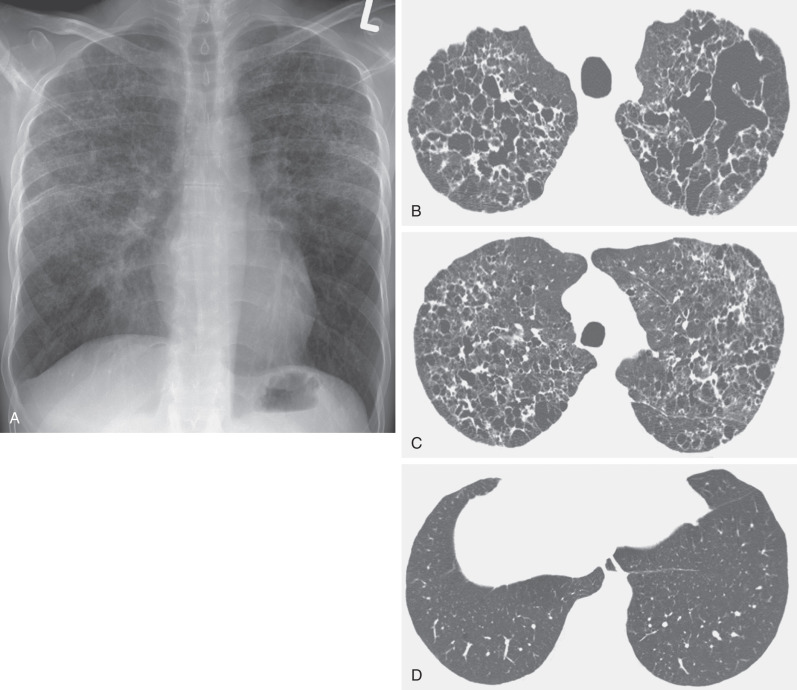
Lymphangioleiomyomatosis is a rare disease limited almost exclusively to women and characterized by cyst formation and an interstitial proliferation of smooth muscle–like cells. The radiographic manifestations consist of a diffuse bilateral reticular pattern and, commonly, evidence of hyperinflation manifested by an increase in the retrosternal clear space or flattening of the diaphragm ( Fig. 5.23 ). Cysts can be identified on the radiograph in 50% to 60% of cases. The characteristic high-resolution CT finding consists of numerous thin-walled, air-filled cysts surrounded by normal lung parenchyma (see Fig. 5.23 ). The cysts can be seen on high-resolution CT in patients who have normal radiographs or radiographs showing only reticular opacities. They usually measure 0.2 to 2 cm in diameter and, unlike pulmonary Langerhans cell histiocytosis, are distributed diffusely throughout the lungs without any zonal predominance.
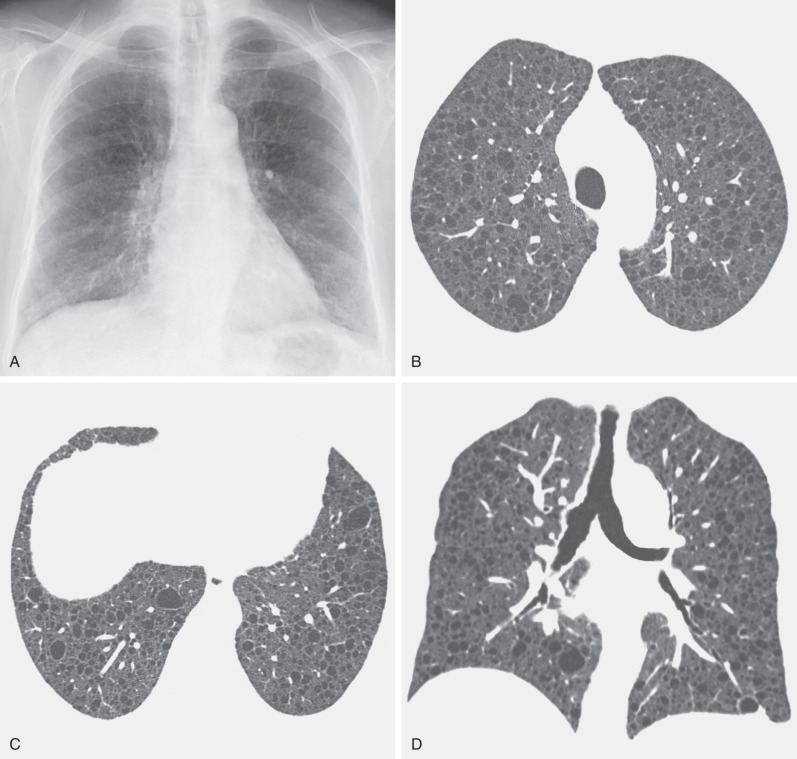
Cystic spaces are seen in approximately 30% of patients with Pneumocystis jirovecii pneumonia. These may be single or multiple, occur mainly in the upper lobes, and are typically superimposed on ground-glass opacities ( Fig. 5.24 ). The cysts in Pneumocystis pneumonia may represent pneumatoceles, premature emphysema, or occasionally cavitated nodules.
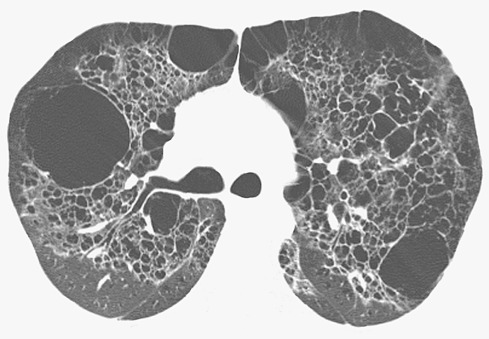
Single or multiple cysts typically superimposed on ground-glass opacities also are seen in approximately 60% of patients with lymphoid interstitial pneumonia and 13% of patients with subacute hypersensitivity pneumonitis. The cysts in lymphoid interstitial pneumonia are usually few in number and tend to involve mainly the lower lung zones ( Fig. 5.25 ); lymphoid interstitial pneumonia may also result in extensive cyst formation. The cysts in subacute hypersensitivity pneumonitis may be single or multiple and have a random distribution. In a study of 36 patients with chronic hypersensitivity pneumonitis, Silva and colleagues showed the presence of a few cysts in 14 patients (39%). In most cases the predominant abnormalities of Pneumocystis pneumonia, lymphoid interstitial pneumonia, and subacute hypersensitivity pneumonitis are ground-glass opacities.
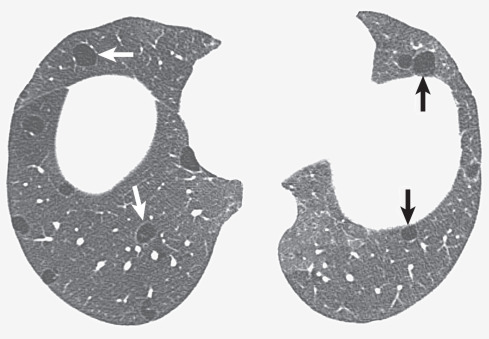
Honeycombing refers to the presence of cystic spaces, usually measuring 0.3 to 1 cm in diameter, whose walls consist of a variable amount of fibrous tissue ( Fig. 5.26 ). Honeycombing typically tends to be most severe in the subpleural regions and along the interlobar fissures. The most common diseases in which the abnormality is identified are idiopathic pulmonary fibrosis ( Fig. 5.27 ), connective tissue disease, and sarcoidosis; however, the process can be seen in the advanced stage of pulmonary fibrosis of any cause. The spaces represent mainly respiratory bronchioles and alveolar ducts that have become dilated as a result of traction by the adjacent fibrous tissue. The presence, distribution, and extent of honeycombing are assessed much more readily on high-resolution CT scan than on chest radiography. The differential diagnosis of conditions associated with honeycombing is similar to that of reticulation.
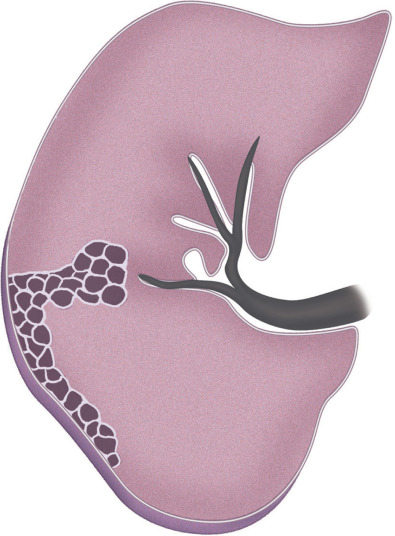
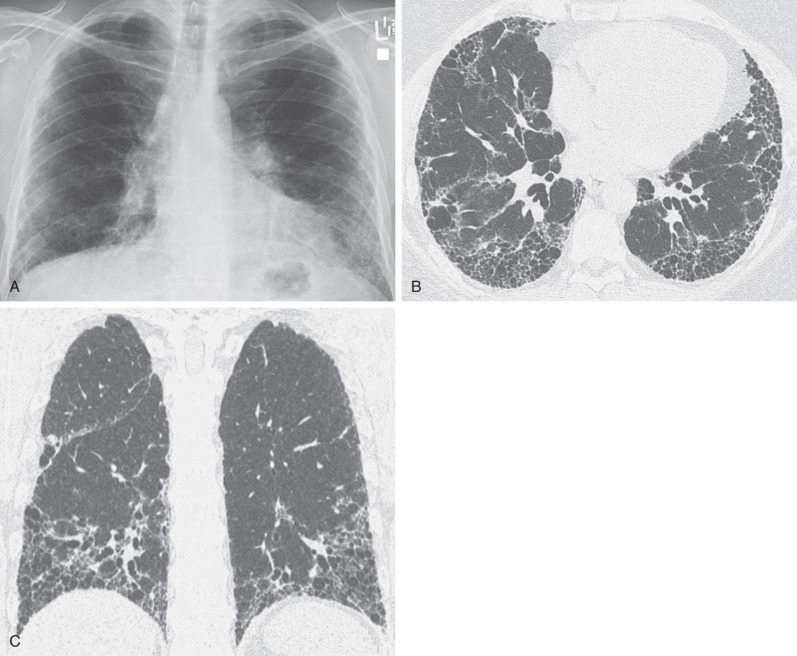
In most patients with cystic abnormalities, a confident diagnosis can be made on high-resolution CT based on the appearance and distribution of the cystic changes and the presence of associated findings, such as nodules and ground-glass opacities ( Fig. 5.28 ). Similar to other interstitial findings, the final diagnosis requires integration of the high-resolution CT findings with the clinical information and laboratory data. Some patients may require lung biopsy for a definitive diagnosis.
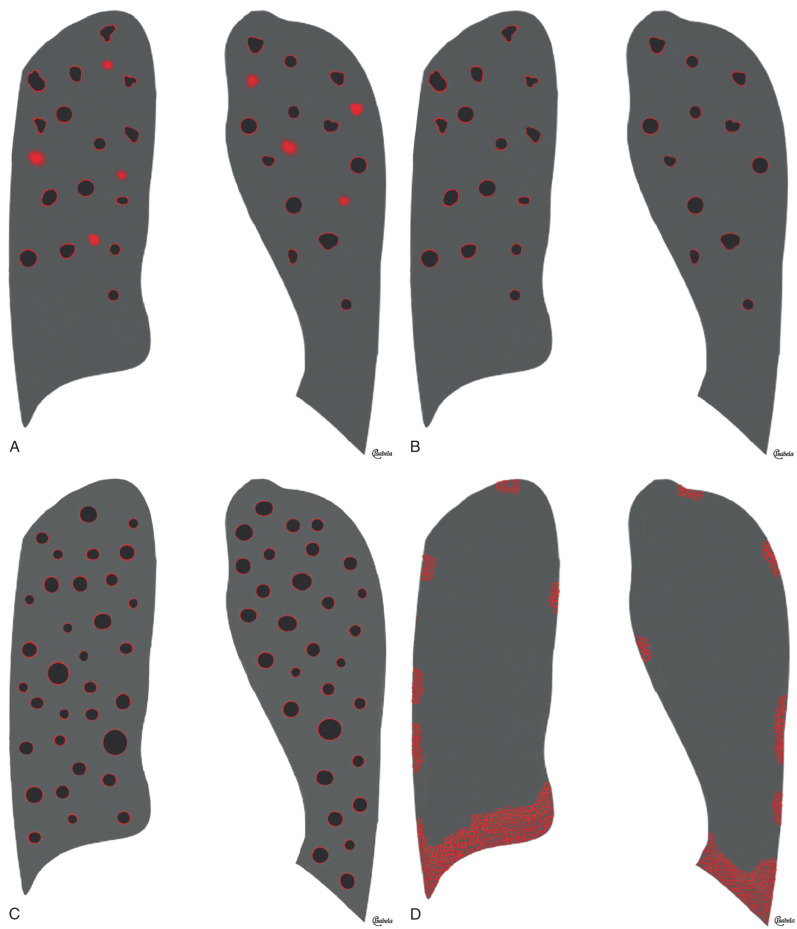
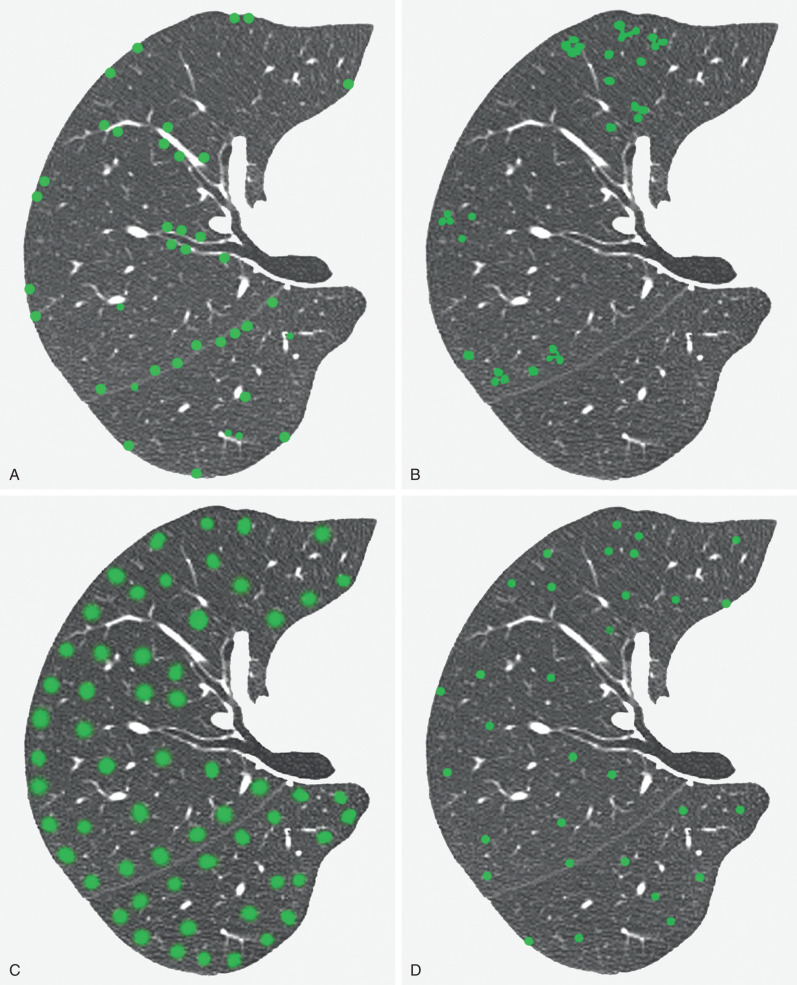
A nodular pattern is characterized by the presence of numerous round opacities measuring less than 1 cm in diameter. The pattern results from expansion of the parenchymal interstitium by a roughly spherical cellular infiltrate, fibrous tissue, or both. The differential diagnosis is based on the pattern and distribution of the nodules, the presence of associated findings such as lymph node enlargement, and the clinical setting. A diffuse small nodular pattern in a febrile patient with acute disease is most suggestive of hematogenous infection, particularly miliary tuberculosis. A similar pattern in a patient with chronic symptoms may result from silicosis, coal workers' pneumoconiosis, intravenous talcosis, metastatic carcinoma (particularly from the thyroid), and adenocarcinoma. The overall distribution of nodules on the radiograph often helps to narrow the differential diagnosis. The nodules in silicosis and coal workers' pneumoconiosis may be diffuse but most commonly involve mainly the middle and upper lung zones, whereas the nodules resulting from hematogenous processes, such as miliary tuberculosis and metastatic carcinoma, are diffuse or involve mainly the lower lung zones (where blood flow is greater). The various diseases resulting in multiple nodules typically result in three main patterns of distribution on high-resolution CT: perilymphatic, centrilobular, and random ( Fig. 5.29 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here