Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The discovery of inhalational agents played a critical role in anesthesia history. The search for the perfect agent capable of rapid induction of anesthesia, one with a high safety profile and minimal side effects, has led research efforts for over a hundred years and still continues. The early reports of the use of nitrous oxide, ether, and chloroform as inhalation anesthetics began to emerge in 1840s ( ), including the first successful public demonstration of ether anesthesia by William Morton on October 16, 1846. Subsequently, a number of unexpected observations led to the discovery of the second generation of inhaled anesthetics: ethyl chloride, ethylene, cyclopropane, and divinyl ether. During World War II, techniques of fluorine chemistry enabled development of compounds halogenated with fluorine, and in the mid-1950s, the first halogenated anesthetic, halothane, replaced pungent, flammable, and toxic earlier agents. Anesthetics with lower solubility, sevoflurane and desflurane, halogenated exclusively with fluorine, were first synthetized in 1960s and 1970s. Modern-day derivatives of ether and nitrous oxide remain the most common anesthetic drugs administered for general anesthesia in children.
The actions of general anesthetics at the molecular, cellular, and neuronal network levels produce a complex set of clinical effects: unconsciousness, amnesia, analgesia, and immobility ( ). Anesthetic effects on memory and consciousness have been shown to involve regulation of thalamic activity and neuronal network trafficking ( ).
The early hypothesis of nonspecific interactions of anesthetics with the lipid bilayer that led to changes in neuronal function was supported by the Meyer-Overton correlation between anesthetic potency and lipid solubility, but more recent studies have demonstrated protein targets for anesthetic actions. Specifically, anesthetics directly interact with membrane channel proteins and receptor proteins without altering lipid bilayer properties at clinically relevant concentrations ( ). Proteins that have been shown to be affected by general anesthetic agents include gamma-aminobutyric acid A (GABA A ), acetylcholine, glycine, glutamate, N-methyl- d -aspartate (NMDA), two-pore domain potassium channels, and voltage-gated sodium channels ( ; ).
GABA A receptor agonism and NMDA receptor antagonism are two principal mechanisms that have been proposed to explain anesthetic-induced unconsciousness. Based on their pharmacologic properties, inhaled anesthetics can be divided into two classes. The first class is the potent inhaled anesthetics, which modulate GABA A receptors and produce anesthesia effects on a number of other receptors and ion channels. The second class is the gaseous inhaled anesthetics, nitrous oxide and xenon, that block NMDA receptors and activate certain ion channels at clinical concentrations ( ). One of the most important targets for potent inhaled anesthetics are the synaptic and extrasynaptic GABA A receptors ( Fig. 10.1 ). Most GABA A receptors consist of two α subunits, two β subunits, and one γ subunit, with the most common pattern for the GABA A receptor being the α1β2γ2 type ( ). The α subunit of the GABA A receptor has been identified as a binding site for inhaled anesthetics. Inhibition of GABA uptake by volatile anesthetics results in higher extracellular GABA concentration, which may lead to prolonged activation of extrasynaptic GABA A receptors ( ). Extrasynaptic GABA A receptors, which differ from synaptic receptors in their subunit composition, have a high affinity for GABA, desensitize slowly, and are remarkably sensitive to many general anesthetics ( ). Increased activity of extrasynaptic GABA A receptors reduces neuronal excitability, impairs synaptic plasticity, and contributes to memory blockade caused by volatile anesthetics ( ).
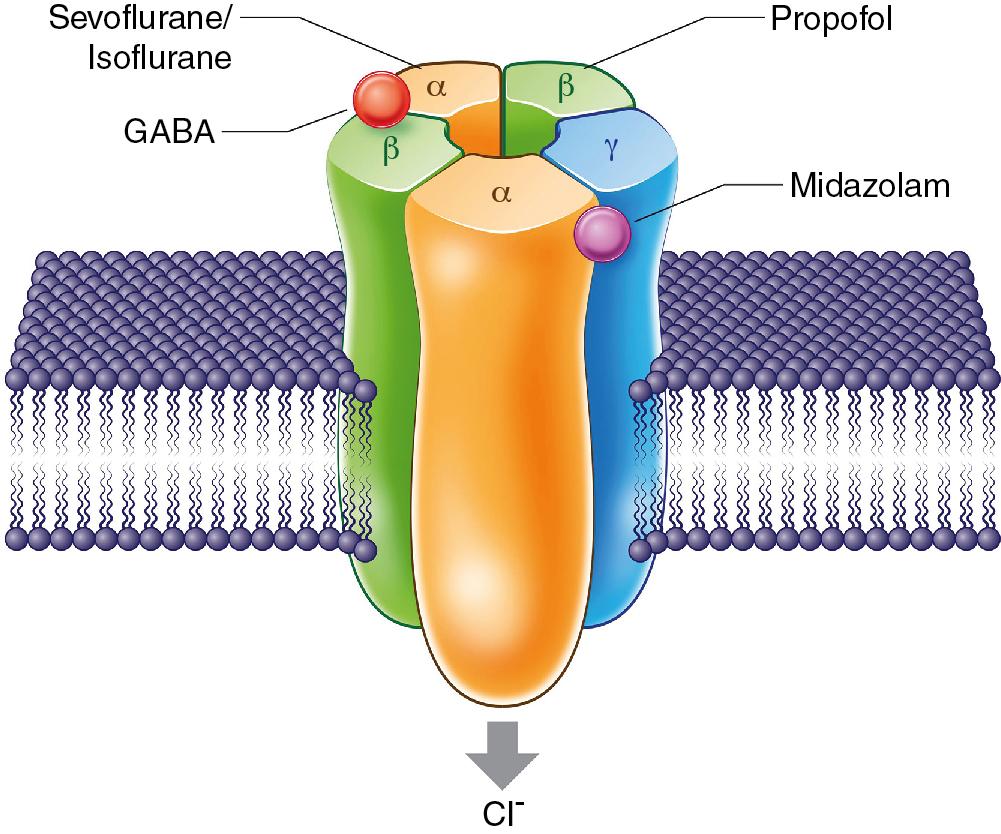
Pertinent to the ontogeny of GABA A receptors system, agonism of GABA A receptors undergoes a developmental switch from excitatory to inhibitory effects. At early stages of development, activation of GABA A receptors produce excitatory effects, resulting in activation of calcium-sensitive signaling processes that are important for the neuronal differentiation and brain development. With maturation, GABA A receptors transmit inhibitory signals . Studies demonstrated that in embryonic and immature neurons, GABA exhibits neurotrophic properties by regulating the neuronal proliferation, migration, differentiation, dendritogenesis, and synaptogenesis ( ; ). In developing brains, transient GABA A receptor-mediated inhibition of excitatory activity of neurons by inhaled anesthetics can potentially affect neurogenesis and may have long-term developmental consequences ( ).
The most widely used inhalational anesthetics are sevoflurane, isoflurane, desflurane, and nitrous oxide. Although halothane is no longer clinically available in North America, it is still widely used worldwide, particularly in developing countries, because of its relatively low cost. Halothane is a polyhalogenated alkane, and sevoflurane, isoflurane, and desflurane are polyhalogenated ethers. Xenon is a naturally occurring noble gas, and though its clinical use has been described, it is not readily available. The properties of the anesthetic agents are summarized in Table 10.1 .
| Halothane | Isoflurane | Sevoflurane | Desflurane | Nitrous Oxide | Xenon | |
|---|---|---|---|---|---|---|
| Molecular weight | 197.4 | 184.5 | 200.1 | 168 | 44 | 131 |
| Boiling point (°C) | 50.2 | 48.5 | 58.6 | 23.5 | –88.5 | –108.1 |
| Vapor pressure (mm Hg) | 244 | 240 | 185 | 664 | — | — |
| Metabolized (%) | 15–25 | 0.2 | 5 | 0.02 | — | — |
| Solubility | ||||||
| Blood: gas partition coefficient | 2.4 | 1.4 | 0.66 | 0.42 | 0.47 | .115 |
| Fat: blood partition coefficient | 51 | 45 | 48 | 27 | 2.3 | Not known |
| MAC | ||||||
| MAC in adults (%) | 0.75 | 1.2 | 2.05 | 7 | 104 | 71 |
| MAC in a 2-year-old (%) | 0.97 | 1.6 | 2.6 | 8.73 | Not known | Not known |
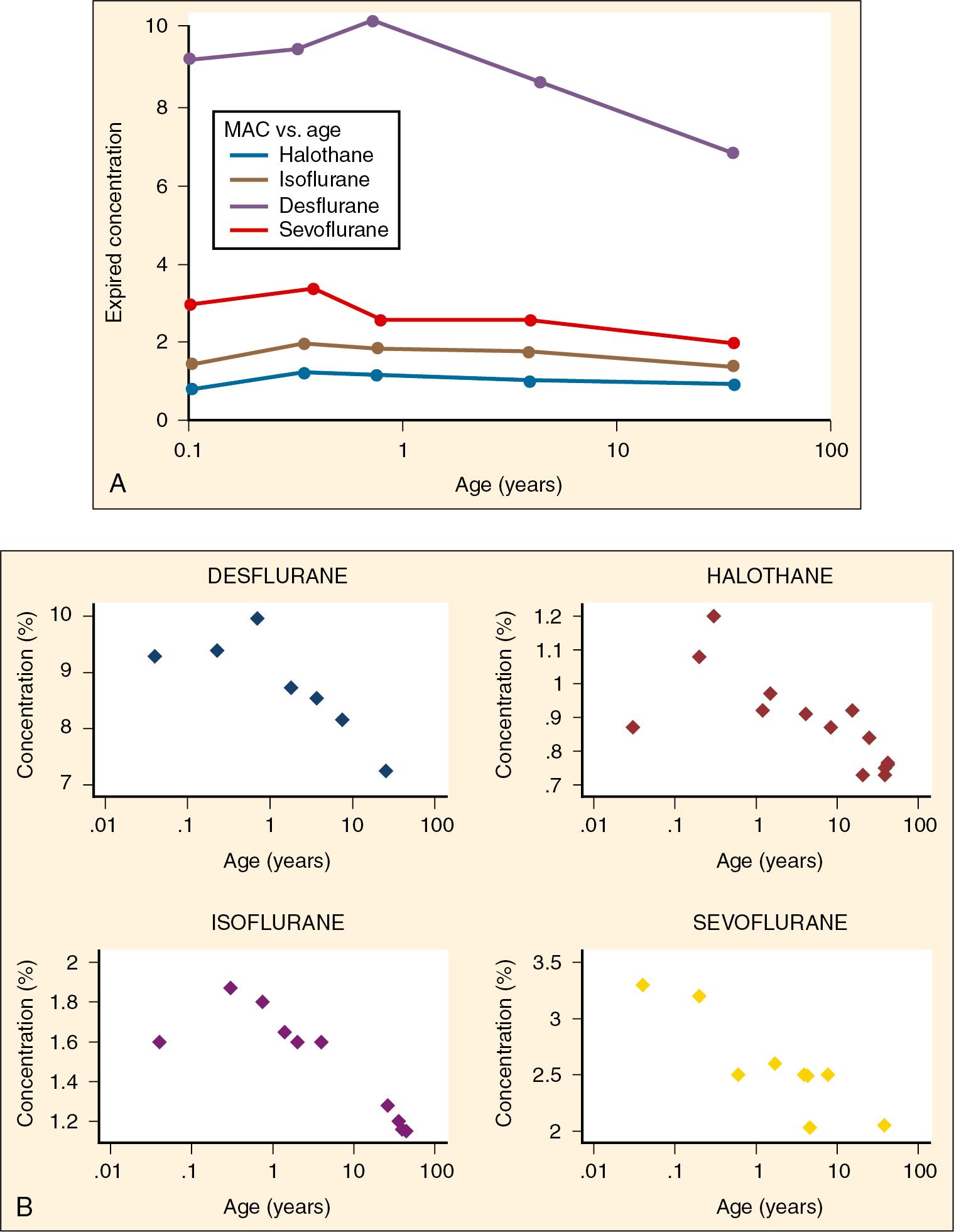
The potency of an inhalation anesthetic agent is defined in terms of MAC, the minimum alveolar concentration, where 1 MAC is the concentration that prevents movement in 50% of subjects in response to a noxious stimulus. Each anesthetic agent has a different MAC, and it is inversely related to lipid solubility, with desflurane being 8.7%, sevoflurane 2.6%, isoflurane 1.6%, and halothane 0.97% for 2-year-olds (see Table 10.1 ). MAC can change with age. It rises during the neonatal period, peaks in infancy, and then declines throughout life ( Table 10.2 and Fig. 10.2 ). MAC is at the lowest values in old age ( Fig. 10.2 ) ( ; ; ; ). It is unknown why MAC is higher in infants compared with older children. MAC or the movement to stimulus may not reflect electroencephalogram (EEG) activity, consciousness, or depth of anesthesia because movement and therefore MAC are determined by spinal motor reflexes rather than by cortical suppression. Age affects BIS inversely such that the younger the child, the higher the BIS for a specific MAC value. In infants less than 1 year of age, preawakening BIS values have been reported to be lower than those obtained in children older than 1 year ( ). Therefore target BIS values of 40 to 60 in infants less than 6 months can be achieved with very low concentrations of sevoflurane.
| Parameter | MAC Anesthetic Concentration |
|---|---|
| Halothane | |
| Neonates | 0.87 |
| 1–6 months | 1.20 |
| 15 ± 7 months | 0.94 |
| Isoflurane | |
| Preterm <32 weeks | 1.28 |
| Preterm 32–37 weeks | 1.41 |
| 7–30 months | 1.69 |
| 4–10 years | 1.69 |
| Sevoflurane | |
| Neonates | 3.3 |
| 1–6 months | 3.2 |
| 6–12 months | 2.5 |
| 1–3 years | 2.6 |
| 2–12 years | 2.3–2.5 |
| Desflurane | |
| Neonates | 9.16 |
| 1–6 months | 9.42 |
| 6–12 months | 9.92 |
| 1–3 years | 8.72 |
| 3–5 years | 8.62 |
| 5–12 years | 7.98 |
MAC-awake is the concentration at which consciousness is both lost and regained. It is the anesthetic concentration needed to suppress a voluntary response to a verbal command (i.e., eye-opening) in 50% of patients and is usually one-third of MAC for the inhaled anesthetic.
MAC-awake for sevoflurane has been reported to be 0.78% in a sample of children aged 2 to 12 years, but it is unclear how MAC-awake changes with age ( ). The dose-response curve showed that the concentration at which 50% of patients respond to verbal commands is markedly lower as the concentration is decreased (emergence from anesthesia) compared with when the concentration is increased (induction of anesthesia). The gap between MAC-awake and MAC-unaware is unpredictable. It is possible that when the volatile concentration drops below the induction MAC-awake value during maintenance of anesthesia, the majority of patients will still remain unaware (see Table 10.3 ).
| Agent | Population | MAC in Air | MAC N 2 O 0.75MAC | MAC Bar 1.6MAC | MAC LMA Insertion | MAC LMA Removal | MAC EI 1.3MAC | MAC BIS50 | MAC Awake Ratio MAC-Awake/MAC 0.3MAC |
|---|---|---|---|---|---|---|---|---|---|
| Sevoflurane | Neonates | 3.3% | |||||||
| Infants | 2.5% | 2.83% | 3.4% | 0.66% 1.4% | |||||
| Children | 2.6% | 1.1% | 4.8% 1.45 MAC | 1.57% | 1.84% | 3.11% 2.9% | 2.83% 2.3% | 0.45% 0.78% | |
| Adults | 1.85% | 0.66% | 4.15% 2.24 MAC | 3.55% | 1.28% | 0.61% | |||
| Desflurane | Neonates | 9.16% | |||||||
| Infants | 9.42% | 7.5% | |||||||
| Children | 8.62% | 6.4% | 1.3 MAC | 3.6% | 3.43% | ||||
| Adults | 6.6% | 2.83% | 5.29% | 5.29% | 2.6% | ||||
| Halothane | Neonates | 0.87% | |||||||
| Infants | 1.2% | 1.16% | |||||||
| Children | 0.94 | 1.12% 1.45 MAC | |||||||
| Adults | 0.75% | 0.29% | 0.41% |
Sevoflurane is currently the most widely used inhalation anesthetic for the induction of anesthesia in children. It is a polyfluorinated methyl isopropyl ether anesthetic that is in liquid form at atmospheric pressures and room temperature, with a much higher MAC than isoflurane and halothane. Sevoflurane is a far less potent anesthetic that is well tolerated in unpremedicated children for the inhalation induction of anesthesia, even in the absence of nitrous oxide. It is a nonirritant gas and less pungent, with a low blood solubility compared with isoflurane, which speeds the equilibration of alveolar and inspired anesthetic partial pressures leading to rapid induction in children ( Fig. 10.3 ). The incidence of breath holding, coughing, laryngospasm, and desaturation during induction of anesthesia with sevoflurane is low ( ). Sevoflurane is a potent respiratory depressant at high doses but cardiovascular homeostasis is maintained in infants and children at 1 MAC. There is rapid elimination along with rapid recovery from sevoflurane compared with that for halothane.
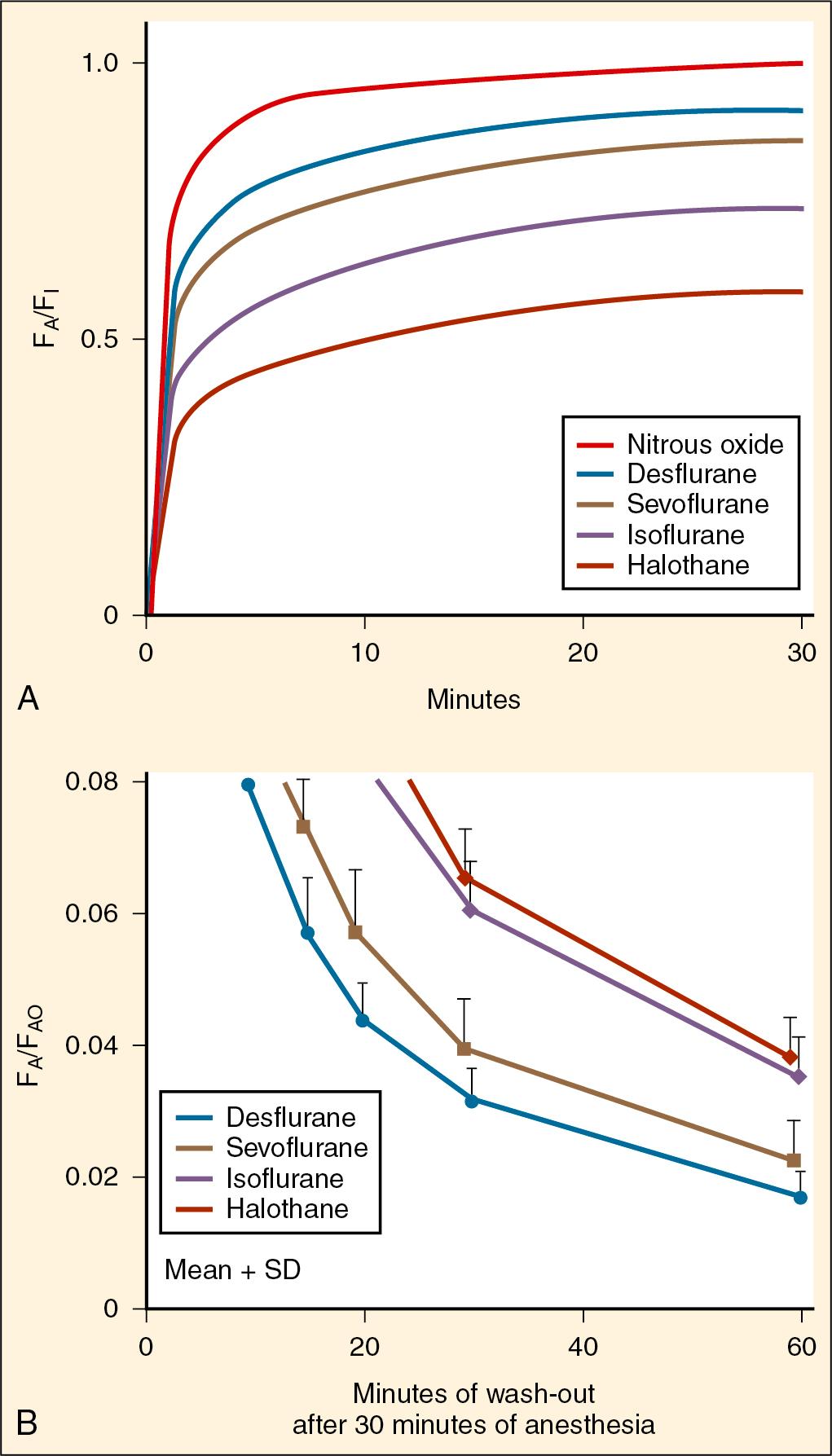
Desflurane is a potent polyfluorinated methyl ethyl ether anesthetic that requires a heated pressurized vaporizer to reliably deliver the agent independent of ambient temperature because its boiling point is close to room temperature. Its molecular structure is similar to that of isoflurane with a single substitution of a chlorine atom with a fluorine atom, which dramatically changes the physicochemical properties of this anesthetic (see Table 10.1 ). Its blood-gas and tissue-blood solubilities are significantly lower than that of other agents such as halothane and isoflurane ( ). As a result, the wash-in and wash-out of desflurane are the fastest of all of the available potent inhalational anesthetics (see Fig. 10.3 ). Because of its low blood solubility, changes in alveolar ventilation and cardiac output will exert small effects on the pharmacokinetics of desflurane, but changes in right-to-left shunting will exert a large effect ( ; ).
Desflurane is not suitable for inhalational induction in children because it is significantly more pungent than halothane and sevoflurane. It may irritate the airway and trigger upper airway reflexes, leading to breath holding and laryngospasm (Taylor and Lerman 1991; Zwass et al. 1992). Therefore sevoflurane is the more widely acceptable agent used in children for inhalational induction of anesthesia, while desflurane is mainly used for maintenance of anesthesia. However, desflurane is not approved for maintenance of anesthesia in children who are not intubated because of an increased incidence of respiratory adverse reactions. This is described in the package insert of desflurane ( ).
Isoflurane is a polyfluorinated methyl ethyl ether anesthetic that shares a molecular structure similar to that of desflurane and it is a liquid at room temperature. It is more soluble in blood and tissues than desflurane so the wash-in and wash-out of isoflurane is slower than that of desflurane ( Fig. 10.3 ). Isoflurane has a pungent odor and may trigger airway reflex responses during inhalational induction like desflurane; therefore, it is not suitable for use as an inhalational induction agent. It is mainly used for maintenance of anesthesia, and children emerge from isoflurane anesthesia without difficulty. Isoflurane does not depress circulation or change the hemodynamics in children, therefore maintaining the blood pressure during surgery.
Halothane was first introduced in 1956 and achieved great success as an inhalational anesthetic agent used for induction and maintenance of anesthesia. It is generally well tolerated and rapidly displaced ether and chloroform, both used since the 1800s, as the surgical anesthetic of choice. However, isolated case reports of hepatotoxicity started to emerge shortly after its introduction ( ; ; ; ). Large-scale review studies eventually yielded identical results of causality between halothane and hepatotoxicity ( ; ), and efforts to develop other halogenated anesthetics with a safer profile led to the introduction of enflurane, isoflurane, desflurane, and sevoflurane. The incidence of halothane-associated hepatitis was found to be much lower in children for unknown reasons ( ; ). When sevoflurane was introduced in 1995, it quickly replaced halothane as the anesthetic agent of choice in children and halothane was virtually eliminated in the United States and Europe. However, halothane continues to be the most commonly used inhalational anesthetic worldwide because of its low cost. Halothane is the most blood-soluble inhaled anesthetic with a slow wash-in. However, the wash-out is similar to that of isoflurane because it is metabolized and can be cleared from the body via the lungs and the liver ( Fig. 10.3 ).
Enflurane is a halogenated inhalational agent that was first used in 1966. It is a structural isomer of isoflurane and is an extremely stable and potent agent. Enflurane is a colorless gas with a pleasant odor and was commonly used as an inhalational anesthetic agent in the 1970s and 1980s but is no longer in common use today. It is relatively insoluble with a blood-gas coefficient of 1.8 and a MAC of 1.68 in 100% O 2 . It can lower the threshold for seizures and its usage is contraindicated in patients with epilepsy. Enflurane undergoes metabolism by hepatic CYP2E1 via oxidative dehalogenation and is excreted in the urine. The extent of metabolism of enflurane is only 2% to 4%, which is markedly lower than that of halothane. There have been isolated reports of severe acute liver injury similar to halothane hepatitis attributed to enflurane exposure, but these are very rare incidences. Enflurane is no longer in use in North America.
Methoxyflurane is a halogenated inhalational agent that was first used clinically in the 1960s into the late 1970s. It can be self-administered via a handheld inhaler by awake patients for relief of moderate or severe trauma pain, and it lasts about 30 minutes with each administration. Methoxyflurane is effective and well tolerated by patients. It is an extremely potent anesthetic agent with a MAC of 0.16 but a very high lipid solubility, which results in a slow induction and slow emergence. Methoxyflurane undergoes extensive hepatic biotransformation, where as much as 50% of the agent is metabolized. It also undergoes significant metabolism in the kidneys to fluoride ions, leading to potential nephrotoxicity from high-serum inorganic fluoride levels. Concurrent use of tetracyclines and methoxyflurane have been reported to lead to renal damage. Manufacturing of methoxyflurane was discontinued in the United States in 1999 and was officially withdrawn from the market by the Food and Drug Administration in 2005. It is still in use in certain European countries and Australia for pain during short medical procedures in the emergency room.
Nitrous oxide is an odorless, inorganic gas at atmospheric pressure and room temperature. It has a MAC value of 104% and is most commonly administered in a concentration of 50% to 75% in oxygen. Nitrous oxide is a weak anesthetic that is typically used as part of a balanced technique with a potent volatile inhalation agent and opioids. It is most commonly used during inhalation induction in children, initially alone as an analgesic and anxiolytic for peripheral intravenous access or mask acceptance and then in combination with sevoflurane to speed up the uptake of the more potent anesthetic agent. It also reduces the anesthetic requirements for the more potent inhalational agents because it contributes to the total MAC during the maintenance of anesthesia. It has a low blood and tissue solubility (partition coefficient of 0.47), resulting in faster equilibration of partial pressures between blood and alveoli and rapid induction and emergence. However, preoxygenation and denitrogenation of the alveoli will not necessarily remove all of the nitrogen molecules from preexisting pockets of air in the body of the patient. Air consists of 21% oxygen and 78% nitrogen, and nitrogen is highly insoluble with a blood-gas partition coefficient 0.015. Therefore nitrogen can get trapped in the gas compartments of the body because it does not pass easily from gas to blood. Nitrous oxide, with a blood-gas coefficient of 0.47, is roughly 34 times more soluble than nitrogen and can quickly and readily transfer across membranes and enter closed gas-filled spaces more than 30 times faster than nitrogen can diffuse out of the space proportionally. The volume of distensible space will increase until the nitrous oxide concentration in the space is equal to the concentration in the alveoli and blood concentration. The use of nitrous oxide should be avoided in middle ear surgery and patients presenting with small bowel obstruction or pneumothorax.
Nitrous oxide was the most widely used anesthetic agent worldwide for decades, owing to its low cost, rapid pharmacokinetics, and minimal cardiopulmonary side effects, but concerns over potential complications have markedly reduced its use. The ENIGMA Trial, which randomized over 2000 patients undergoing noncardiac surgery lasting more than 2 hours to nitrous oxide–based or nitrous oxide–free anesthesia, suggested that exposure to nitrous oxide was associated with increased long-term risk of myocardial infarction but not of death or stroke ( ). A subsequent trial by the same group, the ENIGMA-II Trial, with over 9000 patients randomized to nitrous oxide or not, showed that nitrous oxide did not increase the risk of death and cardiovascular complications or surgical site infection after noncardiac surgery overall or in any subgroup of patients. The emetogenic effect of nitrous oxide can be controlled with antiemetic prophylaxis ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here